耳草属植物化学成分及药理活性研究进展
2014-01-10姜艳艳石任兵
杨 元,姜艳艳,2*,石任兵,2*
1北京中医药大学中药学院;2 北京市教委中药质量控制技术工程中心,北京 100102
耳草属(Hedyotis)为茜草科(Rubiaceae)植物,主要分布于热带和亚热带地区,少数分布于温带。我国有耳草属植物69 种(包括7个变种),主要分布于长江流域及其以南各省区,北部极少[1]。目前临床上常用白花蛇舌草、牛白藤、耳草的地上部分入药,具有清热解毒、消肿止痛等功效,用于感冒发热、咽喉肿痛、咳嗽、疮疖和蛇咬伤。其中白花蛇舌草(H.diffusa)具有良好的抗癌作用,临床常辅助癌症治疗,中国药典一部(2010 年版)附录收载的植物来源为茜草科植物白花蛇舌草Oldenlandia diffusa(willd.)Roxb.的干燥全草[2]。
国内外对耳草属植物报道较多。目前从耳草属中分离出环稀醚萜类、黄酮类、醌类、三萜类、生物碱类、糖类等多种类型数百种化合物,其中环稀醚萜类、黄酮类、醌类、生物碱类为该属植物的主要化学成分。药理学研究表明,该属植物具有抗肿瘤、免疫调节、肝保护、抗菌、抗炎、抗氧化等多种生物活性。
关于耳草属植物化学成分与药理作用的研究,目前,仅见斯建勇[3]等于2007 进行过综述,本文在其基础上总结了2007 年至今最新研究进展,并补充了2007 年之前发表而并未被其收录的部分化合物,如teneoside A、teneoside B、hedyotideaside、capitelline、hedyocapitelline、hedyocapitelline 等,从而更全面深入的对耳草属植物进行介绍,为其进一步研究和应用奠定基础。
1 化学成分
1.1 黄酮类
耳草属植物中含有多种黄酮类成分,主要为黄酮醇及其苷类以及双黄酮类成分,如槲皮素及其苷类成分、山奈酚及其苷类成分、水仙苷、穗花杉双黄酮等。从白花蛇舌草(H.diffusa)中分离得到isoscutellarein、isoetin (2、4)[4];3-methoxy-5,7-dihydroxy-flavonol (5)[5];kaeperferol (6)[7,10];kaempferol-3-O-β-D-glucopyranoside、kaempferol-3-O-(6''-O-α-L-rhamnoseyl)-β-D-glucopyranoside (7、8)[7];kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-Dglucopyranosyl]-β-D-glucopyranoside(9)[8];kaempferol-3-O-[2″-O-(E-6'''-O-feruloyl)-β-D-glucopyranosyl]-β-D-galactopyranoside、kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-galactopyranoside(10、11)[20];kaempferol-3-O-(2-O-β-D-glucopyranosyl)-β-D-glucopyranoside (12)[8];kaempferol-3-O-(2-O-β-D-glucopyranosyl)-β-D-galactopyranoside(13)[20];quercetin(15)[9,17];quercetin-3-O-β-D-glucopyranoside(16)[7];quercetin-3-O-(2''-Oglucopyranosyl)-β-D-glucopyranoside(17)[7];quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-galactopyranoside(18)[8];quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-glucopyranoside(19)[8];5-hydroxy-6,7,3',4' -tetramethoxyflavone(20)[9];amentoflavone(24)[11]。从金毛耳草(H.chrysotricha)中分离得到rutin(1)[31,33,48];isoscutellarein(2)[4];quercetin (15)[9,17];nicotiflorin(22)[31];narcissin(23)[31,48]。从丹草(H.herbacea)中分离得到kaempferol-3-O-rutinoside(14)[45]。从纤花耳草(H.tenellifloa)中分离得到5,7,4'-trihydroxy flavonol-3-O-β-D-glucoside(21)[34]。从该属植物分离得到的黄酮类成分化学结构如图1 所示。
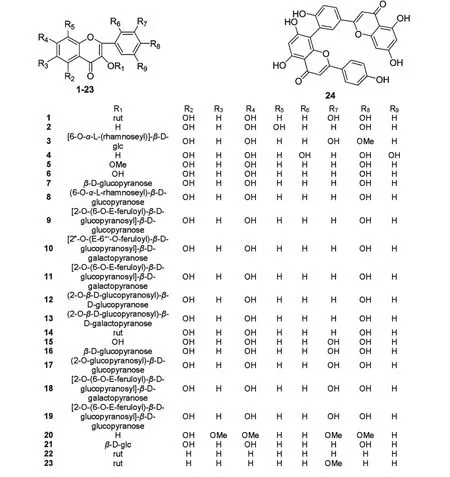
图1 耳草属植物中黄酮类成分化学结构式Fig.1 Chemical structures of flavonoids from genus Hedyotis
1.2 环烯醚萜类
耳草属植物中含有的环稀醚萜类化合物主要为环稀醚萜苷,此类成分为耳草属植物特征化学成分,多数环烯醚萜类成分化学结构中4 位羧基形成甲酯,也有少量4 位无取代基,如harpagoside、harpagide。从金毛耳草(H.chrysotricha)中分离得到asperulosidic acid (25)[48];10-deacetyl asperulosidic acid(27)[28];scandoside methyl ester(28)[31];acetyl scandoside methyl ester(29)[31];6β-hydroxy-genipin(30)[27];asperuloside (hedyotiside B)(32)[48];deacetylasperuloside(33)[48];hedyotiside B (6'-acetyl asperuloside)(34)[28,31];loganin、hedyoside、chrysotosid、hedyotoside、hedyotideaside (36-38、47)[48];6'-acetyl deacetyl asperuloside(54)[31]。从白花蛇舌草中分离得到asperuloside(32)[5,28];diffusoside A、diffusoside B(40、41)[13];E-6-O-p-methoxy-cinnamoyl scandoside methyl ester、Z-6-O-p-methoxy-cinnamoyl scandoside methyl ester、E-6-O-p-feruloyl scandoside methyl ester、Z-6-O-p-feruloyl scandoside methyl ester、E-6-O-p-coumaroyl scandoside methyl ester、Z-6-O-pcoumaroyl scandoside methyl ester(48~53)[21,23]。从纤花耳草(H.tenellifloa)中分离得到asperulosidic acid(25)[34];teneoside A、teneoside B、deacetylasperuloside(31、33、35)[47];teneoside C、harpagoside、harpagide(42、44、45)[35]。从该属植物中分离得到的环烯醚萜结构式见图2。

图2 耳草属植物中环烯醚萜类成分化学结构式Fig.2 Chemical structures of iridoids from genus Hedyotis
1.3 生物碱
从耳草属植物中分离得到的生物碱类成分多为β-carboline 类和吲哚类生物碱。从黄毛耳草中分离得到chrysotricine(55)[31];从头状花耳草(H.capitellata)中 分 离 得 到capitelline (58)[42]、hedyocapitelline、hedyocapitine(56、57)[41]、(-)-isocyclocapitelline、(+)-cyclocapitelline、isochrysotricine(59、60、61)[38]。耳草属植物中生物碱类成分化学结构见图3。
1.4 醌类
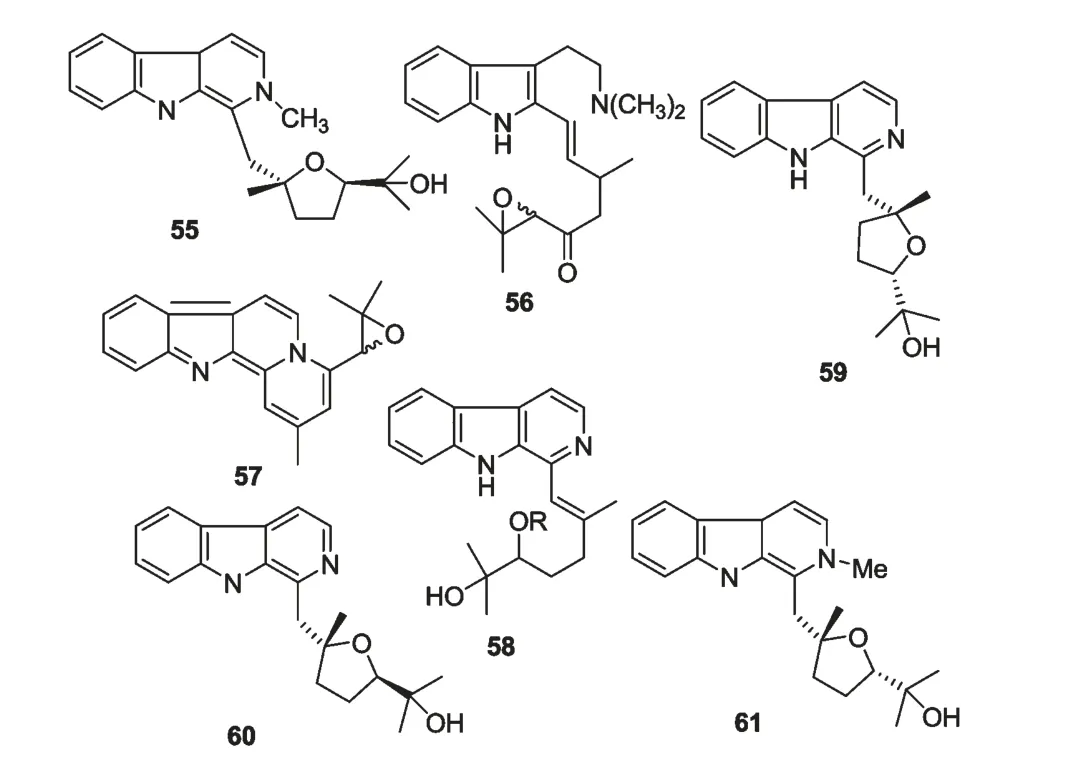
图3 耳草属植物中生物碱类成分化学结构式Fig.3 Chemical structures of alkaloids from genus Hedyotis
据文献报道,目前从耳草属植物中分离得到的醌类成分多数为9,10-蒽醌,另外还有少量1,4-蒽醌和苯醌。取代基多为羟基、甲基、甲氧基和羟甲基,也有邻位环合成吡喃环的;从丹草(H.herbacea)中分离出2-hydroxymethyl-10-hydroxy-1,4-anthraquinone、2,3-dimethoxy-9-hydroxy-1,4-anthraquinone、1,4-dihydroxy-2-hydroxymethyl anthraquinone、1,4-dihydroxy-2-hydroxymethyl anthraquinone (62、63、80,81)[46]。从白花蛇舌草(H.diffusa)中分离得到2-methyl-3-hydroxyanthraquinone、2-methyl-3-methoxy anthraquinone、2,6-dihydroxy-3-methyl-4-methoxy anthraquinone(66、67、75)[17];2-hydroxy-1-methoxy-anthraquinone、2-hydroxy-1,3-dimethoxy anthraquinone(68、70)[11];2-hydroxy-6-methyl-anthraquinone、2-hydroxy-3-methoxy-6-methyl-9,10-anthraquinone (73、78)[26];2-hydroxy-7-methyl-3-methoxy-anthraquinone(69)[6];2,3-dimethoxy-6-methyl anthraquinone(71)[22];2-hydroxy-7-hydroxymethyl-3-methoxy anthraquinone(72)[25];2,7-dihydroxy-3-methyl-anthraquinone (74)[14];2-hydroxy-1-methoxy-3-methyl-anthraquinone (76)[6,16];2,6-dihydroxy-1-methoxy-3-methyl-anthraquinone(77)[16];1,3-dihydroxy-2-methyl-anthraquinone(87)[50];1,7-dihydroxy-6-methoxy-2-methyl-anthraquinone(88)[51]。从 黄 毛 耳 草(H.chrysotricha)中分离得到2,6-dimethoxyl-1,4-benzoquinone(64)[31,48];hydyotanthraquinone(65)[32]。从头状花耳草(H.capitellata)中分离得到2-hydroxymethyl-3,4-[2-(1-hydroxy-1-methylethyl)-dihydrofurano]-8-hydroxyanthraquinone、capitellataquinone A、capitellataquinone B、capitellataquinone C、capitellataquinone D、rubiadin、anthragallol 2-methyl ether、alizarin 1-methyl ether、lucidin-3-O-β-glucoside (79,89-97)[39]。从牛白藤(H.hedyotidea)中分离得到hedanthrosides A、hedanthrosides B、hedanthrosides C、hedanthrosides D、hedanthrosides E(82-86)[44]。从该属中分离得到的蒽醌类成分化学结构见图4。
1.5 其他类
三萜类:从耳草属植物中分离得到的三萜类成分主要为乌苏烷型、齐墩果烷型和羽扇豆烷型。从黄毛耳草分离得到ursolic acid[29]、oleanolic acid[29]、betulic acid[30]。从纤花耳草(虾子草)(H.tenellifloa)中分离得到6个乌苏烷类三萜化合物[50]。从白花蛇舌草中分离得到gypsogenic acid[4]。从牛白藤中分离得到epibetulinic acid[48]。
甾体类:耳草属植物中分离得到的甾体类成分主要为植物甾醇。从白花蛇舌草、牛白藤、金毛耳草、纤花耳草中分离得到β-sitosterol、stigmasterol、stigmasterol-5,2-diene-3β-7α-diol、stigmasterol-5,2-diene-3β-7β-diol、6-hydroxy stigmast-4,22-dien-3-one、3-hydroxy stigmast-5,22-diene -7-one、ergosterol、daucosterol[6,24,25,42,44]。
苯丙素类:从耳草属植物中分离得到的苯丙素类成分主要有简单苯丙素、香豆素和木脂素。从白花蛇舌草和金毛耳草中分离得到esculetin、p-香豆酸、ferulic acid、caffeic acid、scopoletin、liriodendrin、iso-larisiresinol、4,4'-dihydroxy-α-truxillic acid、木脂体紫丁香脂素[4,17,31,32,48,51]。从双花耳草(H.biflora)中分离得到角形吡喃香豆素hedyotiscone A、hedyotiscone B、hedyotiscone C[37]。
挥发性成分:从耳草属植物中分离的挥发油主要包括脂肪族含氧衍生物、芳香族含氧衍生物和萜类含氧衍生物。采用SD、GC 和GC/MS 鉴别出白花蛇舌草中30 多种挥发性成分[24]。采用SD、SFE、GC/MS 从剑叶耳草(H.caudatifolia)中鉴别出60 多种挥发性成分[27]。

图4 耳草属植物中醌类成分化学结构式Fig.4 Chemical structures of anthraquinone from genus Hedyotis
2 药理作用
2.1 抗肿瘤作用
白花蛇舌草水煎液对宫颈癌Hela 细胞有较好的抑制作用,可使Ki-67 蛋白的表达下降[51],降低端粒酶活性、下调hTERT mRNA 的表达水平[52]、使Hela 细胞阻滞于S 期[53],从而诱导、促进肿瘤细胞的凋亡。白花蛇舌草多糖对宫颈癌Hela 细胞也有较好的抑制作用[54]。白花蛇舌草对肝癌具有治疗效果[55],其水提取物通过上调bax 蛋白表达[56],提高CD4+、CT8+淋巴细胞表达[57],以及下调Cdk2 和E2F1 的mRNA 表达,将肝癌细胞株HepG2 细胞阻滞在G0/G1 期[58],从而达到对肝癌细胞的抑制作用。白花蛇舌草提取物可抑制结肠癌HT-29 的细胞增殖,通过上调bax,下调bcl-2[62]、耐药基因ABCG2 的mRNA[63]以及HT-29 细胞Pim-1 和Pim-2 的mRNA 表达[64],从而诱导细胞凋亡,起到抗结肠癌的作用。白花蛇舌草提取物能明显抑制白血病k562 细胞[66]、CEM 细胞[67]、多药耐药白血病细胞HL-60/ADR[68]、HL60 细胞株[67]生长,在一定浓度下,可观察到细胞缩小、染色质明显浓缩、核聚集等典型细胞凋亡特征,在琼脂凝胶电泳中出现明显的DNA 梯形凋亡带,推测白花蛇舌草可能的抗白血病机制为诱导肿瘤细胞凋亡[66]。
白花蛇色花草醇提物对肺腺癌A594 细胞具有一定的抑制作用,其机制可能是将细胞周期阻滞在G1-G0期、上调Bax 和下调Bcl-2 的表达而诱导细胞凋亡[65]。白花蛇舌草中豆甾醇类成分[59]、挥发油类成分[60]、黄酮类成分[61]对肝癌H22、HepG2 具有一定的抑制作用。
2.2 对免疫功能的影响
白花蛇舌草多糖可显著促进溶血素形成、可使脾脏及胸腺增重并且明显提高吞噬能力,从而增强机体的免疫功能[69]。白花蛇舌草总黄酮可以促进免疫功能低下的小鼠由ConA 或LPS-γ 诱导的脾淋巴细胞的增殖反应,促进免疫功能低下小鼠脾脏IgM 抗体形成,并升高抗肿瘤药物所致的小鼠白细胞减少,从而增强机体免疫[70]。
2.3 抗菌作用
白花蛇舌草95%乙醇提取物对革兰氏阳性菌、革兰氏阳性菌具有抑制作用,其中,对格兰氏阴性菌的抑菌作用大于革兰氏阳性菌[71]。白花蛇舌草总黄酮对球菌和杆菌均具有不同程度的抑菌和杀菌作用,且对球菌的作用优于杆菌[72]。头状花耳草提取物对枯草芽孢杆菌B28(突变株)、枯草芽孢杆菌B29(野生型)、铜绿假单胞菌、耐甲氧西林金黄色葡萄球菌有较好的抑制作用[78]。
2.4 抗炎、镇痛作用
白花蛇舌草总黄酮对二甲苯诱导的小鼠耳肿胀和醋酸所致小鼠毛细血管通透性增高有一定的抑制作用,对大鼠松节油气囊肉芽增生和新鲜蛋清诱导大鼠足爪肿胀亦有明显的抑制作用,说明其具有一定的抗炎、镇痛作用[72]。牛白藤石油醚、乙酸乙酯萃取物能明显减轻二甲苯诱导的小鼠耳肿胀程度,抑制热刺激和醋酸引起的小鼠疼痛反应,说明牛白藤有明显的抗炎、镇痛活性[73]。
2.5 其它作用
保肝作用:白花蛇舌草可显著减轻CCl4引起的肝组织病理损伤程度,提高外周血CD4+T 细胞的百分比和CD4+T 细胞/CD8+T 细胞的比值,降低CD8+T 细胞的百分比,降低血浆中TNF-γ 和IL-6的水平,对肝损伤有一定的治疗作用[74]。
治疗哮喘作用:白花蛇舌草通过阻断NF -κBp65 表达,下调哮喘小鼠BALF 中IL-4、IL-5、IL-13 水平,同时上调BAIF 中IFN-γ 水平以及降低炎症细胞数量,从而抑制气道炎症,达到治疗哮喘的作用[75]。
抗氧化作用:白花蛇舌草多糖和总黄酮、丹草提取物均可以清除DPPH 自由基,且氧化能力随着与浓度呈现量效关系[76-78]。
神经保护作用:白花蛇舌草中的黄酮类和环稀醚萜类成分可减弱谷氨酸盐诱导的神经毒性,有一定的神经保护作用[8]。
3 结语
耳草属植物具有丰富的植物资源和显著的药理作用,在民间大多作白花蛇舌草使用。目前,除了白花蛇舌草(Hedyotis diffusa willd.)外,对耳草属其它植物的研究与开发应用还较少,因此,亟待对本属植物的化学成分和药理作用进行进一步深入研究,从而阐明药效物质基础,为耳草属植物的进一步药用植物资源开发应用提供科学依据。
1 Delectis Florae Reipublicae Popularis Sinica Agendae Academiae Sinicae Edita(中国科学院中国植物志编辑委员会).Flora Reipublicae Popularis Sinicae(中国植物志).Beijing:Science Press,1999.71:26-32.
2 Chinese Pharmacopoeia Commission(国家药典委员会).Pharmacopoeia of the People’s Republic of China(中华人民共和国药典).Beijing:China Medical Science Press,2010.Appendix 23.
3 Si JY(斯建勇),et al.Advance on chemistry and bioactivity of genus Hedyotis.Nat Prod Res Dev(天然产物研究与开发),2007,19:517-523.
4 Huang WH(黄卫华),et al.Chemical constituents from Hedyotis diffusa.Chin J Chin Mater Med(中国中药杂志),2009,34:712-714.
5 Yang YB(杨亚滨),et al.Chemical constituents of Hedyotis diffusa willd.J Yunnan Univ(云南大学学报),2007,29:187-189.
6 Kang XD(康兴东),et al.Chemical constituents of Hedyotis diffusa willd.J Shenyang Pharm Univ(沈阳药科大学学报),2007,24:479-481.
7 Zhang HJ(张海娟),et al.Study on flavonoids from Hedyotis diffusa willd.J Chin Med Mater(中药材),2005,28:385-387.
8 Kim YL,et al.neuroprotective constituents from Hedyotis diffusa.J Nat Prod,2001,64:75-78.
9 Liu JZ(刘晶芝),Wang L(王莉).Studies on chemical constituents of Hedyotis diffusa willd.Hebei Med Univ(河北医科大学学报),2007,28:188-190.
10 Ren FZ(任风芝),et al.Study on flavonoids from Hedyotis diffusa willd.Chin J Chin Mater Med(中国药学杂志),2005,40:502-504.
11 Wu KS(吴孔松),et al.Studies on constituents of Oldenladia diffusa.Chin J Chin Mater Med(中国药学杂志),2005,40:817-818.
12 Zhang YY(张永勇),Luo JB(罗佳波).Analysis of the chemical constituents of Hedyotis diffusa.J Chin Med Mater(中药材),2008,31:522-524.
13 Zhang Y,et al.Two new iridoid gluco-sides from Hedyotis diffusa.Fitoterapia,2010,81:515-517.
14 Yu L(于莉),et al.A new anthraquinone from Hedyotis diffusa.Chin J Med Chem(中国药物化学杂志),2008,18:298-299.
15 Kang XD(康兴东),et al.A new anthraquinone from Hedyotis diffusa willd.Chin J Med Chem(中国药物化学杂志),2006,16:368-369.
16 Zhou YJ(周应军),et al.Studies on constituents of Oldenlandia diffusa.Chin J Chin Mater Med(中国中药杂志),2007,32:590-593.
17 Si JY(斯建勇),et al.Chemical constituents of Hedyotis diffusa.Nat Prod Res Dev(天然产物研究与开发),2006,18:942-944.
18 Tan NH(谭宁华),et al.Anticancer activity and principles of Hedyotis diffusa.Nat Prod Res Dev(天然产物研究与开发),2004,14(5):33-36.
19 Lu CM,et al.A new acylated flavonol glycoside and antioxidant effects of Hedyotis diffusa.Planta Med,2000,66:374-377.
20 Kim YL,et al.Neuroprotective constituents from Hedyotis diffusa.J Nat Prod,2001,64:75-78.
21 Nishihama Y,et al.Three new iridoid glucosides from Hedyotis diffusa.Planta Med,1981,43:28-33.
22 Ho TI,et al.An anthraquinone from Hedyotis diffusa.Phytochemistry,1986,25:1988-1989.
23 Wu HM,et al.Iridoids from Hedyotis diffusa.J Nat Prod,1991,54:254-256.
24 Wong KC,Tan GL.Composition of the essential oil of Hedyotis diffusa Willd.J Eessential Oil Res,1995,7:537-539.
25 Kang XD,et al.Two new anthraquinones from Hedyotis diffusa.J Nat Prod,2008,10:193-197.
26 Huang WH,et al.Four anthraquinones from Hedyotis diffusa.Nat Prod Lett,2008,10:193-197.
27 Pan WG(潘为高),et al.GC-MS analysis of volatile oil from Hedyotis lancea.Chin J Exp Tradit Med Form(中国实验方剂学杂志),2012,18:130-134.
28 Peng JN(彭江南),et al.Chemical investigation of cenus hedyotis II.isolation and identification of iridoids from Hedyotis chrysotricha.Acta Pharm Sin(药学学报),1997,32:908-913.
29 Fang ZP(方乍浦),Yang YF(杨义芳).Isolation and identification of chemical constituents from Hedyotis chrysotricha(Palib.)merr.Chin J Chin Mater Med(中国中药杂志),1992,17:98-100.
30 Fang ZP(方乍浦),Yang YF(杨义芳).Isolation and identification of chemical constituents from Hedyotis chrysotricha(Palib.)merr.Jiangxi Med Coll(江西医学院学报),1991,32:51-53.
31 Peng JN(彭江南),et al.Chemical studies on Hediotis chrysotricha.Chin J Exp Tradit Med Form(中国新药杂志),1999,8:741-743.
32 Lin LE(林隆泽),Zhang JS(张金生).The isolation and identification of hydyotanthraquinone.Acta Botan Sin(植物学报),1988,30:670-672.
33 Shang HT(尚海涛).Analysis of flavonoids in herba hedyotids chrysotridhae with liquid chromatogram and mass chromatogram(LC-MC).Anim Husb Feed Sci(畜牧与饲料科学),2009,30(6):26-27.
34 Yuan QM(袁青梅),et al.Isolation and identification of chemical constituents from Hedyotis tenellifloa.Chin Tradit Herb Drugs(中草药),2004,35:981-982.
35 Yuan QM(袁青梅),et al.One new iridoid glycoside from Hedyotis tenelliflora.Chin Tradit Herb Drugs(中草药),2011,42:1464-1466.
36 Peng JN(彭江南),et al.Chemical investigation of genus hedyotis II.isolation and identification of iridoids from Hedyotis chrysotricha.Chin Tradit Herb Drugs(中草药),1999,30:170-172.
37 Chen YH,et al.New cytotoxic 6-oxygenated 8,9-dihydrofurocoumarins,hedyotiscone A-C,from Hedyotis biflora.Planta Med,2006,72:75-78.
38 Nguyen MP,et al.β-Carboline alkaloids from Hedyotis capitellata.Phytochemistry,1999,52:1725-1729.
39 Ahmada R,et al.Anthraquinones from Hedyotis capitellata.Phytochemistry,2005,66:1141-1147.
40 Phuong NM,et al.Capitelline-A new indole alkaloid from Hedyotis capitellata.Nat Prod Lett,1998,11:93-100.
41 Phuong NM,et al.Two new β-carboline alkaloids from Hedyotis capitellata var.mollis.Planta Med,1999,65:761-762.
42 Peng JN,et al.A β-carboline alkaloid from Hedyotis chrysotricha.Phytochemistry,1997,46:1119-1121.
43 Peng JN,et al.Iridoids from Hedyotis hedyotidea.Phytochemistry,1998,46:1657-1659.
44 Hu XP,et al.New anthraquinone and iridoid glycosides from the stems of Hedyotis hedyotidea.Helv Chim Acta,2011,94:675-685.
45 Hamzah AS,Lajis NH.Chemical constituents of Hedyotis herbacea.ASEAN Rev Biodiver Envir Conserv(ARBEC),ArticleⅡ1-6.
46 Permana D,et al.Anthraquinones from Hedyotis herbacea.J Nat Prod,1999,62:1430-1431.
47 Zhao JF,et al.Two new iridoid glycosides from Hedyotis tenelliflora Blume.Helv Chim Acta,2005,88:2532-2536.
48 Peng JN(彭江南).Chemical studies on herbs of genus hedyotis.Beijing:Chinese Peking Union Medical College(中国协和医科大学),PhD.1995.
49 Zhao JF(赵静峰),et al.Chemical studies on Xiazicao.Chin J Chem(有机化学),2005,25:687.
50 Huang WH(黄卫华),et al.Chemical constituents from Hedyotis diffusa.Chin J Chin Mater Med(中国中药杂志),2008,33:524-526.
51 Zhang PY(张培影),et al.The study of Hedyotis diffusa for its effects on proliferation and apoptosis of the cervical tumor in nude mouse model.Chin Mod Med(中国当代医药),2010,17(30):5-8.
52 Liu Y(刘颖),et al.Effect of Hedyotic diffusa on telomerase activity and hTERT gene expression in human cervical carcinom a Hela cells.J Basic Clin Oncol(肿瘤基础与临床),2010,23:103-106.
53 Gao C(高超),et al.Effect of Hedyotic diffusa on cell cycle,apoptosis and telomerase activity in human cervical cancer Hela cells.Acta Acad Med Xuzhou(徐州医学院学报),2010,30:466-468.
54 Yang PM(杨培民),et al.Effect of polysaccharides of Oldenlandia diffusa on growth of bel-7402 and hela cells.Chin Med J Res Prac(现代中药研究与实践),2010,24(3):32-34.
55 Hu L(胡玲),et al.Effect of Herba Hedyotis Diffusae on expression of heat shock protein70 and p16 in mice with hepatoma cell-transplanted tumor.Trad Chin Drug Res Clin Pharm(中药新药与临床药理),2009,20:18-20.
56 Hu L(胡玲),et al.Effect of Herba Hedyotis diffusa on bcl-2 and bax expression in bearing neoplasia of H22 hepatoma cells.Chin J Integr Trad West Med dig(中国中西医结合消化杂志),2011,19:378-381.
57 Gu XW(古学文),et al.Effect of H8P70 expression induced by Herba Hedyotis diffusae on T lymphocytes in mice with H22 liver cancer cell xenograft.Trad Chin Drug Res Clin Pharm(中药新药与临床药理),2010,21:393-395.
58 Chen XZ(陈旭征),et al.Effect of Cdk2 and E2F1 mRNA expression induced by hedyotis difusa on human hepatoma carcinoma HepG2 cell lines.Fujian J TCM(福建中医药),2012,43(2):32-34.
59 Zhang Y(张硕),et al.Inhibitive effect of stigmasterol from Hedyotis diffusa on hepatoma cell in vivo vitro and its influence on transplanted H22 tumor cell’s multiplication cycle,apoptosis.Prog Mod Biomed(现代生物医学进展),2008,8:2016-2017.
60 Wang LL(王丽丽),et al.Anti-cancer effect of volatile oil from Hedyotis diffusa on human hepatoma carcinoma hepG2 cell lines.Fujian J TCM(福建中医药),2013,44(3):60-61.
61 Zhou YX(周忆新),et al.Effect of flavone from(Willd.)Roxb.on human hepatoma carcinoma cell hepG-2 Oldenlandia diffusa in vitro.Anti Infect Pharm(抗感染药学),2009,6:179-181.
62 Peng J(彭军),et al.Effect of Hedyotic diffusa extract on expression of Bcl-2 and Bax in human colon cancer HT-29 cells.J Fujian Univ TCM(福建中医药大学学报),2010,20(5):23-26.
63 Lin JM(林久茂),et al.Anti-cancer effect of Hedyotis diffusa on colon cancer 5-Fu cell lines.Fujian J TCM(福建中医药),2013,44:53-55.
64 Wei LH(魏丽慧),et al.Effect of Herba Hedyotis diffusa ethanol extract on mRNA expression of Pim-1 and Pim-2 in human colon cancer HT-29 cells.World J Int Trad West Med(世界中西医结合杂志),2011,6:284-287.
65 Gai BA(高宝安),et al.Anti-cancer effect of ethanol extract from Hedyotis diffusa on lung adenocarcinoma A549 cell lines.Lishizhen Med Mater Med Res(时珍国医国药),2009,20:1392-1394.
66 Zhu DC(朱大诚),et al.Anti-cancer effect of aqueous extract from Hedyotis diffusa on lung adenocarcinoma K562 cell lines.Lishizhen Med Mater Med Res(时珍国医国药),2011,22:334-336.
67 Chen XZ(陈秀珍),et al.Research on the inhibitory function and mechanism of the aqueous extract of Hedyotis diffusa willd on CEM cells.Lishizhen Med Mater Med Res(时珍国医国药),2010,21:573-574.
68 Chen XZ(陈秀珍),et al.Anti-cancer effect of Hedyotis diffusa on leukemia CEM cell lines.Lishizhen Med Mater Med Res(时珍国医国药),2009,20:2461-2463.
69 Chen HX(陈浩许),et al.Immunomodulatory effect of polysaccharide and total flavonoids from Hedyotis diffusa.Veterin Sci Chin(中国兽医科学),2008,2:4-6.
70 Wang YL(王宇翎),et al.Immunomodulatory effects of total flavones of Oldenlandia diffusa willd.Chin Pharm log Bull(中国药理学通报),2005,21:444-447.
71 Li T(李涛),et al.Study on the antimicrobial effect of extract from Oldenlandia diffusa willd.Lishizhen Med Mater Med Res(时珍国医国药),2008,19:1335-1336.
72 Wang YL(王宇翎),et al.Anti-inflammatory and antibacterial effects of total flavones of Oldenlandia diffusa willd.Chin Pharm logl Bull(中国药理学通报),2005,21:348-350.
73 Chen YF(陈艳芬),et al.Screening of anti-inflammatory analgesic effective parts of Hedyotis hedyotidea.Trad Chin Drug Res Clin Pharm(中药新药与临床药理),2012,23:17-19.
74 Li XP(李秀萍),et al.Effects of Hedyotis diffusa willd on tlymphocytes subgroup tnf-and il-6 in mice with liver in jury.Ningxia Med J(宁夏医学杂志),2010,32:495-497.
75 Pu HM(朴红梅),et al.Effects of Hedyotis diffusa on cytokines level of type th1/th2 in mice model with asthma.Chin Hosp Pharm(中国医院药学杂志),2013,33:1381-1385.
76 Jiang JP(蒋剑平),et al.Antioxidant activity of polysaccharide from Hedyotis diffusa willd.Chin Arch Trad Chin Med(中华中医药学刊),2012,30:1076-1078.
77 Xu HS(许海顺),et al.Study on antioxidant activity of different extractant from Hedyotis diffusa willd.J Gansu Coll TCM(甘肃中医学院学报),2012,29(2):48-51.
78 Ahmad R,et al.Antioxidant,radical-scavenging,anti-inflammatory,cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species.Life Sci,2005,76:1953-1964.
