Comparison of Physiological Properties Between Dwarf and Vinetype Cucumbers (Cucumis sativus Linn.)
2014-03-07XinMingQinZhiweiZhouXuiyanandWuTao
Xin Ming, Qin Zhi-wei, Zhou Xui-yan, and Wu Tao
College of Horticulture, Northeast Agricultural University, Harbin 150030, China
Comparison of Physiological Properties Between Dwarf and Vinetype Cucumbers (Cucumis sativus Linn.)
Xin Ming, Qin Zhi-wei*, Zhou Xui-yan, and Wu Tao
College of Horticulture, Northeast Agricultural University, Harbin 150030, China
Dwarfism is one of the most important traits in crop breeding. In this study, a dwarf cucumber D0462 and a vine NA129 were taken as experimental materials. The metabolism of hydrogen peroxide (H2O2) andsuperoxide anion radical (O2-•), activities of antioxidant enzymes, and the levels of endogenous hormones of the two cultivars were compared. In the whole growth period, D0462 maintained higher H2O2and O2-• levels than NA129. The activities of SOD (superoxide dismutase), CAT (catalase), APX (ascorbate peroxidase) and IAAO (indoleacetic acid oxidase) in D0462 were found higher than those in NA129. The contents of gibberellic acid (GA3), indole-3-acetic acid (IAA), zeatin riboside (ZR) and jasmonic acid (JA) in internode were determined by enzyme-linked immunosorbent assay (ELISA). The internode of D0462 remained lower levels of GA3, IAA, and ZR. In contrast, JA level in internode of D0462 was higher than that of NA129. Compared with NA129, the cell mumbers in internode of D0462 did not change, but cell size evidently decreased. The chloroplasts in mature mesophyll D0462 exhibited swollen filled with starch grains instead of regular oval ones, and thylakoids of chloroplast swelled and became ambiguous.
dwarf, cucumber, antioxidant enzyme, endogenous hormone
Introduction
Lodging has been a problem impacting most crop and vegetable productivity (Sameri et al., 2009). The fungal infection and spindling extensively occur, and the inefficiency of mechanical harvesting are serious problems due to plant lodging (Tar'an et al., 2003). However, short plant height can reduce the lodging risk as well as improve yield and commodity (Hellewell et al., 2000). Dwarf is an important agronomic trait in crop breeding. And dwarf mutants have been utilized extensively in plant breeding. Dwarf genotypes have been reported to improve lodging resistance, increase yield, enhance fertility, improve early maturity, and induce high tillering capacity (Ren et al., 2010).
Cucumber (Cucumis sativus L. 2n=2x=14) is an important vegetable. The compact (dwarf) characterization is an important trait in cucumber breeding. Selecting directly for yield is proposed common and the most effective approach to screen excellent varieties of cucumbers (Cramer and Wehner, 2000). A large and well known dwarf genotypes or mutants in agriculture attributes to hormone-deficience (Hedden et al., 2000). Many plant endogenous hormones have been found to be the key elements involved in dwarfism, such as IAA, GA3, JA and ZR. Plant development is a complicated process, whichis regulated by interaction of physicochemical properties, including antioxidants and hormone. The plant hormones have been reported to involve in regulating antioxidant activitiy (Buran et al., 2012). As previously reported, the hormones such as IAA, GA, ABA (abscisic acid), JA and salicylic acid (SA) in plants affected the levels of antioxidase, and changed the antioxidant capacities of plants (Zhang et al., 2007; Sun et al., 2012; Agarwal et al., 2005). Under lead stress, application of exogenous hormones ABA could significantly increase SOD, CAT, POD, and APX activities in Atractylodes macrocephala (Wang et al. 2013). On the contrary, antioxidant levels acting as free radical scavengers could affect the metabolism of hormone (Li et al., 2007). However, there are only few studies focusing on the differences between endogenous hormones and antiocidant of the dwarf and vine cucumber.
Materials and Methods
Plant materials and growth conditions
The vine (NA129) and the dwarf cultivars (D0462) were taken as materials in this study. D0462 has visible shorter height and smaller number of internode than NA129. Seeds of both lines were immersed in water overnight and sown in a nursery bed. One-month old seedlings were transplanted to the experimental Station of Horticulture at Northeast Agricultural University.
The first internode samples were randomly collected from 10 plants of D0462 and NA129 at different developmental phases after germination: 23 (trophophase), 30 (flowering phase), 37 (fruiting phase), 44 (full bearing period), and 51 days (senescence phase), respectively. Samples were immediately frozen in liquid nitrogen, and then stored at –80℃. Plant height was monitored during different growth periods.
Analysis of antioxidant enzyme activity and H2O2and O2-• contents
The extracts of 1.0 g young internode were suspended with 50 mmol • L-1phosphate buffer (pH 7.5) containing 2% polyvinylpyrrolidone (PVP). SOD activity was measured according to the method of Beyer and Fridovich (1987). One unit of SOD activity was defined as the amount of the extract that caused 50% nitroblue tetrazolium (NBT) reduction under the experimental conditions. CAT activity was assayed by monitoring the consumption of H2O2as described by Aebi (1984). One unit of CAT was expressed as l mol of H2O2oxidized mg-1protein min-1. APX activity was determined by monitoring the decrease in absorbance at 290 nm, as the method given by Nakano and Asada (1981). APX activity was expressed as l mol ascorbate oxidized mg-1protein min-1. IAAO activity was measured as the method of Zhang et al. (2009). IAAO activity was presented in μmol IAA destructed mg-1protein min-1. The level of H2O2and O2-• were determined according to the method described by Yiu et al (2009).
Determination of endogenous hormone
The extraction and purification of endogenous IAA, GA3, JA and ZR were measured by modified method of Arkhipova et al (2005). Homogenized internode samples were conducted in cold 80% (v/v) methanol for 4 h at 4℃. The supernatant was collected after centrifugation at 10 000 g for 15 min. Then the crude extract was passed through a C-18 sep-column (Waters, Milford, MA). The efflux was collected and dried with a stream of N2, the residue was then dissolved in 2 mL phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5). IAA, GA3, JA and ZR were determined by ELISA kits based on monoclonal antibodies (China Agricultural University) according to the protocol provided by the manufacturer. ELISA tests were performed with 96-well microtitration plates. Each hormone was tested in triplicates.
Observation of plant structure
Young internodes and leaves of D0462 and NA129 were cut into small fragments (1 mm×2 mm) anddoubly fixed with 2.5% glutaraldehyde (pH 6.8) and 1.2% osmic acid. The prepared internode samples were subsequently dehydrated in a graded ethanol series and embedded with an Epon812 (E Micron Technologies Limited, China). Transverse sections were sliced using a microtome RMC POWERTOME XL (RMC, US) and leaf ultrastructure was observed using a transmission electron microscope H-7650 (Hitachi, Japan).
Statistical analysis
SPSS 19.0 statistical software package (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. The data was represented as mean±standard deviation (SD). Differences of mean values were valued by using the one-way analysis of variance (ANOVA) and Duncan's multiple range tests. Statistically significant differences between individual means were assumed at P<0.05.
Results and Discussion
Plant morphology
From the seedling stage, D0462 was easily identified. Obvious differences in morphology were found between D0462 and NA129. Compared with NA129, dwarf plants D0462 had shorter and less internodes, and less branches. However, there was no difference in the leaf size between the two cucumber cultivars (Fig. 1). The plant heights at different development stages were presented in Table 1. The result showed that D0462 remained significantly shorter height than NA129. The adult plant height of D0462 was over 60% shorter than that of NA129 at the aging stage (Table 1).

Fig. 1 Growth character of D0462 (left) and NA129 (right)
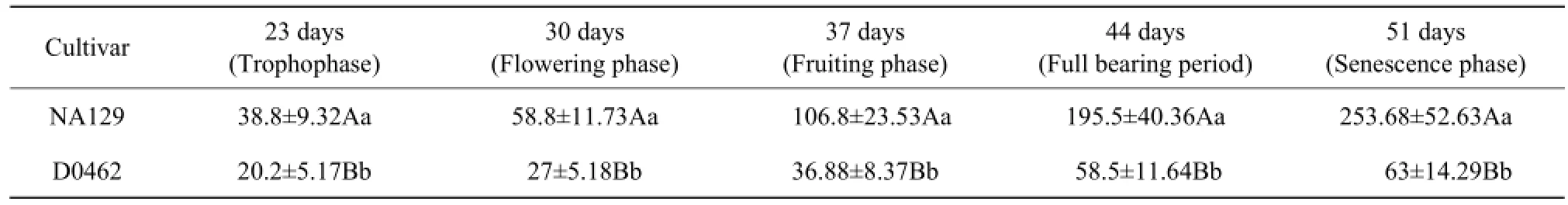
Table 1 Evaluation of plant height of D0462 and NA129 during growth stage (cm)
Contents of H2O2and O2-•
The increase in ROS, such as H2O2and O2-•, was concluded to play a role in cell senescence (Zhang et al., 2011). In our present study, O2-• and H2O2levels of D0462 were higher than those of NA129. The contents of O2-• and H2O2in D0462 showed similar patterns as those in NA129 (Fig. 2). The maximum H2O2content of D0462 was 2.94 μmol • g-1FW, which was 23.82% more than that of NA129 at the same sampling time. H2O2content of D0462 increased until full bearing period (44 days) after gerneration and then decreased (Fig. 2B). There was no obvious differences in O2-• contents between D0462 and NA129 in the fruiting phase (37 days). The maximum O2-• content of D0462 was (49.71±5.72)μmol • g-1FW, approximately 8.69% more than that of NA129 (Fig. 2A). Wu et al. (2008) observed that the high level O2-• was linked to the inhibition of internode elongation in pumpkin.
Antioxidant enzymatic activity
Antioxidant enzyme system, such as SOD, CAT and APX, significantly increased resulting from ROS overproduction (Tartoura and Youssef, 2011). The results of the present study indicated that the activity of SOD, CAT and IAAO in D0462 remained much higher than those in NA129 during growth period (Fig. 3). SOD activities of D0462 and NA129 increased gradually, and peaked at full bearing period (44 days) after germination. Maximum SOD activity of D0462 was (293.92±45.32) U • g-1, which was 66.14% more than that of NA129 (Fig. 3A). Similarly, maximum activity of CAT in D0462 and NA129 was discovered at full bearing period (44 days), reaching (126.33±36.36) U • g-1and (81.79±34.27) U • g-1, respectively. In both lines, the activity of CAT showed a similar tendency. CAT activities in D0462 and NA129 at full bearing period (44 days) increased 4.48 and 7.76 times more than those at trophophase (23 days), respectively (Fig. 3B). APX activities in leaves of both D0462 and NA129 decreased slightly at first, and then increased. APX activities of both D0462 and NA129 reached maximum (617.17±32.44 and 576.49±43.65 U • g-1, respectively) at full bearing period (44 days) and then dropped down a little (Fig. 3C). Maximum enhancement in the activity of IAAO (1 086±87.29 U • g-1FW ) was observed in leaves of D0462 at full bearing period (44 days). However, IAAO activity in leaves of NA129 changed little during growth (Fig. 3D). The growth and development of plants is a complicated process, which is regulated by some plant regulators, such as endogenous hormone and antioxidant enzyme (Yan et al., 2010). It was reported that antioxidant levels acting as free radical scavengers could affect the metabolism of hormone (Li et al., 2007). IAAO has been documented to participate in regulating plant endogenous hormone content, especially IAA.
Endogenous hormone concentrations
Zhao et al. (2010) concluded that plant hormones interacted in a very complex way. Balancing hormone metabolisms played critical roles in proper regulation of plant growth and development, and controlled plant hight, growth and development (Peng et al., 2009). Endogenous hormone contents in inernodes of both genotypes were investigated during growth. The results showed that the endogenous hormones (GA3, IAA, ZRand JA) contents were found to be higher in NA129 than those in D0462 at all the stages of development (Fig. 4).
In NA129, GA3and IAA significantly increased at fruiting phase (37 days), and then decreased. The endogenous GA3level in NA129 reached 571.53 ng • g-1FW, and 1.6 times higher than that in D0462 (Fig. 4A) at fruiting phase (37 days). It was reported that GA3could promote cell elongation in either excised or intact internode tissue (Yaxley et al., 2001) and acted as a regulator of stem elongation (Fujioka et al., 1988). Many dwarf mutants have been characterised as gibberellic acid (GA) deficient or insensitive mutants (Zhang et al., 2011). Patel and Thaker (2007) found that GA3involved in the internode elongation.
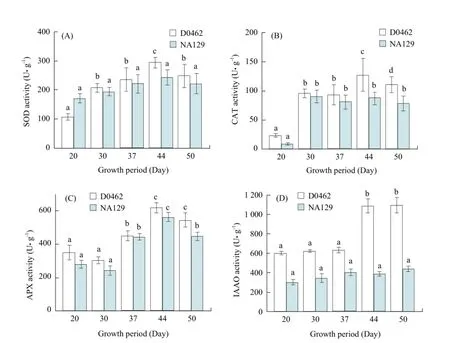
Fig. 3 Activities of SOD (A), CAT (B), APX (C) and IAAO (D) in internode during development period of D0462 and NA129
IAA content in internode of NA129 rapidly increased, peaked at fruiting phase (1070.23±42.47 ng • g-1FW), and then sharply decreased later. In D0462, however, no significant change of IAA content in internode was observed, and plant hormone in D0462 still remained lower level (Fig. 4B). IAA was also reported to play essential role in plant growth (Mahouachi et al., 2007). IAA was demonstrated to promote normal stem elongation (Wu et al., 2009). The result showed that both GA3and IAA levels remained significantly greater in D0462 than those in NA129 during growth period except the full bearing period (44 days). It suggested an essential involvement of GA3and IAA in the height characterization of cucumber (Fig. 4B). And GA3and IAA probably related to the induction elongation and plant growth.
ZR content in both NA129 and D0462 increased and reached the peak at full bearing period (577± 65.27 ng • g-1FW and 379.61±20.87 ng • g-1FW, respectively), and then decreased slightly at the end ofthe experimental period (Fig. 4). These data suggested that ZR might involve in fruiting, whereas the effect of ZR on plant height was ambiguous. The same conclusion of rice has been described by Yang et al (2000).
JA was demonstrated as a significant regulator to control the elongation growth of plants (Ueda et al., 1994; Maciejewska and Kopcewicz, 2002). In the previous reports, exogenous JA application not only diminished the effect of GA3on the elongation of the second leaf sheath of dwarf rice seedlings and lettuce hypocotyls (Yamane et al., 1981), but also reduced IAA-induced elongation of etiolated oat coleoptile (Ueda et al., 1994). In this paper, JA levels in D0462 stems were higher than those in NA129 throughout the growth period (Fig. 4D). This result was supported by Kong et al. (2005), who found that JA probably inhibited stem elongation of Pharbitis nil.
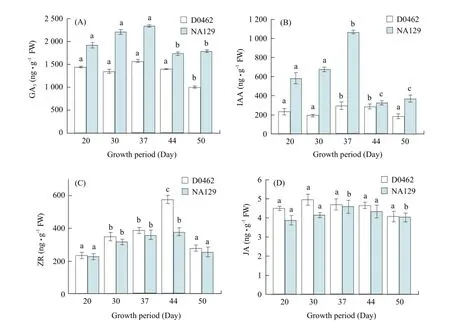
Fig. 4 Leavels of GA3(A), IAA (B), ZR (C) and JA (D) in internode of D0462 and NA129 during growth stage
Microstructure and ultrastructure of internode
The young internodes and leaves from D0462 and NA129 were investigated by SEM (Fig. 5). Microscopic observation showed that there were no obvious differences in the number the number of collenchyma cell layer and in internode between D0462 and NA129. However, the length of cells in internode was reduced noticeably in D0462. Otherwise, the number of vascular bundle increased, as well the number of secondary thickened vessel in D0462 increased. It investigated that the size of cells in internode significantly decreased, whereas the cell well thickened in collenchyma cells in D0462 (Fig. 5A). Compared with NA129, elongation of parenchyma cells retarded markedly at the early stage in D0462, which mightcontribute to the dwarfism. Previous studies have shown that dwarfism in rice and pumpkin mutant could be attributed to a defect in elongation of parenchyma cells (Gao et al., 2009; Wu et al., 2007).
When observed by TEM, the mesophyll cells of mature leaves (50-day-old) in NA129 were somewhat vacuolated and filled with chloroplasts and grains. And nucleus was close to chloroplasts. The chloroplast possessed well developed grana and stromal thylakoids (Fig. 6a and c).
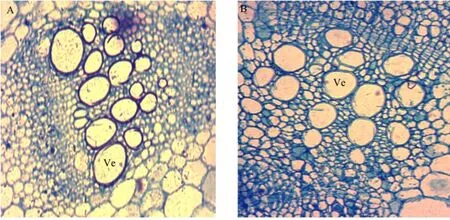
Fig. 5 Microstructure of main stem in D0462 (A) and NA129 (B) on 40 days
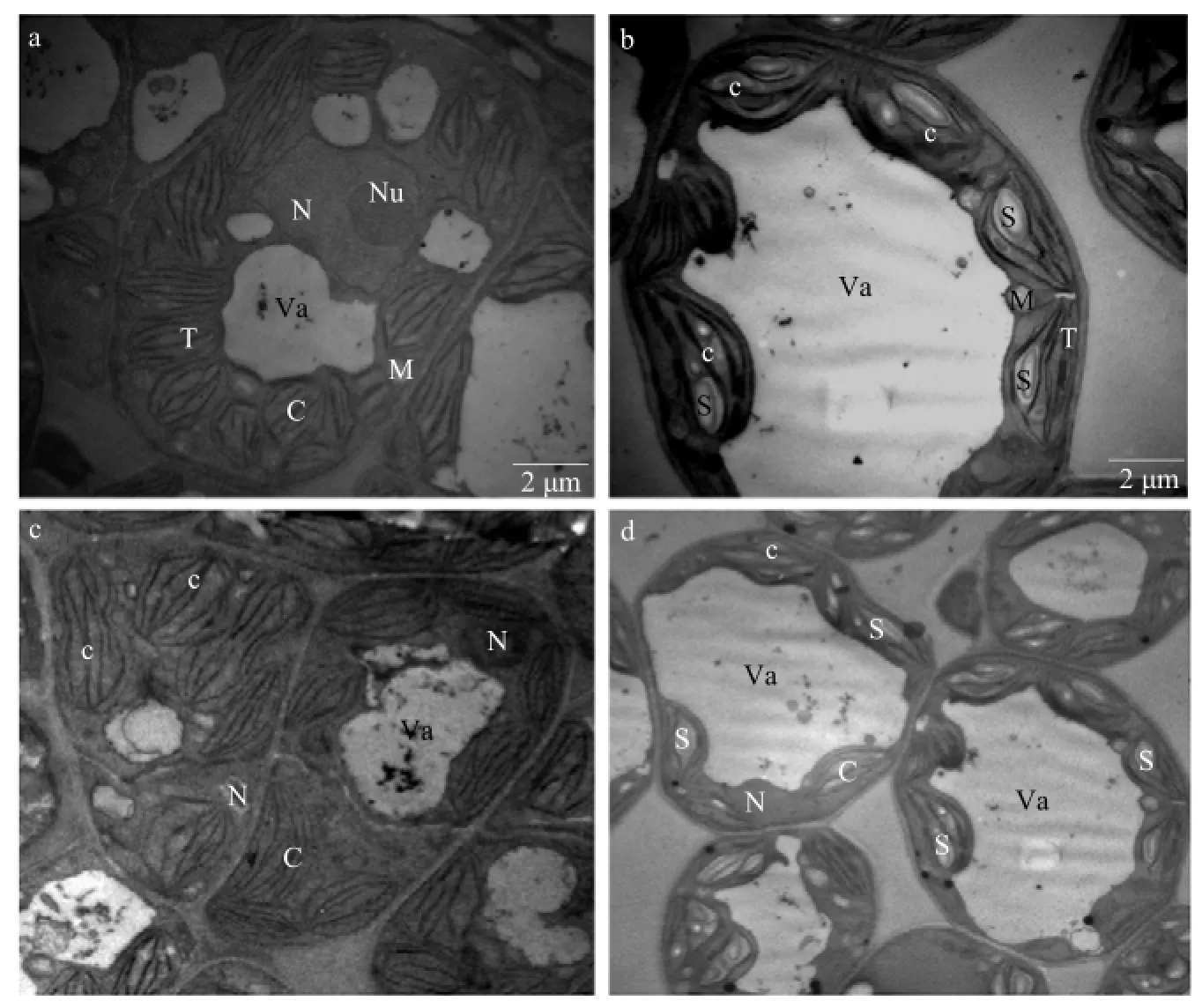
Fig. 6 Transmission electron microscopy (TEM) micrographic images of D0462 and NA129
Chloroplast was a main producer of ROS (Sun et al., 2002), which was known as one of most sensitive organelles in leaf tissues (Krause and Santarius, 1975). Therefore, it might be inevitably affected on ultrastructure of chloroplasts as a result of much accumulation of ROS (Asada et al., 1998). Therefore, an increase in the number of plastoglobuli with different shapes could be observed in chloroplasts with swollen thylakoids in D0462 (Fig. 6b and d). The thylakoids were found ambiguous or disappeared in some chloroplasts. Compared to NA129, an increase of enlarged starch grains in the chloroplasts was detected in D0462. Finally, an increase in vacuole volume with a large quantity of electron-dense points could be seen.
Aebi H. 1984. Catalase in vitro. Methods Enzymol, 105: 121-126.
Agarwal S, Sairam R K, Srivastava G C, et al. 2005. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Science, 169: 559-570.
Arkhipova T N, Veselov S U, Melentiev A I, et al. 2005. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant and Soil, 272: 201-209.
Asada K, Endo T, Mano J, et al. 1998. Molecular mechanisms for relaxation of and protection from light stress. in: Satoh K, Murata N. ed. Stress responses of photosynthetic organisms. Elsevier Science Publishing, Amsterdam. pp. 37-52.
Jr Beyer W F, Fridovich I. 1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry, 161: 559-566.
Buran T J, Sandhu A K, Azeredo A M, et al. 2012. Effects of exogenous abscisic acid on fruit quality, antioxidant capacities, and phytochemical contents of southern high bush blueberries. Food Chemistry, 132: 1375-1381.
Cramer C S, Wehner T C. 2000. Fruit yield and yield component correlations of four pickling cucumber populations. Cucurbit Genitics Coop Report, 23: 12-15.
Fujioka S, Yamane H, Spray C R, et al. 1988. The dominant nongibberellin-responding dwarf mutant (D8) of maize accumulates native gibberellins. Proceedings of the National Academy of Sciences of the United States of America, 85: 9031-9035.
Gao Z, Qian Q, Liu X, et al. 2009. Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Molecular Biology, 71: 265-276.
Hedden P, Phillips A L. 2000. Gibberellin metabolism: new insights revealed by the genes. Trends in Plant Science, 5: 523-530.
Hellewell K B, Rasmusson D C, Gallo-Meagher M. 2000. Enhancing yield of semi-dwarf barley. Crop Science, 40: 352-358.
Kong F J, Gao X Q, Nam K H, et al. 2005. Theobroxide inhibits stem elongation in Pharbitis nil by regulating jasmonic acid and gibberellin biosynthesis. Plant Science, 169: 721-725.
Krause G H, Santarius K A. 1975. Relative thermostability of the chloroplast envelope. Planta, 127: 285-299.
Li X M, He X Y, Chen W, et al. 2007. Effects of elevated CO2and/or O3on hormone IAA in needles of Chinese pine. Plant Growth Regulation, 53: 25-31.
Maciejewska B, Kopcewicz J. 2002. Inhibitory effect of methyl jasmonate on flowering and elongation growth in Pharbitis nil. Journal of Plant Growth Regulation, 21: 216-223.
Mahouachi J, Arbona V, Go'mez-Cadenas A. 2007. Hormonal changes in papaya seedlings subjected to progressive water stress and rewatering. Plant Growth Regulation, 53: 43-51.
Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxides in spinach chloroplast. Plant and Cell Physiology, 22: 867-880.
Patel D, Thaker V S. 2007. Estimation of endogenous contents of phytohormones during internode development in Merremia emarginata. Plant Biology, 51: 75-79.
Peng Z, Zhou X, Li L, et al. 2009. Arabidopsis hormone database: a comprehensive genetic and phenotypic information database for plant hormone research in Arabidopsis. Nucleic Acids Research, 37: 975-982.
Ren X F, Sun D F, Guan W W, et al. 2010. Inheritance and identification of molecular markers associated with a novel dwarfing gene in barley. BMC Genetics, 11: 89.
Sameri M, Nakamura S, Nair S K, et al. 2009. A quantitative trait locus for reduced culm internode length in barley segregates as a Mendelian gene. Theoretical and Applied Genetics, 118: 643-652.
Sun B, Yan H Z, Zhang F, et al. 2012. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Research International, 48: 359-366.
Sun W, Montagu M V, Verbruggen N. 2002. Small heat shock proteinsand stress tolerance in plants. Biochim Biochimica et Biophysica Acta, 1577: 1-9.
Tar'an B, Warkentin T, Somers D J, et al. 2003. Quantitative trait loci for lodging resistance, plant height and partial resistance to mycosphaerella blight in field pea (Pisum sativum L.). Theoretical and Applied Genetics, 107: 1482-1491.
Tartoura K A H, Youssef S A. 2011. Stimulation of ROS-scavenging systems in squash (Cucurbita pepo L.) plants by ompost supplementation under normal and low temperature conditions. Scientia Horticulturae, 130: 862-868.
Ueda J, Miyamoto K, Aoki M. 1994. Jasmonic acid inhibits the IAAinduced elongation of oat coleoptile segments: a possible mechanism involving the metabolism of cell wall polysaccharides. Plant and Cell Physiology, 35: 1065-1070.
Wang J, Chen J, Pan K. 2013. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environmental Science and Pollution Research International, 20: 1441-1449.
Wu C T, Zhou B L, Zhang T Z. 2009. Isolation and characterization of a sterile-dwarf mutant in Asian cotton (Gossypium arboreum L.). Journal of Genetics and Genomics, 36: 343-353.
Wu T, Cao J S, Zhou J H. 2008. Comparison of antioxidant activities and endogenous hormone levels between bush and vine-type tropical pumpkin (Cucurbita moschata Duchesne). Scientia Horticulturae, 116: 27-33.
Wu T, Zhou J H, Zhang Y F, et al. 2007. Characterization and inheritance of a bush-type in tropical pumpkin (Cucurbita moschata Duchesne). Scientia Horticulturae, 114: 1-4.
Yamane H, Takagi H, Abe H, et al. 1981. Identification of jasmonic acid in three species of higher plants and its biological activities. Plant and Cell Physiology, 22: 689-697.
Yan K, Chen W, Zhang G Y, et al. 2010. Elevated CO2ameliorated oxidative stress induced by elevated O3in Quercus mongolica. Acta Physiologiae Plantrum, 32: 375-385.
Yang J C, Peng S B, Visperas R M, et al. 2000. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regulation, 30: 261-270.
Yaxley J L, Jablonski W, Reid J B. 2001. Leaf and flower development in pea (Pisum sativum L.): mutants cochleata and unifoliata. Annals of Botany, 88: 225-234.
Yiu J C, Liu C W, Fang D Y T, et al. 2009. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiology and Biochemistry, 47: 710-716.
Zhang H, Hu S L, Zhang Z J, et al. 2011. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology, 60: 251-257.
Zhang H, Shao M, Qiao Z, et al. 2009. Effect of phytohormones on fiber initiation of cotton ovule. Acta Physiologiae Plantarum, 31: 979-986.
Zhang M, Duan L, Tian X, et al. 2007. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. Journal of Plant Physiology, 164: 709-717.
Zhao H F, Qiu K, Ren G D, et al. 2010. A pleiotropic phenotype is associated with altered endogenous hormone balance in the developmentally stunted mutant (dsm1). Journal of Plant Biology, 53: 79-87.
S642.2
A
1006-8104(2014)-02-0019-09
Received 29 October 2013
Supported by the China Postdoctoral Science Foundation (2012M520701); the Key Laboratory Biology and Genetic Improvement of Horticultural Crops (Northeast Region), Ministry of Agriculture (CVB2012-001)
Xin Ming (1981-), male, Ph. D, lecturer, engaged in the research of vegetable breeding. E-mail: xinmingneau@126.com
* Corresponding author. Qin Zhi-wei, professor, supervisor of Ph. D student, engaged in the research of cucumber breeding. E-mail: qzw303@126. com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regulation of Foliar Application DCPTA on Growth and Development of Maize Seedling Leaves in Heilongjiang Province
- Effects of Substitute Media on Development of Potted Cyclamen percicum Mill.
- Expression of HSP72 in Mouse Preimplantation Embryos with Heat Shock
- Effects of Maternal Dietary Energy Restriction on Fat Deposition of Offspring
- Effects of Dietary Protein and Temperature on Growth and Flesh Quality of Songpu Mirror Carp
- Synthesis and Identification of SG-BSA and SG-OVA
