Effects of Maternal Dietary Energy Restriction on Fat Deposition of Offspring
2014-03-07LiJingfengXuLiangmeiZhangYanyunJiangDanZhangJingZhangHuiandLiJie
Li Jing-feng, Xu Liang-mei, Zhang Yan-yun, Jiang Dan, Zhang Jing, Zhang Hui, and Li Jie
Institute of Animal Nutrition, Northeast Agricultural University, Harbin 150030, China
Effects of Maternal Dietary Energy Restriction on Fat Deposition of Offspring
Li Jing-feng, Xu Liang-mei*, Zhang Yan-yun, Jiang Dan, Zhang Jing, Zhang Hui, and Li Jie
Institute of Animal Nutrition, Northeast Agricultural University, Harbin 150030, China
The study was carried out to investigate the effects of maternal dietary energy restriction on growth performance, serum indices and fat deposition of offspring. A total of 400 female Arbor Acres (AA) broiler breeders were studied. These female birds involved three experimental treatments and a control group with normal dietary energy diets (ND, 11.7 MJ of ME • kg-1during the laying). In treatments 2, 3 and 4, the energies of diets were 20%, 30% and 50% (LD20, LD30 and LD50) lower than those of the control, respectively. The study commenced at the beginning of the laying period when the total egg production reached 5% of the flock. All the broiler offspring were fed the same diets. The results showed that in low energy diets, offspring showed decreased 1-day-old weight, but 49-day-old weight was higher in LD20 diet (P<0.05). For offspring during days 1-49, the average daily gain (ADG) in LD20 group and the feed conversion ratio in LD50 group were improved as compared with those of the control (P<0.05). Compared with the control, abdominal fat percentage increased in 49-day-old offspring from LD30 diet (P<0.05); the fat content of breast muscle in offspring increased in broilers fed low energy diets (P<0.05). In 28-day-old offspring from breeders given LD20 and LD50 diets, liver fat percentages were higher compared with ND (P<0.05). The subcutaneous fat thickness in 28-day-old offspring from LD50 group and 49-day-old offspring from LD30 group was higher (P<0.05). On day 49, the serum cholesterol (CHO) of offspring from breeders fed LD20 diet and serum high-density lipoprotein (HDL) of offspring from breeders fed LD50 diet reduced compared with those of the control (P<0.05). In addition, a higher triiodothyronine (T3) content in serum was found in offspring from broiler breeders given LD20 and LD30 diets (P<0.05). Serum thyroxine (T4) in offspring significantly decreased with the decrease of diet energy (P<0.05). In conclusion, to a certain extent, dietary energy restriction in breeders could improve growth performance and promote lipid metabolism of offspring.
energy restriction, growth performance of offspring, blood indice of offspring, broiler breeder, fat deposition and metabolism
Introduction
With the rapid development of poultry industry, decline of meat quality and energy waste is being more and more serious. For broiler breeders, diet energy could affect egg quality, and then may influence embryo development, early growth and fat metabolism in offspring (Tian et al., 2012). Broiler chicken performance is highly dependent on the genetic potential of these birds (Havenstein et al., 2003), but other factors, such as breeders' age, levels of fed intake, and dietary composition of broiler breeders, may also influence the performance of offspring (Triyuwanta et al., 1992;Lopez & Leeson, 1994; 1995; Hossain et al., 1998; Peebles et al., 1999a; 1999b). A series of reports from Enting et al. (2007) showed that low energy broiler breeders' diets resulted in an increase in egg production and could affect embryonic development, and performance and mortality of their offspring. Spratt et al. (1987) reported that diet energy would influence 20-day-old body weight of the male offspring. Maternal feed restriction could affect blood biochemical indices and hormone levels of embryo (Zhang et al., 2011) and fat deposition of offspring by changing the activity and mRNA expression levels of liver fatty acid synthase (Hu et al., 2010). However, few studies have approached to the effects of broiler breeder energy restriction on performance of offspring. The present study was conducted to investigate the effects of broiler breeder energy restriction on growth performance, fat deposition and serum indices of offspring.
Materials and Methods
Dietary treatments of broiler breeders
This experiment was conducted at the Experimental Poultry Farm of Northeast Agricultural University. A total of 400 females Arbor Acres broiler breeders and 20 males at 20 weeks of the age were selected from the breeder stock. These female birds were divided into four treatments randomly (each treatment represented by five replicates of 20 birds), including three experimental treatments and one control. Treatment 1 was the control, in which normal energy density diets were fed during the experiment (ND, 11.70 MJ • kg-1of ME). In the treatments 2, 3 and 4, the levels of energy decreased by 20%, 30% and 50% (LD20, LD30 and LD50), respectively.
The experimental period started when egg production reached 5%, and ended in week 45. Each bird was housed in the individual cage measuring 48 cm× 34 cm×39 cm, with feed supply restricted and water fed ad litibum. The nutritional requirements of the female diets are shown in Table 1.
From 21 weeks old, the lighting program was started combining natural and artificial light. The lighting time was increased by 30 min per week from 14 h per day until reaching 16 h, and then maintained at 16 h per day thereafter.

Table 1 Composition and nutrient levels of experimental diets (air-dry basis) (g • kg-1)
Dietary treatments of chicks
Birds were fertilized at 40 weeks of the age. During 41 to 42 weeks of the age, 900 eggs were collected and then stored at 10℃ until incubation. Eggs were incubated at a constant eggshell temperature of 37.8℃during the entire incubation period. After hatching, all male broiler chickens were selected and transported to the Experimental Poultry Farm of Northeast Agricultural University, China. Birds were reared under the same normal conditions in stair-step cages with five replicates per treatment according to broiler feeding schedule. In all the broiler chicken experiments, light was provided 23 h per day. The environmental temperature was maintained at 32-34℃ for the first week and then decreased gradually to 21℃ by 2-3℃ per week and the room temperature was controlled at 18-24℃ thereafter. The whole experimental period lasted for 49 days. Birds were fed the same cornsoybean-based diets meeting or exceeding the nutrition standard of Chinese NY/T 33-2004 (1-3 weeks, ME 12.54 MJ • kg-1, CP 21.52%; 4-6, ME 12.96 MJ • kg-1, CP 19.98%; 7 weeks, ME 13.17 MJ • kg-1, CP 18.00%). Chickens had ad libitum access to water and feed during the trial.
Sample collection
On days 28 and 49, the broiler offspring were fasted for 12 h and then the birds and feed were then weighed to determine the average daily gain (ADG), the average daily feed intake (ADFI), and the feed conversion ratio. On days 28 and 49, two birds randomly from each replicate were selected and euthanized for sampling. Blood was collected (5 mL) by cardiac puncture into a 10-mL anticoagulant-free Vacu-tainer tube (Greiner Bio-One GmbH, Kremsmunster, Austria) and then centrifuged at 3 000×g for 10 min to obtain serum. The serum samples were stored at -20℃ until analyses. Abdominal fat and fat tissues surrounding the proventriculus and gizzard lying against the inside abdominal wall and around the cloaca, was collected as described by Ain Baziz et al (1996). Subcutaneous fat thickness and intermuscular fat width were measured by vernier caliper according to Ricard et al. (1983) and Baziz et al (1983). At last, liver and right breast muscle were removed and stored for further analyses. The fat content of liver and breast muscle were determined by Soxhlet extractor method (Zhang, 2003).
The serum indices, including the total cholesterol (C), triglyceride (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were assayed by an automatic biochemical analyzer (RA-1000, Bayer Corp., Tarrytown, NY.) with commercial reagent kits (Zhongsheng Beikong Bio-Technology and Science, Inc. Beijing, China). The serum triiodothyronine (T3) and thyroxine (T4) were measured by radioimmunoassay kit (Diagnostic Systems Laboratories Inc., Webster, TX).
Statistical analysis
Data was analyzed by ANOVA by using GLM procedure of SAS institute (2000). Duncan's multiple range test and critical difference were determined at 5% significance level. The results of statistical analyses were shown as means±standard deviation.
Results
The 1-day-old chicken weight reduced in offspring from breeders fed low energy diets (Table 2), comparing with the control diet. No significant difference was found on 28-day-old chicken weight. Compared with the control, 49-day-old body weight in offspring from breeders fed LD20 diets increased (P<0.05). ADFI and ADG in offspring were not significantly affected during days 1-28, and offspring from breeders fed LD50 diet markedly exhibited an increasing ADFI during days 1-49 (P<0.05). ADG in offspring from breeders fed LD20 diet was higher compared with those from breeders fed ND (P<0.05). Feed conversion ratio of offspring from broiler breeders fed LD50 diet significantly decreased during the experimental period (days 1-49) as compared with that of the control (P<0.05).
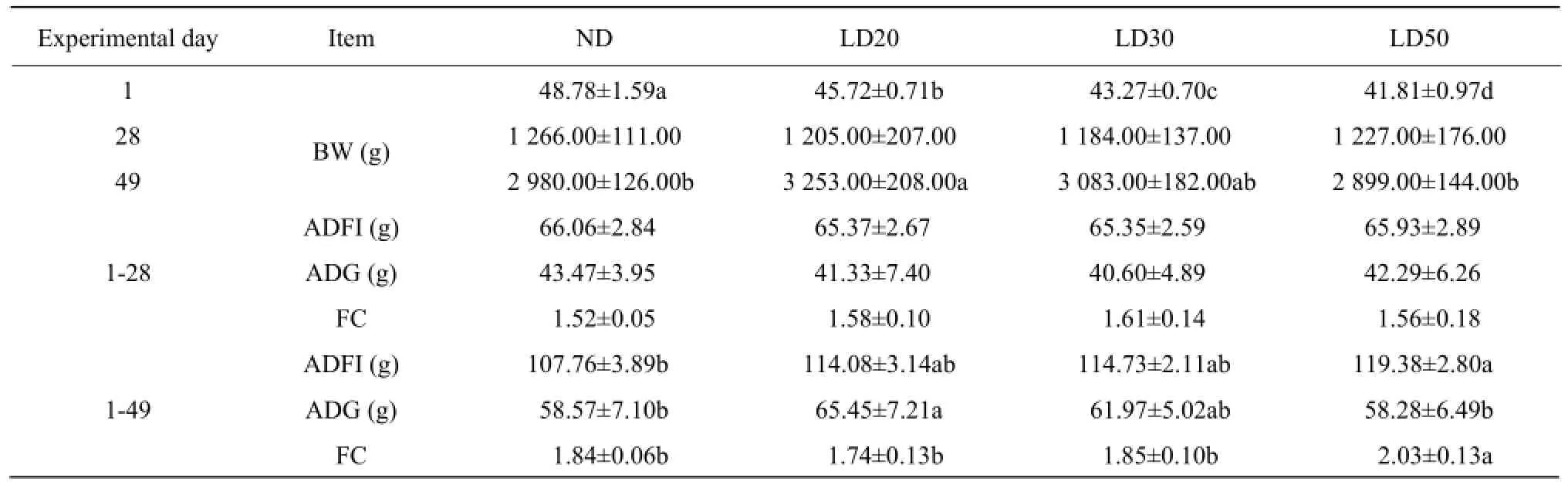
Table 2 Effects of maternal dietary energy restriction on growth performance of offspring
Dietary treatments on serum indices of offspring are presented in Table 3. On 28 days of the age, both serum TG and T4levels in offspring of breeders fed LD50 diet were higher than those from breeders fed LD20 and LD30 diets (P<0.05), whereas no differences were observed in comparison with ND. There was no difference in C, T3, LDL, and HDL levels in offspring among treatments, and the same result was obtained on 49-day-old serum TG and LDL levels. On 49 days of the age, serum C level in offspring of breeders fed LD20 diet and serum HDL level in offspring from breeders received LD50 diet were lower than those in offspring from broiler breeders given ND (P<0.05). In addition, a higher T3content in serum was found in the offspring of breeder fed LD20 and LD30 diets (P<0.05). Serum T4level in offspring significantly decreased with the decreasing of breeder energy in diets (P<0.05).

Table 3 Effects of maternal dietary energy restriction on serum indices of offspring
Effects of maternal energy restriction on fat deposition of offspring are shown in Table 4. The difference in intermuscular fat width among treatments was not found on days 28 and 49. Compared with ND, theabdominal fat percentage did not differ in offspring of 28-day-old; however, it had a significant increase in 49-day-old offspring of breeders fed LD30 diet (P<0.05). On 28 days of the age, the fat content of breast muscle was higher in experimental treatments (P<0.05); whereas, only offspring from breeders given LD50 diet was higher on 49-day-old (P<0.05). In offspring from broiler breeders fed LD20 and LD50 diets, liver fat percentage was higher on day 28 than that fed ND (P<0.05), but no difference was found on day 49 among treatments. On 28 days of the age, there was an increasing trend in subcutaneous fat thickness in offspring. In addition, on 49 days of the age, the subcutaneous fat thickness in offspring was significantly improved from LD30 diet as compared with that in the control (P<0.05).
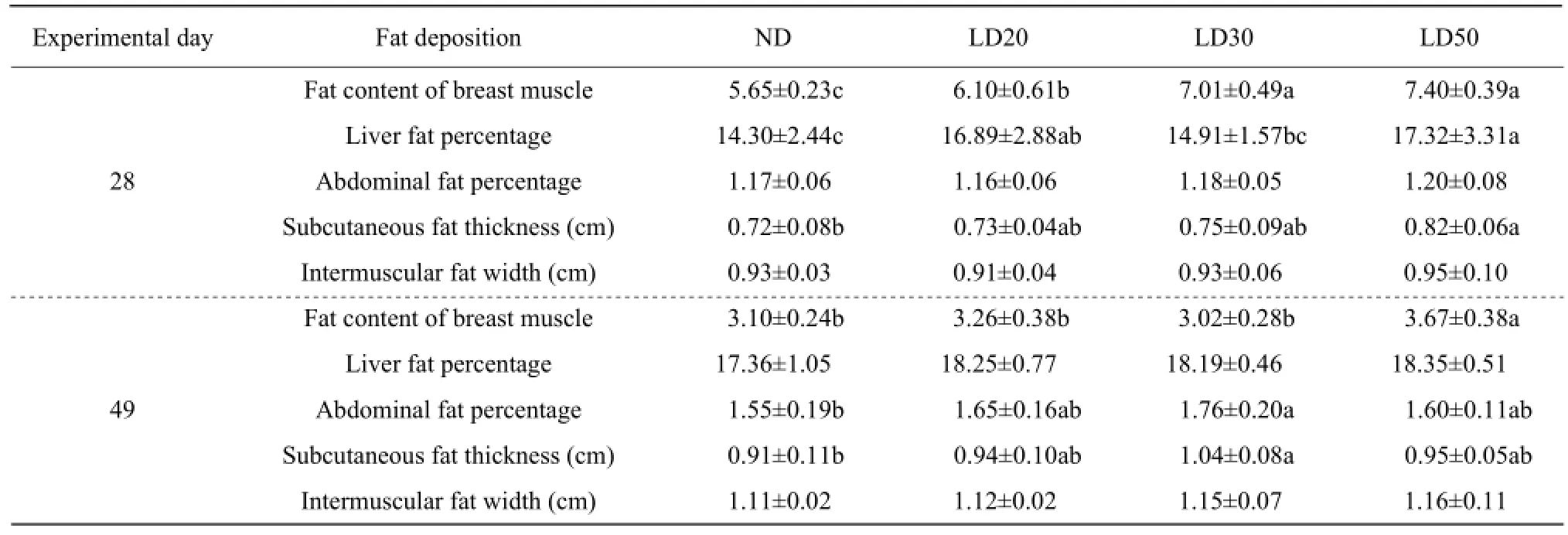
Table 4 Effects of maternal energy restriction on fat deposition of offspring
Discussion
The results of this experiment showed that maternal energy restriction affected the performance of their offspring.
Our results showed that low energy diets would decrease 1-day-old body weight in offspring of breeders, which was in accordance with the findings of Xu et al. (2010) that broiler offspring tended to have a lower 1-day-old body weight from broiler breeders fed low energy diets. However, the offspring were able to compensate for changes when normal postnatal nutrition was given. A higher 49-day-old body weight was found in offspring from breeders given LD20 diet; however, no significant difference was found for low energy diets. ADG in offspring of breeders fed LD20 diet and ADFI and feed conversion ratio of offspring from breeders given LD50 diet were higher during days from 1 to 49, these results were consistent with the findings of Enting et al. (2007) that ADFI was higher in offspring from broiler breeders fed limited feed. Triyuwanta et al. (1992) found that the effects of maternal diets tended to disappear as offspring age increased.
C, TG, HDL and LDL are important indices of lipid metabolism. The fat deposition of poultry relays on TG contention in blood. LDL, the major carriers of cholesterol, can take cholesterol to liver. And serum HDL represents the clearing situation of C, which can combine the redundant blood lipids and take it out of the body (Triyuwanta et al., 1992; Xu et al., 2010). Our results showed that the offspring from breeders fed LD20 diet had a much lower content of C and those from LD50 group had a lower content of HDL in serum on day 49 than those from ND group. These results were against the findings of Li et al. (2010) that C increased in lean line 28-day-old offspring frombroiler breeders fed less feed during laying period. The contradictory results of the two studies might come from different hen breeder strains and ages, dietary treatment feed, management protocols, and environmental factors. Taken together, the present results suggested that offspring from breeders given LD20 and LD50 diets had a much more robust lipid metabolism.
Maternal nutrition may alter the nutrient supply to the fetus and thereby affect the fetal production of essential growth regulatory factors in the blood and tissues, which may in turn modify nutrient availability to the fetus (Rehfeldt et al., 2004). Thyroid hormone as an important regulator in the growth and fat metabolism of poultry could promote the transformation and the decomposition of fat, and most T4act as taking off the iodine to T3in peripheral tissue (Smith and Freund, 2002). In this study, the contention of T3significantly increased in offspring from breeders received LD20 and LD30 diets. These results were consistent with the findings of Li et al. (2010) that T4and T3contents were respectively lower and higher for lean line hens fed limited food.
Previous studies suggested that low protein intake of pregnant rodents had a permanent effect on the growth capacity of their offspring (Strakovsky et al., 2010). In our study, the percentage of abdominal fat and liver fat, fat content of the breast muscle and subcutaneous fat thickness increased in offspring when low energy diets were given to broiler breeders. These results were consistent with Fontana et al. (1992) that the abdominal fat increased when the broiler recovered to ad libitum after feeding restriction. These results were also consistent with the findings of Anguita et al. (1993) that the offspring tended to deposit large amounts of fat in later growth process when maternal was fed limited food during pregnancy. The possible reason may be long time energy restriction affecting the expression of children growth regulatory factors in body and the nutrient efficiency, and then increasing the body fat deposition of their offspring by altering the nutrition deposition in eggs (Lippens et al., 2000).
Conclusions
In conclusion, with a lower 1-day-old weight, the offspring showed a better growth performance and a promoted lipid metabolism for broiler breeders fed low energy diets. The offspring of broiler breeders fed LD20 had the optimum performance.
Anguita R M, Sigulem D M, Sawaya A L. 1993. Intrauterine food restriction is associated with obesity in young rats. The Journal of Nutrition, 123: 1421-1428.
Baziz H A, Geraert P A, Padilha J C, et al. 1996. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poultry Science, 75: 505-513.
Enting H, Boersma W J, Cornelissen J B, et al. 2007. The effect of lowdensity broiler breeder diets on performance and immune status of their offspring. Poultry Science, 86: 282-290.
Fontana E A, Weaver W D, Watkins B A, et al. 1992. Effect of early feed restriction on growth, feed conversion, and mortality in broiler chickens. Poultry Science, 71: 1296-1305.
Havenstein G B, Ferket P R, Qureshi M A. 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Science, 82: 1500-1508.
Hossain S M, Barreto S L, Bertechini A G, et al. 1998 Influence of dietary vitamin E level on egg production of broiler breeders, and on the growth and immune response of progeny in comparison with the progeny from eggs injected with vitamin E. Animal Feed Science and Technology, 73: 307-317.
Hu J W, Shan A S, Li F, et al. 2010. Influence of feed restriction of broiler breeder hens on the fat deposition, gene expression and activity of related enzymes of offspring. Scientia Agricultura Sinica, 43: 3230-3236.
Li F, Shan A S, Hu J W, et al. 2010. Effect of feed intake of broiler hens on the relative expression of mstn and four genes of myod family in body tissues of offspring. The 6th National Conference on Feed Nutrition, Harbin.
Lippens M, Room G, De Groote G, et al. 2000 Early and temporary quantitative food restriction of broiler chickens. 1. Effects on performance characteristics, mortality and meat quality. British Poultry Science, 41: 343-354.
Lopez G, Leeson S. 1994. Egg weight and offspring performance of older broiler breeders fed low-protein diets. The Journal of Applied Poultry Research, 3: 164-170.
Lopez G, Leeson S. 1995. Response of broiler breeders to low-protein diets. 2. Offspring performance. Poultry Science, 74: 696-701.
Peebles E D, Doyle S M, Pansky T, et al. 1999a. Effects of breeder age and dietary fat on subsequent broiler performance. 1. Growth, mortality, and feed conversion. Poultry Science, 78: 505-511.
Peebles E D, Doyle S M, Pansky T, et al. 1999b. Effects of breeder age and dietary fat on subsequent broiler performance. 2. Slaughter yield. Poultry Science, 78: 512-515.
Rehfeldt C, Nissen P M, Kuhn G, et al. 2004. Effects of maternal nutrition and porcine growth hormone (pGH) treatment during gestation on endocrine and metabolic factors in sows, fetuses and pigs, skeletal muscle development, and postnatal growth. Domestic Animal Endocrinology, 27: 267-285.
Ricard F H, Leclercq B, Touraille C. 1983. Selecting broilers for low or high abdominal fat: distribution of carcass fat and quality of meat. British Poultry Science, 24: 511-516.
SAS Institute. 2000. SAS/STAT user's guide. SAS Institute Inc., Cary, NC.
Smith J, Freund A M. 2002. The dynamics of possible selves in old age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57: 492-500.
Spratt R S, Leeson S. 1987. Effect of protein and energy intake of broiler breeder hens on performance of broiler chicken offspring. Poultry Science, 66: 1489-1494.
Strakovsky R S, Zhou D, Pan Y X. 2010. A low-protein diet during gestation in rats activates the placental mammalian amino acid response pathway and programs the growth capacity of offspring. The Journal of Nutrition, 140: 2116-2120.
Tian B, Huang F F, Xu L M, et al. 2012. Dietary energy level affects laying performance, egg quality an egg component of broiler breeders during the early laying period. Chinese Journal of Animal Nutrition, 24: 327-333.
Triyuwanta L C, Brillard J P, Nys Y. 1992. Maternal body weight and feed allowance of breeders affect performance of dwarf broiler breeders and tibial ossification of their progeny. Poultry Science, 71: 244-254.
Xu L M, Chen Z H, Li F, et al. 2010. Effects of maternal dietary energy restriction on growth performance, carcass and meat quality of broilers. Chinese Journal of Animal Nutrition, 22: 894-903.
Zhang L Y. 2003. Feed analysis and examination technology of feed quality. Chinese Agricultural Publisher, Beijing.
Zhang Y Y, Shan A S, Li F, et al. 2011. Effect of maternal feed restriction on serum lipid metabolism during embryo period. Scientia Agricultura Sinica, 44: 4088-4095.
S831.5
A
1006-8104(2014)-02-0046-07
Received 16 December 2013
Supported by the Education Department Research Program of Heilongjiang Province (12531036); Doctor Science Foundation of Northeast Agricultural University (2009RC28)
Li Jin-feng (1987-), male, Master, engaged in the research of animal nutrition and feed science. E-mail: jinfeng_125@sina.com
* Corresponding author. Xu Liang-mei, Ph. D, professor, engaged in the research of animal nutrition and feed science. E-mail: xuliangmei@sohu.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regulation of Foliar Application DCPTA on Growth and Development of Maize Seedling Leaves in Heilongjiang Province
- Comparison of Physiological Properties Between Dwarf and Vinetype Cucumbers (Cucumis sativus Linn.)
- Effects of Substitute Media on Development of Potted Cyclamen percicum Mill.
- Expression of HSP72 in Mouse Preimplantation Embryos with Heat Shock
- Effects of Dietary Protein and Temperature on Growth and Flesh Quality of Songpu Mirror Carp
- Synthesis and Identification of SG-BSA and SG-OVA
