低分子量卡拉胶马来酰化衍生物抗氧化性能研究
2014-01-10王亚珍周冬香
孙 涛,王亚珍,谢 晶,周冬香,薛 斌
1上海海洋大学食品学院;2 上海海洋大学海洋科学研究院,上海 201306
Introduction
Carrageenans are sulfated anionic polymers composed of D-galactose units linked alternately with α-1,4 and β-1,3 linkages.They can be divided into different types(κ-,ζ-,and λ-carrageenan)depending on the number and position of sulfate groups[1].κ-Carrageenan is an economical important kind and widely used in food industry.It has been found to have valuable biological functions,such as anti-cancer,lowering blood pressure,stimulating the growth of connective tissue and bone absorption of calcium[2].However,the further utilization of carrageenan was limited for its high molecular weight and poor water solubility.Degradation and structural modification are useful to improve the water solubility and to enlarge the utilization of carrageenan.It was reported that the acylation of LMW κ-carrageenans followed by sulfation resulted in potentiation of anti-HIV activity[3].Since then,the synthesis and characterization of succinyl LMW κ-carrageenans,maleoyl LMW κ-carrageenans as well as their structure-activity relationship were studied[4].The antioxidant activity of κ-carrageenan oligosaccharides and their derivatives was also studied in recent years[5].However,the research on the antioxidant activity of maleoyl κ-carrageenan oligosaccharides derivatives was comparatively deficient.Moreover,the research on the influence of hydroxyl groups and the property of substituting groups on the antioxidant activity of maleoyl κ-carrageenan oligosaccharides derivatives was fewer.In the present study,maleic anhydride was introduced into LMW κ-carrageenans,and the antioxidant activities of the derivatives were investigated.
Materials and Methods
Chemicals
Carrageenan polysaccharides were purchased from Shanghai United Food Additives Co.,Ltd.(Shanghai,China).Luminol and 1,1-diphenyl-2-picrylhrazyl(DPPH)were purchased from Sigma Chemicals Co.(Shanghai,China).All other chemicals were of analytical grade and supplied by Shanghai Chemicals Co.(Shanghai,China).
Preparation of A,B,MA and MB
Water-soluble LMW κ-carrageenans were prepared according to the reported method with some modifications[6].κ-Carrageenans (5.0 g)was added to distilled water (200 mL),and then heated to 80 ℃.50.0 mL of H2O2solution (30%,w/w)was dropped into the κ-carrageenans solution within 30 minutes.After degradation for 10 h,white powder was obtained by lyophilization.The white powder was dissolved in distilled water and filtered through 0.45 μm microporous filter.Different LMW κ-carrageenans (A and B)were collected by dialyzing (Spectra/porl,molecular weight cut-off 3500-7000,7000)against distilled water for 6 days.
Two maleoyl derivatives of A and B (MA and MB)were prepared according to Jiang Yun-peng’s method[4].Compound A and B were percolated through a column (100 mL)of 732 type cation-exchange resin at 4 ℃.The pH of the solution was adjusted to 8.0 by tetrabutylammonium (TBA)to afford TBA salt by lyophilization.κ-Carrageenans TBA salt (1 g)were dissolved in N,N-dimethylformamide (DMF)(25 mL)under N2flow,and then 4-dimethylaminopyridine (147 mg),maleic anhydride (1.44 g)and tributylamine (6 mL)were added.The solution was heated at 60 ℃for 6 h.After the reaction,the mixture was cooled to 4 ℃,and then a cold saturated ethanolic solution of sodium acetate was added.The mixture was allowed to stand for 1 h at 4 ℃.After centrifugation,the precipitate was dissolved in distilled water,and dialyzed against 5% sodium hydrogen carbonate for 2 days,followed by distilled water for 4 days.The solution was lyophilized to afford maleoyl LWM κ-carrageenans (MA and MB).
Characterization of A,B,MA and MB
FT-IR spectra of A,B,MA and MB were acquired with KBr pellets on an EQUNOX55 FT-IR-Laman spectrophotometer with a revolution of 0.8 cm-1in the range of 500-4000 cm-1.
The molecular weight of LMW κ-carrageenans and their maleoyl derivatives was measured by Gel Permeation Chromatography (GPC).The GPC was performed on a Waters-515 chromatograph equipped with Waters 2410 refractive index detector and Ultrahydrogel 500 and 120.Elution was carried out using 0.07% Na2SO4solution as the mobile phase at a flow rate of 0.5 mL/min.The temperatures of the column and detector were both maintained at 40 ℃ during the determination process.
Superoxide anion radical scavenging activity
The superoxide anion scavenging activity of A,B,MA and MB was determined using chemiluminescent method.The assay was carried out on a chemical luminometer (IFFM-D,Xi’an,China).The chemiluminescent reaction was processed in a Na2CO3-NaHCO3(pH=10.20,0.05 mol/L)buffer solution at ambient temperature.The samples were dissolved in Na2CO3-NaHCO3buffer solution to prepare scavenger solutions at different concentrations (from 0.11 mmol/L to 3.26 mmol/L).The scavenging activity of samples against superoxide anion was evaluated according to their quenching effects on the chemilumescence (CL)signal of luminol-pyrogallol system.The inhibition efficacy for superoxide anion was calculated as scavenging effect (%)=(A0-Ai)/A0×100%,where A0and Airepresented CL peak areas of the blank group and test group,respectively.The free radical produced in the system was proved to be superoxide anion tested by superoxide dismutase (SOD),catalase and mannitol.Ascorbic acid was used as the positive control[7].
DPPH radical scavenging activity
The DPPH scavenging activity of the samples was measured using the modified method of Yamaguchi,et al[8].2.0 mL of DPPH solution (0.1 mmol/L in ethanol)was incubated with 2.0 mL of testing samples at different concentrations (0.1-2.0 mg/mL,in water),respectively.The reaction mixture was shaken well and incubated for 30 min at 33 ℃and the absorbance of the resulting solution was read at 517 nm against a blank.The radical scavenging activity was measured as a decrease in the absorbance of DPPH and was calculated using the following equation:Scavenging effect(%)=(1-Asample/Acontrol)× 100%.
Reducing power assay
The reducing power of all samples was determined by the method of Oyaizu[9].Different concentrations of testing samples (2.0 mL,in water)were mixed with 2.5 mL sodium phosphate buffer (pH=6.60,0.2 mol/L)and 2.5 mL potassium ferricyanide (1% W/V).The mixtures were incubated for 20 min at 50 ℃,then 2.5 mL trichloroacetic acid (10% W/V)was added to the mixture,followed by centrifugation at 3000 rpm for 10 min.The supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of ferric chloride solution (0.1% W/V)and the absorbance was measured at 700 nm.Increased absorbance of the reaction mixture indicated increased reducing power.
Statistical analysis
All analyses were performed in triplicate.Data of antioxidant evaluation were expressed as mean ± standard error of the mean.SPSS 11.0 (SPSS Inc.,Chicago,USA)was used to evaluate the significant difference by the method of student’s t-test when P<0.05.
Results and Discussion
Structural characterization of κ-carrageenan polysaccharide and its maleoyl derivatives
The FT-IR spectra of κ-carrageenan polysaccharides(KC),the LMW κ-carrageenans (A)and its maleoyl derivative (MA)were shown in Fig.1.Compounds A and MA maintained three characteristic absorption peaks.Peaks appearing at 1250 cm-1and 850 cm-1were contributed to S=O of sulfate esters and C-O-S of axial secondary sulfate on C-4 of galactose,respectively.The band at 930 cm-1was characteristic absorption of C-O on 3,6-anhydro-D-galactose,which indicated the structural integrity of κ-carrageenans were maintained in the degradation and maleoylation process[9].A new absorption band appeared at 1740 cm-1of A possibly suggested the formation of carbonyl group in the degradation process.The appearance of this new absorption was a common character of oxidative degraded polysaccharides[7].Compared with the spectrum of A,the absorption band at 1590 and 1430 cm-1of MA were attributed to asymmetrical stretching vibration and symmetrical stretching vibration of -COO-of carboxylate,and the absorption peak appearing at 1640 cm-1was assigned to C=C[4].These indicated that the maleoyl derivatives of LMW κ-carrageenans were prepared.
The molecular weight of κ-carrageenan polysaccharide(KC),LMW κ-carrageenans (A,B)and maleoyl LMW κ-carrageenans (MA,MB)was 3.5 ×105Da,1890 Da,4550 Da,3600 Da and 6210 Da,respectively.

Fig.1 FT-IR spectra of κ-carrageenan polysaccharide(KC),LMW κ-carrageenan (A)and its maleoyl derivative (MA)
Superoxide anion radical scavenging activity
The scavenging effects of LMW κ-carrageenans and their maleoyl LMW κ-carrageenans on superoxide anion were shown in Fig.2.A concentration-dependent inhibition against superoxide anion was observed.The IC50values of A,B,MA and MB were 1.86,1.36,0.46 and 0.43 mmol/L,respectively.The results indicated that the order of their scavenging efficacy on superoxide anion was:MA >A,MB >B;B >A,MB >MA.This indicated that maleoyl LMW κ-carrageenans showed the stronger superoxide anion scavenging activity than LMW κ-carrageenans,and scavenging activity of LMW κ-carrageenans and their maleoyl LMW κ-carrageenans on superoxide anion was increased with the increasing of molecular weight.

Fig.2 Superoxide anion scavenging effect of LMW κcarrageenans and their maleoyl derivatives
DPPH scavenging activity
Fig.3 depicted the DPPH radical scavenging effect of LMW κ-carrageenans and their maleoyl LMW derivatives.Scavenging activity of DPPH radical was evident at all tested concentrations.The IC50values of A,B,MA and MB were 1.56,1.00,1.31 and 0.45 mmol/L,respectively.The results indicated that the order of the scavenging efficacy on DPPH radicals was:MA > A,MB > B;B > A,MB > MA,which was consistent with the results of superoxide anion scavenging assay.

Fig.3 DPPH radical scavenging effect of LMW κ-carrageenans and their maleoyl derivatives
Reducing power
Fig.4 depicted the reducing power of LMW κ-carrageenans and their maleoyl LMW derivatives.The absorbances of A,B,MA and MB increased with the increasing of their concentrations.At a concentration of 2.0 mmol/L,their absorbances were 0.326,0.494,0.795 and 0.905,respectively.The results indicated that the order of their reducing power was:MA >A,MB >B;B >A,MB >MA,which was consistent with the results of above two antioxidant assays.
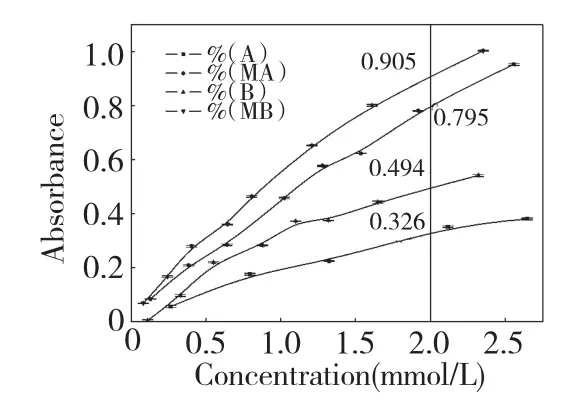
Fig.4 Reducing power of LMW κ-carrageenans and their maleoyl LMW derivatives
Discussion
The antioxidant activity of natural polysaccharides has indicated that the active hydroxyl and amino groups in the polymer chains may take part in free radicals scavenging and contributed to the antioxidant activity[10].The antioxidant activity of κ-carrageenans may also be owed to hydroxyl groups in the polymer chain,the maleoylation took place regioselectively at C-2 of the κcarrageenan 3-linked unit.Although the content of hydroxyl groups was decreased by the maleoylation process,the electron-withdrawing effect of maleoyl group could destroy the intermolecular and intramolecular hydrogen bonds,and enhanced the reaction ability of hydroxyl groups with free radical.Thus MA and MB showed better antioxidant activity than A and B,respectively.At the same time,the antioxidant activity of LMW κ-carrageenans and their maleoyl LMW κ-carrageenans showed in following order:B >A,MB >MA.With lower molecular weights,LMW κ-carrageenans and their maleoyl LMW κ-carrageenans derivatives had shorter polymer chain,and their ability to form intramolecular and intermolecular hydrogen bonds declined sharply.In this situation,the hydroxyl groups were more active,and it was helpful to antioxidant activity.But,on the other hand,the hydroxyl groups in polymer chain were decreased with the decreasing of molecular weights.In other words,the number of active groups that react with free radicals were decreased in LMW κcarrageenans and it was harmful to antioxidant activity.The antioxidant activity was controlled by both above mentioned facts.Thus,the antioxidant activity of LMW κ-carrageenans and their maleoyl LMW κ-carrageenans derivatives increased with the increasing of the molecular weights.
Conclusion
The antioxidant activity of LMW κ-carrageenans and their maleoyl LMW κ-carrageenans derivatives may be mainly attributed to the hydroxyl groups and the property of substituting group.The introduction of substituting groups will decrease the intramolecular and intermolecular hydrogen bonds,and thus will be helpful to increase the antioxidant activity of κ-carrageenan maleoyl derivatives.However,the detailed structure-antioxidant activity relationship still needs further investigation.The antioxidant activity study of the maleoyl derivatives will be helpful to expand the applications of κcarrageenans in biomedicine.
1 Mclean MW,Williamson FB.Glycosulphatase from Pseudomonas carrageenovora.Eur J Biochem,1979,101:497-505.
2 Yamada T,Ogamo A,Saito T,et al.Preparation and anti-HIV activity of low-molecular-weight carrageenans and their sulfated derivatives.Carbohyd Polym,1997,32:51-55.
3 Yamada T,Ogamo A,Saito T,et al.Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect.Carbohyd Polym,2000,41:115-120.
4 Jiang YP,Guo XK.O-maleoyl derivative of low-molecularweight κ-carrageenan:Synthesis and characterization.Carbohyd Polym,2005,61:441-445.
5 Yuan HM,Song JM,Zhang WW,et al.Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives.Bioorg Med Chem Lett,2006,16:1329-1334.
6 Sun T,Tao HN,Xie J,et al.Degradation and antioxidant activity of κ-carrageenans.J Appl Polym Sci,2010,117:194-199.
7 Sun T,Zhou DX,Mao F,et al.Preparation of low-molecularweight carboxymethyl chitosan and their superoxide anion scavenging activity.Eur Polym J,2007,43:652-656.
8 Yamaguchi T,Takamura H,Matoba T,et al.HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl.Biosci Biotech Bioch,1998,62:1201-1204.
9 Oyaizu M.Studies on products of browning reaction-antioxidative activities of products of browning reaction prepared from glucosamine.Jpn J Nutr,1986,44:307-315.
10 Xie WM,Xu PX,Liu Q.Antioxidant activity of water-soluble chitosan derivatives.Bioorg Med Chem Lett,2001,11:1699-1701.
