Regulation of Foliar Application DCPTA on Growth and Development of Maize Seedling Leaves in Heilongjiang Province
2014-03-07GuWanrongMengYaoZhangJunbaoJiBiaoWangYongchaoLiJingandWeiShi
Gu Wan-rong, Meng Yao, Zhang Jun-bao, Ji Biao, Wang Yong-chao, Li Jing and Wei Shi*
1College of Agriculture, Northeast Agricultural University, Harbin 150030, China
2Observation Experiment Station of Ministry of Agriculture for Crop Cultivation Science in Northeast Area, Harbin 150030, China
3Heilongjiang Academy of Land Reclamation Sciences, Jiamusi 154002, Heilongjiang, China
Regulation of Foliar Application DCPTA on Growth and Development of Maize Seedling Leaves in Heilongjiang Province
Gu Wan-rong1,2, Meng Yao3, Zhang Jun-bao1,2, Ji Biao1,2, Wang Yong-chao1,2, Li Jing1, and Wei Shi1*
1College of Agriculture, Northeast Agricultural University, Harbin 150030, China
2Observation Experiment Station of Ministry of Agriculture for Crop Cultivation Science in Northeast Area, Harbin 150030, China
3Heilongjiang Academy of Land Reclamation Sciences, Jiamusi 154002, Heilongjiang, China
DCPTA (2-diethylaminoethyl-3, 4-dichlorophenylether) is a new plant regulator which can be used to regulate growth and development for crops. Experiments on maize seedlings were conducted in the growth chamber to study the effects of foliar applied DCPTA. The plant pots were placed in a completely randomized design with three replicates. The maize seedlings were treated with 0 mg • L-1(control), 20 mg • L-1and 40 mg • L-1DCPTA solution. The effects of DCPTA on the photosynthetic characteristics (photosynthesis, stomata conductance, intercellular CO2, and transpiration rate), related physiological characteristics (contents of soluble sugar and starch), chlorophyll fluorescence parameters (Fo, Fm, Fv/Fm, Fv/Fo, qP, and qN) and the weight of dry matter in maize seedling were studied. The results showed that DCPTA enhanced photosynthesis of maize seedling. In general, photosynthetic rate in leaves was significantly promoted through spraying DCPTA solution, and 40 mg • L-1DCPTA was found to be the best concentration for maize. The relationship between stomata conductance and transpiration rate in maize leaves could be described as linear. With regard to the chlorophyll fluorescence parameters, our findings showed that 40 mg • L-1DCPTA in maize seedling caused an increase in Fm, Fv/Fm, Fm/Fo, qP and a decrease in Fo and qN at some time points checked. It is suggested that DCPTA increased photosynthetic rate by raising both the content of chlorophyll and activities of PSII and the contents of sugar and starch. Compared with the control, the treated maize seedling caused an increase in plant height, root length, shoot dry mass, root dry mass, or the total (root plus shoot) dry mass.
DCPTA, photosynthesis, growth and development, maize seedling
Introduction
Tertiary amine substance is a kind of high bioactive regulator with small molecular mass. DCPTA (2-diethylaminoethyl-3, 4-dichlorophenylether) and DTA-6 (the analog of DCPTA) are the two representative regulators. DCPTA is one of the most representative tertiary compounds (Ren et al., 2003), which has been used widely in recent years. DCPTA has various influences on physiological process in plants, which has strong abilities to regulate photosynthesis of the plants and the activity of relative enzymes (Yokoyama et al., 1977; Gausman et al.,1991). The study has proved that tertiary amines substance can promote the effectiveness and the ratio of utilization of carbohydrate in the process of synthesis and metabolism (Chen, 1995; Keithly et al., 1991). Previous work has confirmed that DCPTA can manipulate and promote many kinds of plant growth from the concentration 1 mg • L-1to 40 mg • L-1(Stephen et al., 1977; Stephen et al., 1982; Lu et al., 2000). Some studies show that DCPTA can increase the ability of root activity and the ability to resist disease and stress conditions are increased (Keithly et al., 1988). The yield and the nutritional quality are improved (Keithly et al., 1990; Zhang et al., 2001). It can also induce β-carotenoid biosynthesis and increase its content (Poling et al., 1977). Spraying DCPTA can speed up seedling growth, chlorophyll content increase, and the ability of photosynthesis in different plants, which indicates that DCPTA may be involved in regulation of some photosynthetic reactions (Keithly et al., 1988; Gausman et al., 1985; Liang et al., 1998; Gu et al., 2008; Zhou et al., 2004).
Up to now, few has been known about the mechanism of the promotive effects of DCPTA on plant photosynthesis. However, detailed understanding of the effect of DCPTA on key physiological processes determining plant productivity is warranted prior to practical application. The main objective of study was to investigate the response of photosynthetic responses of maize after foliar application of DCPTA. 0 mg • L-1(CK), 20 mg • L-1and 40 mg • L-1DCPTA concentrations were selected to determine which could be expected to induce a strong regulation of photosynthesis in maize. In this research, we investigated the effects of DCPTA on photosynthesis, stomata conductance, intercellular CO2, transpiration rate, the content of soluble sugar and starch, chlorophyll fluorescence parameters and the weight of dry matter in maize. These results might be useful for researchers attempting to study the effects of DCPTA, which might pave the way for wider use in maize production, especially in Heilongjiang Province.
Materials and Methods
Materials
The research was conducted in a growth chamber under 30℃/20℃ and 10 h day/14 h night conditions with a relative humidity of 75% in Northeast Agricultural University in 2012. Maize hybrid Zhengdan 958 plants were grown in small pots (30 cm diameter, 30 cm deep) containing soil and vermiculite. Hoagland nutrient solution and water were supplied sufficiently throughout the pots.
Experiment design
The plant pots were placed in a completely randomized design with three replicates. Nine seedlings per pot were grown at the beginning, and then thinned to three seedlings per pot at the third-leaf stage of maize. Leaf samples were taken from each pot of plants and used for the measurements. At three-leaf stage, seedlings were sprayed with DCPTA and used in the following measurements. Each treatment consisted of 12 pots, which were later on used for sampling and analysis. Each measurement consisted of at least three separate samplings.
DCPTA was obtained from China Zhengzhou Zhengsi Chemical Limited Company, and was dissolved with distilled water containing 0.02% (v/v) Tween-20. The young plants were sprayed with the solutions for two sides until dripping with an atomizer. Each plant required 5 mL of solution. The treatments were as follows: CK (sprayed with water containing 0.02% Tween-20; 20 mg • L-1DCPTA; 40 mg • L-1DCPTA).
Methods
Photosynthetic rate measurement
Gas exchange was measured with a portable photosynthesis system Li-6400 equipped with the standard leaf chamber (encloses 6 cm2of leaf area). All the photosynthetic measurements were taken with a constant airflow rate of 500 μmol • s-1. The concentration of CO2was (400±5) mm3• dm-3and the temperature was(27±2)℃. The net rate of photosynthesis (A), stomata conductance (g) and intercellular CO2concentration (Ci) were determined at light saturation level with incident photosynthetic photo flux density (PPFD) of 1 000 μmol • m-2s. WUE (water use efficiency) was measured by the ratio of A/E. All the measurements for plants were made at 8:00 a.m. and 11:00 a.m. to avoid any effects of photo-inhibition and were repeated six times using different fully expanded leaves. All these parameters were measured every three days for five times after foliar application with different concentrations of DCPTA.
Chlorophyll fluorescence measurements
Modulated chlorophyll fluorescence was analyzed with PAM-2000 (a pulse amplitude modulation fluorescence), an instrument that has been used widely in basic and applied fluorescence research. Procedures used for measuring some parameters were based on standard methodologies as documented in PAM-2000 manual.
Fv/Fm quantified the maximal efficiency of photon capture by open PSII reaction centers, and was one of the most widely used chlorophyll fluorescence features. It was calculated from the equation Fv/ Fm=(Fm-Fo)/Fm, where Fm was the maximal fluorescence yield of a dark-adapted sample, with all PSII reaction centers fully closed, and Fo was the minimum fluorescence yield of a dark-adapted sample, with all PSII reaction centers fully open. Effective quantum yield, which denoted the actual efficiency of PSII photon capture in the light by closed PSII reaction centers, was determined as ΔF/Fm'=(Fm'-Ft)/Fm', where Fm' was the maximal fluorescence of a preilluminated sample with PSII centers closed, and Ft was the fluorescence at steady-state. For measurement of maximal fluorescence induction Fv/Fm, leaves were dark-adapted for at least 25 min. For a period of 300 s, saturation pulses were triggered every 20 s to determine Fs' and Fm' until a quasi steadystate was reached. Finally, Fo' was recorded on the again darkened leaves exposed to a weak modulated measuring beam. qP and qN were calculated by fomulas qP=(Fm′–Ft)/(Fm′–Fo′), and qN=1-(Fm′–Fo′)/(Fm–Fo) (Schreiber et al., 1995).
Chlorophyll content (leaf greenness) measurements
A portable chlorophyll meter SPAD-502 was used to measure leaf greenness of the maize plants. Measured each fully expanded leaf at eight locations near the midrib and then averaged.
Assay of relative water content and electrolyte leakage
Leaf relative water content (RWC) was estimated according to the following method. Leaf samples were weighed (fresh weight) immediately and placed in a water vapour-saturated vial at 5℃ for 48 h and weighed (turgid weight). The samples were dried in an oven at 80℃ for 48 h and their dry weights were determined. RWC was calculated by the following fomula: RWC= [(fresh weight–dry weight)/(turgid weight–dry weight)]*100.
Electrolyte leakage was measured as an indicator of the ability of cellular membranes to maintain integrity. Membrane permeability of the leaves was measured by using the young leaf discs of nine plants for each treatment (three/replicate). The samples were washed three times with deionized water to remove surface-adhered electrolytes. Leaf discs were placed in closed vials containing 10 mL deionized water and incubated at 25℃ on a rotary shaker for 24 h. Subsequently, electrical conductivity of the solution (C1) was determined. Samples were then autoclaved at 120℃ for 20 min and the last electrical conductivity (C2) was obtained after equilibration at 25℃. The conductivity of the solution was measured using a conductivity meter. Electrolyte leakage (%)=(C1/ C2)*100.
Seedling growth
The plant height, leaf area, shoot dry mass, root length and root dry mass were measured after spraying for 25 days, and the dry weights were taken after the samples were oven-dried at 80℃ for 48 h.
Assay of contents of soluble sugar and starch
Soluble sugars were extracted from 1 g of dry pow-dered material with acetone and were determined calorimetrically according to the phenol sulphuric acid method (Dubois et al., 1956). Quantitative determination of starch content was accomplished with the residue after the extraction of sugars using the enthrone method (Mccready et al., 1950).
Statistical analysis
There were three uniform seedlings for each replication. The experiments were repeated three times. There was no interaction among repetitions of the experiments, so the results were pooled. The experimental data was analyzed with SPSS statistical package. Then all the data were subject to ANOVA, and means were compared using the appropriate Fisher's protected LSD (p<0.05).
Results
Effect of foliar application DCPTA on leaf photosynthesis in maize seedling leaves
Fig. 1 showed the effect of 20 mg • L-1and 40 mg • L-1DCPTA on A, g, E and Ci 15 days after spraying. The results indicated that the effect of DCPTA on gas exchange occurred around day 6 in maize seedling. The significant promotive effect of DCPTA on A was observed 6-9 days after spraying and declined by day 9 after spraying. The foliar application of 40 mg • L-1DCPTA caused 24.3% and 27.1% increases in A 6 and 9 days after spraying, whereas 20 mg • L-1DCPTA resulted a 10.4% and 17.1% increase. These results indicated that A was enhanced significantly by the application of 40 mg • L-1DCPTA, which showed that the optimum concentration of DCPTA for enhancement of A in maize was 40 mg • L-1. A did not differ from the control plants from day 12 to 15, though A was a little higher than the control. A similar relationship and a corresponding shift of the optimum CO2concentration were also observed for the stomata conductance (g) and transpiration rate (E). DCPTA treated plants always maintained a higher g and a higher E than the control. With days increasing, g increased continuously and reached the peak on day 6, then declined gradually. While E increased continuously and reached the peak on day 9, then declined gradually, showing a same trend with A. The intercellular CO2concentration (Ci) was lower for treated plants than for the controls. The further analysis showed that the relationship between g and E in maize leaves could be described as linear, and the regression line was y=12.099x+0.1355 (R=0.689**) (Fig. 2).
The chlorophyll fluorescence parameters Fo, Fm, Fv/Fm, Fv/Fo, qP, and qN were characterized 20 mg • L-1and 40 mg • L-1DCPTA by 15 days after spraying. In maize, on day 3, day 6 and day 9, there were no significant differences in Fo of the treated plants. On day 9, the difference between 40 mg • L-1DCPTA treated maize and the control in Fo was no significant (p>0.05), while Fo was significantly increased (p<0.05) by 20 mg • L-1DCPTA treated maize (Table 1). On day 15, Fo of the treated plants was significantly lower (p<0.05) than that of the control. With regard to maximal fluorescence in the dark Fm, Fm of the treated maize seedling increased continuously and reached the peak on day 9, then declined gradually (Table 1). Compared with the control, there were significant increases (p<0.05) at all times except for the day 15 in 40 mg • L-1DCPTA treated maize. On day 6 and day 9, the difference between 20 mg • L-1DCPTA treated maize and the control in Fm was also significant (p<0.05).
Compared with the control, the treated maize seedling caused an increase of Fv/Fm. The decrease in Fv/Fm resulted from the increase in Fo and the decrease in Fm. The application of 20 mg • L-1DCPTA treated maize seedling and 40 mg • L-1DCPTA treated maize seedling reached their peak on day 9 and day 12 respectively, then declined gradually. The application of 40 mg • L-1DCPTA treated maize seedling showed a significant increase (p<0.05) in comparison to the control. On day 6 and day 15, the difference between 20 mg • L-1DCPTA treated maize and the control in Fv/Fm was also significant (p<0.05). There was no difference between 20 mg • L-1DCPTA treated maizeseedling and the control on day 3, day 9 and day 12. A similar trend was found in the value of Fv/Fo in the treated maize seedling (Table 1). With days increasing, qP and qN values with the foliar 40 mg • L-1DCPTA application of maize leaves increased (p<0.05) significantly, while there was no significant increase with the concentration of 20 mg • L-1DCPTA as compared to the control.
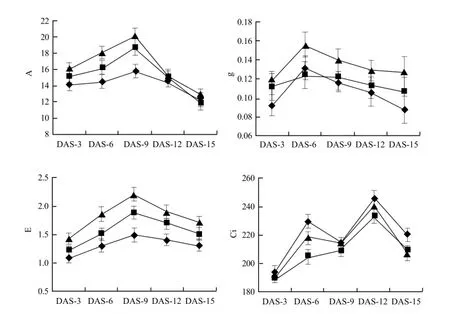
Fig. 1 Effect of foliar application DCPTA on A, g, E, and Ci in maize seedling leaves
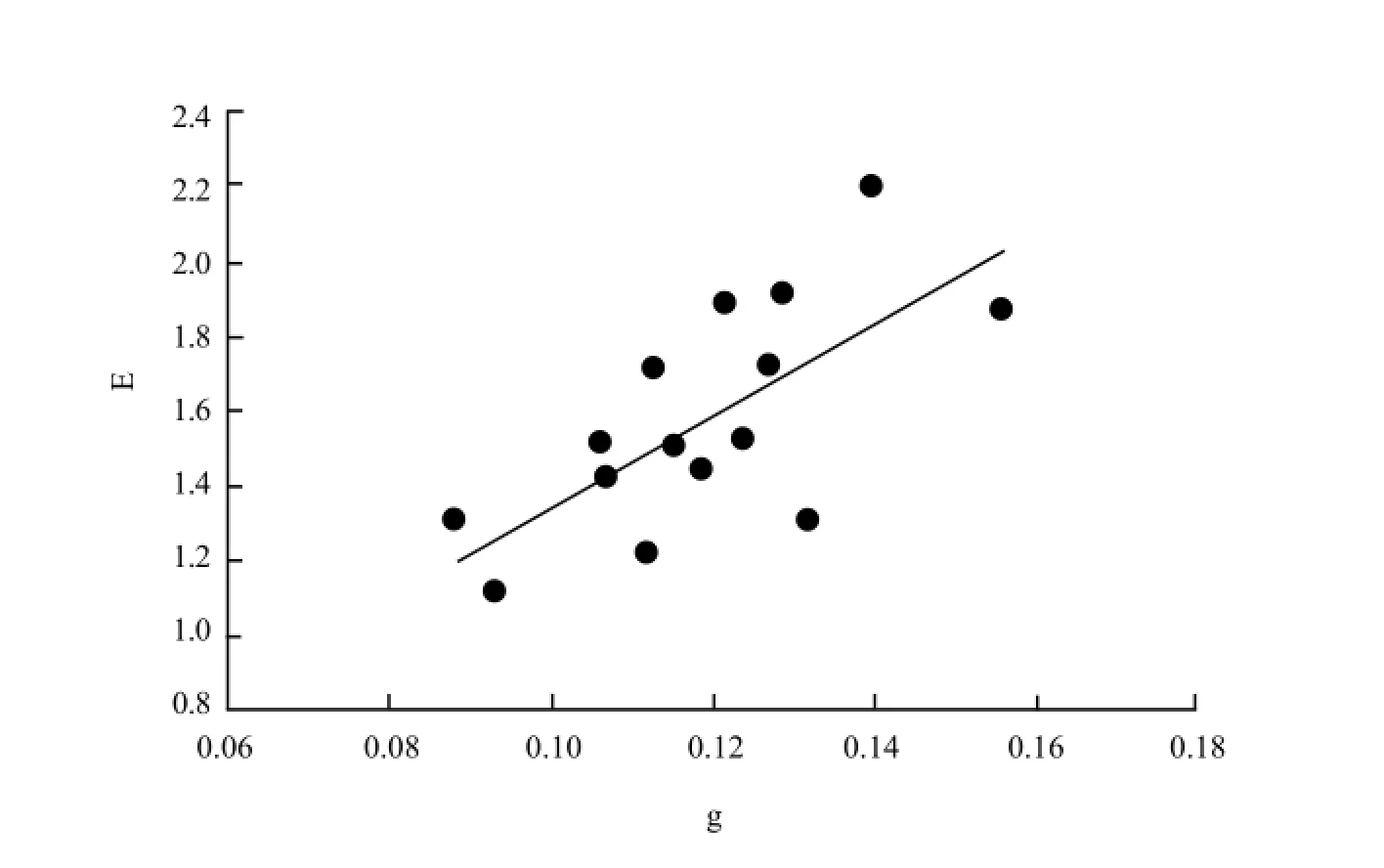
Fig. 2 Relationship between stomata conductance and transpiration rate of young expanded leaves on different days after DCPTA treatment

Table 1 Effect of foliar application DCPTA on chlorophyll parameters Fo, Fm, Fv/Fm, qP, and qN in maize seedling leaves
Effect of foliar application DCPTA on content of leaf chlorophyll, chla, chlb, chla/chlb and SPAD value
Compared with the control, the treated maize seedling caused an increase content of chla, chlb, the ratio of chla/chlb and chlt (Table 2). The foliar 40 mg • L-1DCPTA application of maize leaves showed a higher increase than the concentration of 20 mg • L-1DCPTA. For these parameters, there was no significant difference between the treated maize seedling and the control. Further measurements showed that the chlorophyll metre readings (SPAD) in maize did not differ from the control.
Effect of DCPTA on relative water content and electrolyte leakage 25 days after foliar application
The measurements showed that DCPTA treated plants always maintained higher relative water content, while a lower relative conductivity than the control (Table 3). The application of 40 mg • L-1and 20 mg • L-1DCPTA caused relative water content 10.3% and 4.3% increases respectively compared to the control. With the increase concentration of foliar application DCPTA, the electrolyte leakage decreased significantly, which showed 17.5% and 8.8% decreases after 40 mg • L-1and 20 mg • L-1DCPTA foliar applications.
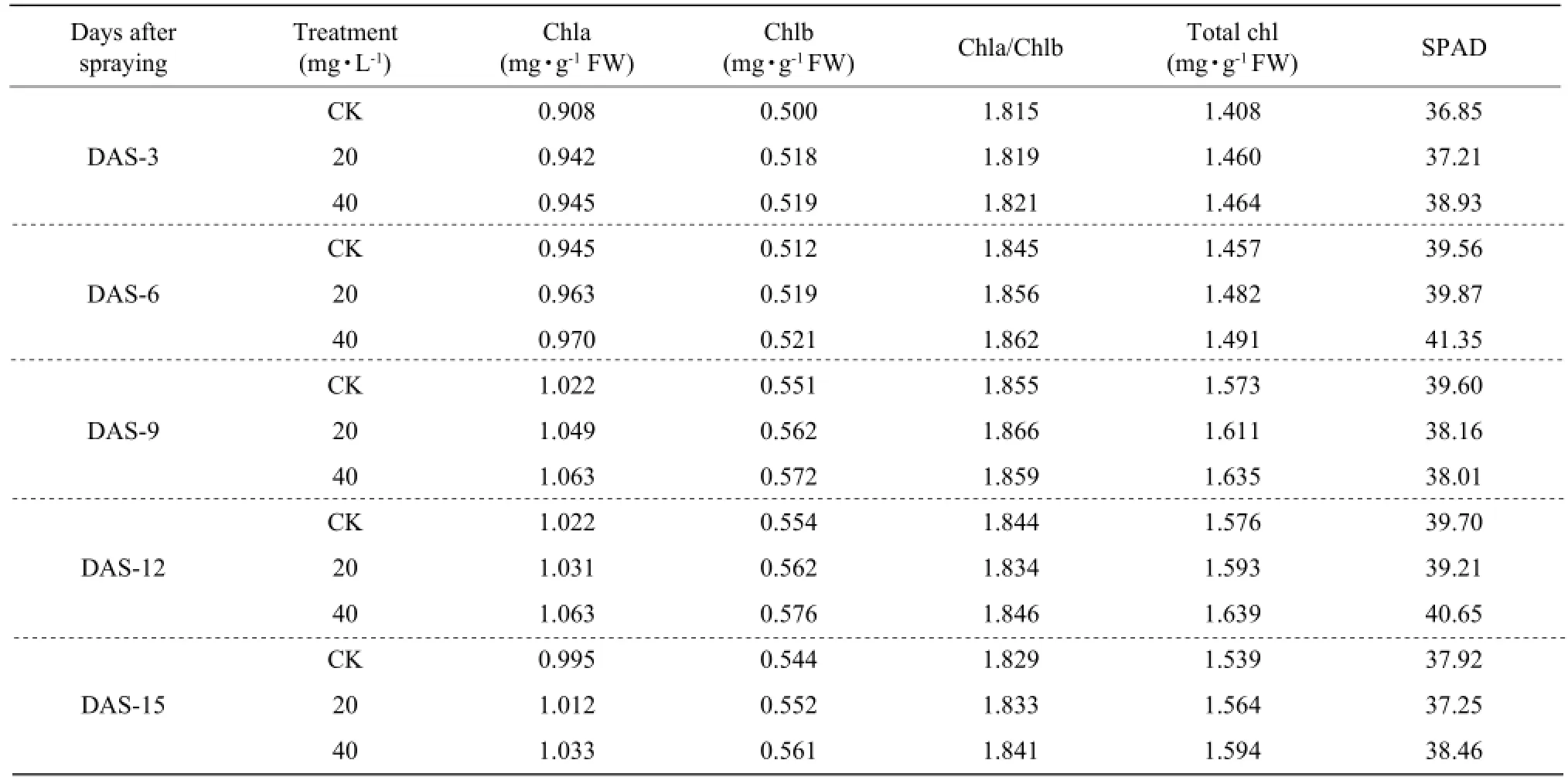
Table 2 Effect of foliar application DCPTA on chlorophyll in maize seedling leaves
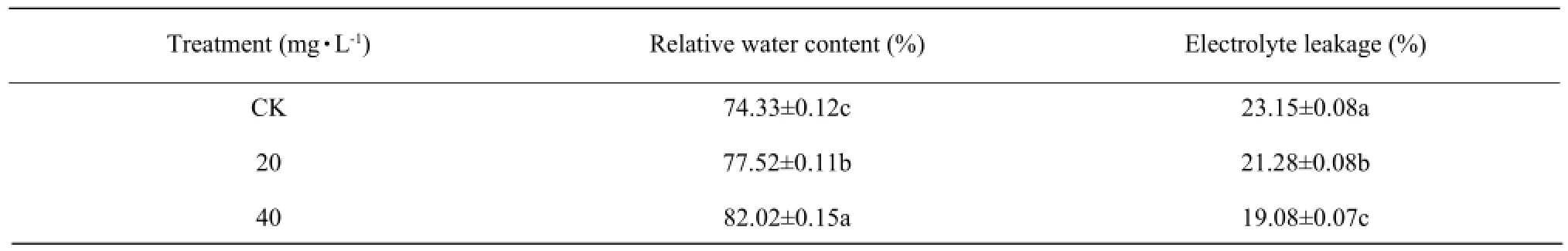
Table 3 Effect of DCPTA on relative water content and electrolyte leakage 25 days after foliar application
Effect of DCPTA on soluble sugar content and starch content in dry matter
Compared with the control, the treated maize seedling caused an increase of soluble sugar and starch contents in the dry leaves (Fig. 3). There was great significance between treated plants and the controls at the concentration 40 mg • L-1for maize. In the treated maize, the foliar application of 40 mg • L-1DCPTA caused 15.4% and 12.6% increases of soluble sugar and starch contents respectively than in the control.
Effect of DCPTA on growth of maize seedling 25 days after foliar application
Compared with the control, the treated maize seedling caused an increase in plant height, root length, shoot dry mass, root dry mass, or total (root plus shoot) dry mass (Table 4).
The foliar application of DCPTA affected the plant height, root length, shoot dry mass, root dry mass, ortotal (root plus shoot) dry mass. With regard to the total dry mass, the applications of 20 mg • L-1DCPTA and 40 mg • L-1DCPTA increased 10.3% and 19.5% respectively in comparison to the control.

Fig. 3 Effect of DCPTA on contents of soluble sugar and starch 25 days after foliar application in dry matter
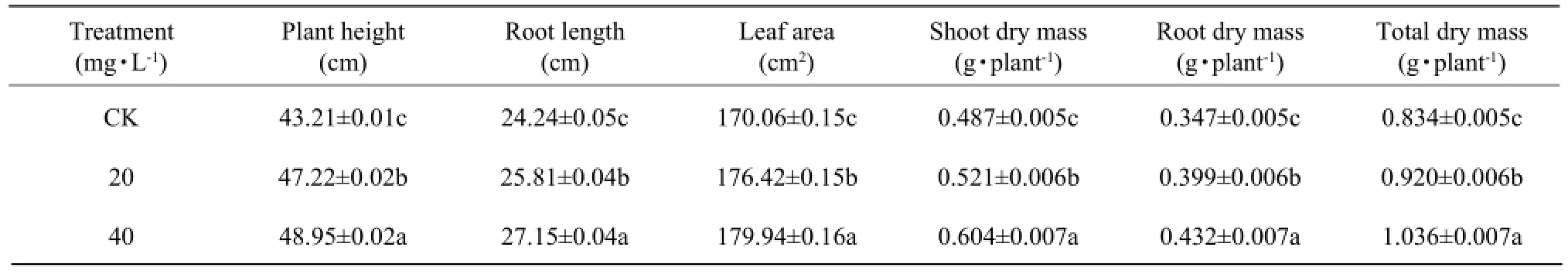
Table 4 Effect of DCPTA on growth of maize seedling 25 days after foliar application
Discussion
The tertiary amine substance DCPTA increased net photosynthetic rates in maize seedling for at least 12 days, beginning 3 days after foliar application. It seemed that DCPTA generally increased photosynthetic rates following their foliar application to these two crop species. Given that maize belongs to gramineae poaceae from different plant families and represents a different photosynthetic physiologies (C4for maize), and this phenomenon seems likely to occur in a wide range of plant species for C3, such as soybean species. Several researchers have confirmed that spraying optimum concentration of DCPTA can increase the ability of photosynthesis in different plants. Some results showed that the spraying DTA-6 on wild barely at four-leaf stage increased the photosynthetic rate greatly (Liang et al., 1998; Gu et al., 2008). It appears that DCPTA can alter physiological process involved in photosynthesis and growth of plants.
Stomata behavior and regulation are very important factors in the control of photosynthetic rate. The increase in photosynthetic rates seen on day 9 after application of DCPTA was generally accompanied by increased or unchanged stomata conductance levels and transpiration; however, the intercellular CO2concentrations of treated plants were generally lower than those of the control. This suggests thatthe increases in photosynthetic rates following spray applications of DCPTA were the result of increased CO2uptake activity at the chloroplast level, rather than simple increase in stomata opening (reduced resistance to entry of CO2in the leaves). With regard to WUE value, DCPTA had no significant effect on water use efficiency. The further analysis showed that there is a linear relationship between g and E in the maize leaves at time points checked. Previous work has shown that increased stomata opening is the primary cause of increased photosynthetic activity, an increase in Ci would be expected (Wajahatullah et al., 2003). The results suggested that the increase in photosynthetic rate is probably due to enzyme related activities at the chlorophyll level (Keithly et al., 1991; Gausman et al., 1985).
Chlorophyll a fluorescence quenching analysis has been shown to be a non-invasive, powerful and reliable method to assess the changes in the function of PSII under different environmental conditions (Krause and Weis, 1991; Mohammed et al., 1995). Fv/Fm value in the dark-adapted state showed an increase with the optimum concentration in maize seedling leaves. The decrease of Fv/Fm is the remarkable characteristics of photosynthesis inhibition (Dickmann et al., 1992). Fm is maximal fluorescence in the dark, and the decrease of the value is another characteristic of photosynthesis inhibition. The decrease of Fo is the evidence of the increasing dissipation of radiant energy (Krause et al., 1991; Ma et al., 2011). With regard to the chlorophyll a fluorescence parameters, we found that 40 mg • L-1DCPTA in maize seedling caused the increase in Fm, Fv/Fm, Fm/Fo, qP and the decrease in qN. The results showed that the light transformation efficiency increased in the reaction centre of PSII and the primary reaction of photosynthesis was promoted and promoted the excited energy transformation from LHC II to PSII after spraying optimum concentration DCPTA on plants. The potential activities of PSII showed the similar trend with Fv/Fm. The higher value of qP means higher electron transferring rate (Van et al., 1990). Our results also found that DCPTA treated plants increased qP value, which increased PSII opening degree. qN value showed a similar characteristic with qP. Our results suggested that the up-down regulation of PSII might be a mechanism to regulate the photosynthetic electron transport to match the higher demand for ATP and NADPH in the dark reactions of Calvin cycle in the treated plants in comparison to the controls.
There is no significant difference in chla, chlb, chlt and the ratio of chla/chlb between the treated plants and the control. The optimum concentration of DCPTA can increase the content of chla, chlb, chlt and the ratio chla/chlb in maize and soybean, which is benefic for the photosynthesis. Previous work has confirmed the same results about DCPTA in different plants. The results were in accordance with other researchers, which showed that DCPTA could increase the content of chlorophyll and there was no change in the ratio of chla/chlb (James et al., 1991). The further study showed that the content increase of chlorophyll after the treatment was the result of the volume increase of chlorophyll, not the increasing synthesis of chla and chlb. The content of LHCP II increased by DCPTA treatment by the electrophoresis analysis. DCPTA spraying on the leaves of Stevia rebaudiana Bertoni caused cell organs changed differently by using electron microscopy technique, which showed that DCPTA treatment can promote the differentiation of thylakoid lamella in the young leaves, and increase the numbers of granum and oppressed region (Shen et al., 1994). These results showed that the changes in chlorophyll levels were not directly responsible for photosynthetic rate changes.
The results showed that DCPTA treated plants maintained a higher relative water content while a lower relative conductivity than the control, which was good to the plants to resist the stress condition. Meanwhile, higher relative water content and lower relative conductivity in leaves would keep good environment for the plant growth. Other studies reported that the rice seedlings which had been presoaked with different concentrations of DCPTA showed higher resistance to cold temperature, theincrease of seedling survive percentage and the reduction of cell electrolyte leakage (Zhang et al., 2001; Wang et al., 2003; Liu and Yang, 2005). Our foundlings showed that the treated maize seedling caused an increase of soluble sugar and starch contents in the dry leaves, which gave a good foundation for the higher dry matter accumulation and transportation. It is corresponded with the characteristics of high ability of photosynthesis rate in leaves after spraying DCPTA, and the yield and quality increased significantly (Zhang et al., 2001; Lu et al., 2000; Tong et al., 2011). Biomass of plants can be a direct reflection on the situation of development and growth. We could directly see the effect of plant growth regulators. DCPTA affected development and growth of maize seedling when measured 25 days after foliar application. In general, the treated maize seedling caused an increase in plant height, root length, shoot dry mass, root dry mass, or the total (root plus shoot) dry mass. Compared with the control, an increment in the proportion of root and seedling with 1 mg • L-1DCPTA pretreatment has been found in rice (Xu et al., 2001; Zhang et al., 2001).
Our results showed that the optimum concentration DCPTA (40 mg • L-1) increased photosynthetic rate by raising both the contents of chlorophyll and the activities of PSII and the contents of sugar and starch. Compared with the control, the treated maize seedling caused an increase in plant height, root length, shoot dry mass, root dry mass, or the total (root plus shoot) dry mass. We assume that DCPTA will have similar effects on many other crop species.
Chen M Z. 1995. Effects of N, N-diethylaminoethyl hexanote on the physiological activity in Matthiola incana R.Br. Acta Horticulturae Sinica, 22(2): 201-202
Dubois M, Gilles K A, Hamilton J K, et al. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem, 28: 350-356.
Dickmann D I, Liu Nguyen P V. 1992. Photosynthesis, water relations and growth of two hybrid popolus genotypes during a severe drought. Canadian Journal of Forest Research, 22(8): 1094-1106.
Gausman H W, Quisenberry J E, Yokoyama H. 1991. Introduction to effects of plant biochemical regulators. In: Gausman H W. Plant biochemical regulator. Marcel Dekker. Inc., New York. pp. 1-16.
Gausman, H W, Burd J D, Quisenberry J, et al. 1985. Effects of 2-diethylaminoethyl-3, 4-dichlorophenylether on cotton (Gossypium hirsutum L) growth and phenology. Biotchnology, 3: 255-257.
Gu W R, Li Z H, Zhai Z X, et al. 2008. Regulation of tertiary amine bioregulator on photosynthesis and chlorophyll fluorescence parameters of corn leaves. Acta Agriculture Boreali-Sinica, 23(3): 85-89.
Gu W R, Li Z H, Zhang M C. 2008. Physiological functions of tertiary amines DCPTA and DTA-6 and their applications in agriculture. Journal of Anhui Agri Sci, 36(28): 12105-12107, 12111.
Gu W R, Li Z H, Zhai Z X, et al. 2009. Effect of DCPTA and DTA-6 on the endogenous hormone and free radicals in corn and soybean seedling leaves. Journal of Plant Genetic Resources, 10(2): 300-305.
James H, Keithly J H, Yokoyama H. 1991. Regulation of crop growth and yield by tertiary amine bioregulators. In: Gausman H W. Plant biochemical regulator. Marcel Dekker. Inc., New York. pp. 223-246.
Keithly J H, Yokoyama H, Gausman H W. 1990. Effect of 2-diethylaminoethyl-3, 4-dichlorophenylether (DCPTA) on growth and development of sugarbeet. Plant Science, 68(1): 57-64.
Keithly J H, Yokoyama H, Gausman H W. 1991. Regulation of crop growth and yield by tertiary amino bioregulators. In: Gausman H W. Plant biochemical regulator. Marcel Dekker. Inc., New York. pp. 222-245.
Krause G H, Weis E. 1991. Chlorophyll fluorescence and photo-synthesis: the basics. Annu Rev Plant Physiol Plant Mol Bio, 42: 313-349.
Lu J Z, Xue X C, Zhang A L. 2000. The regulation of DA-6 on the growth and physiology activity in Sabina Chinese. Bulletin of Botanical Research, 20(1): 73-78.
Liang G J, Li Y Y, Shao L. 1998. Effect of DA-6 and BR+GA3on growth and photosynthetic rate in Spinach. Acta Horticulturae Sinica, 25(4): 356-360.
Liu X Y, Yang B L. 2005. Studies on the action of DA-6 reducing the phytotoxicity of ethametsulfuron on rice. Modern Agrochemicals, 4(3): 31-35.
Ma Q H, Tan X H, Liang L S, et al. 2011. Effects of plant growth regulators DA-6 and DCPTA on reactive oxygen species and related physiological indices of Ziziphus jujuba mill cv Dongzao during growth. Food Science, 32(8): 296-299.
Mccready R M, Guggolz J, Silviera V, et al. 1950. Determination of starch and amylose in vegetables. Anal Chem, 22: 1156-1158.
Mohammed G H, Binder W D, Gillies S L. 1995. Chlorophyll fluorescence: a review of its practical forestry applications and instrumentation. J For Res, 10: 383-410.
Poling S M, Hsu W J, Koehrn F J. 1977. Chemical regulation of β-carotenoid biosynthesis. Phytochemistry, 16(5): 551-555.
Ren X J, Fu S K, Gao H G, et al. 2003. Synthesis of 2-(Diethylamino) ethyl caproate by using lewis acid. Journal of Shenyang Institute of Chemical Technology, 17(3): 204-206.
Shen M S, Lin Y J, Chen M C, et al. 1999. Studies on influence of DTA-6 regulation on leaf cells ultrastructure in Stevia rebaudiana Bertoni. Sugar Crops of China, 4: 1-3.
Stephen M, Poling W, Jean H, et al. 1977. Chemical induction of B-carotene biosynthesis. Phytochemistry, 16(5): 551-555.
Stephen M, Poling Wan, Jean H, et al. 1982. Syhthetic bioregulators of poly-cis carotenoid biosynthesis. Phytochemistry, 21(3): 601-604.
Tong R J, Liu X Q, Geng H M, et al. 2011. Effects of DCPTA on the seedling growth of kidney bean. Hubei Agricultural Sciences, 50(9): 1823-1825.
Van K, Snelj F H. 1990. The use of chlorophyll fluorescence nom enclature in plant stress physiology. Photosynth Res, 25: 147-150.
Wajahatullah K, Balakrishnan P, Donald L S. 2003. Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol, 160(5): 485-492.
Wang J P, Zhai Z X, He Z P, et al. 2003. Effect of DTA-6 on crude and amino acid content of alfalfa. Journal of China Agricultural University, 8(3): 25-28.
Xu Q M, Chen J S, Gao H. 2001. Preliminary investigation into DTA-6 physiological effect on rice seedling. Journal of Tianjin Normal University, 21(2): 57-60.
Yokoyama H, Hayman E P, Hsu W J, et al. 1977. Chemical bioinduction of rubber in guayule plant. Science, 197(4308): 1076-1077.
Zhou T, Hu Y J , Zhou X M, et al. 2004. Effect of DTA-6 on seedling photosynthesis and growth of wild barley Hordeum brevisubulatum. Pratacul Tural Science, 21(4): 31-34.
Zhang M C, He Z P, Wang Y Q, et al. 2001. Effect of plant growth regulator DTA-6 on sweet pea. Chinese Journal of Pesticide Science, 3(4): 53-58.
Zhang Z L. 2001. Effect of DTA-6 on seedling growth and its coldresistance in rice. Guizhou Agricultural Science, 29(4): 14-16.
Zhou T, Hu Y J, Zhou X M, et al. 2004. Effect of DTA-6 on seedling photosynthesis and growth of wild barely. Pratacul Science, 21(4): 31-34.
S513
A
1006-8104(2014)-02-0001-11
Received 3 May 2013
Supported by the National Natural Science Foundation of China (31201164); the Program of Science and Technology of Education Department of Heilongjiang Province (12521036); China Postdoctoral Science Foundation (2012M511434); Heilongjiang Province Postdoctoral Science Foundation (LBH-Z12036); the Doctoral Starting Up Foundation of Northeast Agricultural University (2012RCB01)
Gu Wan-rong (1980-), male, assistant researcher, post-doctor of crop science, engaged in the research of plant high yield production and macro agriculture. E-mail: wanronggu@163.com
* Corresponding author. Wei Shi, professor, supervisor of Ph. D student, engaged in the research of plant high yield production and macro agriculture. E-mail: weishi5608@163.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Comparison of Physiological Properties Between Dwarf and Vinetype Cucumbers (Cucumis sativus Linn.)
- Effects of Substitute Media on Development of Potted Cyclamen percicum Mill.
- Expression of HSP72 in Mouse Preimplantation Embryos with Heat Shock
- Effects of Maternal Dietary Energy Restriction on Fat Deposition of Offspring
- Effects of Dietary Protein and Temperature on Growth and Flesh Quality of Songpu Mirror Carp
- Synthesis and Identification of SG-BSA and SG-OVA
