苦楝化学成分及抗糖尿病活性研究
2014-01-09谭钦刚赖春华张贵杰陈毅飞覃德绩王恒山
谭钦刚 ,赖春华,张贵杰,陈毅飞,覃德绩,王恒山
1桂林医学院;2 广西师范大学,桂林 541004
苦楝(Melia azedarach L.)为楝科(Meliaceae)楝属(Melia)植物,其树皮因良好的药理活性曾收载于2010 年《中国药典》一部[1]。研究表明,苦楝叶提取物能使糖尿病小鼠的血糖水平显著下降且呈量效关系[2],为了深入研究苦楝皮的化学成分及抗糖尿病活性,本实验对苦楝皮乙醇提取物的化学成分进行了研究,共分离得到5 个三萜类成分和3 个酚性化合物,并对分离得到的部分化合物进行了体外抗糖尿病活性测试。研究结果表明,化合物1~3,6,7 为首次从该植物中分离得到。受试化合物2~5 均未表现出葡萄糖激酶(glucokinase,GK)、组蛋白去乙酰化酶(sirtuin1,SIRT1)体外激动活性和二肽基肽酶IV(dipeptidyl peptidase IV,DPPIV)抑制活性,其中化合物2 对人11β-羟基类固醇脱氢酶1(11βhydroxysteroid dehydrogenase 1,11β-HSD1)具有显著的抑制作用,并对人11β-HSD2 有良好的选择性。
1 仪器与材料
FAB-MS 用VG Auto Spec-3000,EI-MS 用VG ZAB-SH 质谱仪(英国VG 公司);NMR 用Bruker AM-400 或DRX-500 核磁共振仪测定(德国Bruker公司,TMS 为内标);酶标仪(SpectraMax 190,美国Molecular Device 公司);PHS-3TC 型精密数显pH 计(上海天达仪器有限公司);薄层层析检测用UV-210 紫外分光光度计(上海元析仪器有限公司)。
柱层析硅胶(200~300 目),GF254薄层板为青岛海洋化工厂生产;Rp-18(40~65 μm)为德国Merck公司产品;Sephadex LH-20(40~70 μm)为瑞典Pharmacia 公司产品;分离纯化用试剂为分析纯,10%的硫酸-乙醇溶液(加热)检测;阳性对照物PSN-GK1、MK0431(FW:505)、白黎芦醇(RSV)、甘草次酸购自Sigma 公司,小鼠或人11β-HSD 基因购自NIH Mammalian Gene Collection。
样品购自云南昆明,由中国科学院昆明植物研究所曾春霞博士鉴定为苦楝(Melia azedarach L.)的皮。
2 实验方法
2.1 提取与分离
苦楝皮干燥品(10 kg)粉碎,用95%的乙醇加热回流提取3 次,滤液减压回收得提取物570 g,水分散后用乙酸乙酯萃取3 次,得乙酸乙酯萃取物260 g,上硅胶柱层析,用氯仿∶丙酮(1∶0~1∶1)系统梯度洗脱,得到七个组分(Fr.I -VII)。Fr.III 经硅胶柱层析,以石油醚-丙酮(6∶1~3∶1)洗脱,再经甲醇结晶得化合物1(73.0 mg),6(5.8 mg)。Fr.IV 经Rp-18 柱层析,以甲醇-水(7 ∶3~10 ∶0)洗脱,以Sephadex LH-20(氯仿-甲醇=1∶1)过滤,甲醇结晶得化合物2(56.1 mg),3(24.8 mg),4(29.5 mg)。Fr.V 经Rp-18 柱层析,以甲醇-水(5∶5~10∶0)洗脱,甲醇结晶得化合物5(383.2 mg)。Fr.VI 经Rp-18 柱层析,以甲醇-水(4∶6~10∶0)洗脱,甲醇结晶得化合物7(5.0 mg),丙酮结晶得化合物8(9.3 mg)。
2.2 体外抗糖尿病活性研究
糖尿病发病原因复杂,某些酶与其发生发展密切相关,如GK 在糖尿病患者血糖平衡控制中有减少肝脏葡萄糖生成和促胰岛素分泌的双重作用[3],SIRT1 激动剂可改善肥胖糖尿病大鼠的胰岛素敏感性[4],DPPIV 的活性增高与糖尿病的发生存在相关性[5],抑制11β-HSD 的活性可使糖尿病患者的代谢作用正常等[6]。
采用文献[7]描述的酶偶联分析法,测定化合物2-5 对GK 和SIRT1 的激动活性;按文献[8]方法构建体外筛选模型并测定上述化合物抑制DPPIV 的活性;按文献[9]方法,测定上述化合物对小鼠和人11β-HSD 的抑制作用。
3 实验结果
3.1 化合物结构鉴定
化合物1 无色针状结晶(MeOH),mp.194~195 ℃;FAB-MS(negative):m/z 457[M-1]-;分子式C30H50O3;1H NMR(CDCl3,500 MHz)δ:5.10(1H,t,J=6.9 Hz,H-24),3.56(1H,m,H-12),1.66(3H,s,H-26),1.61(3H,s,H-27),1.15(3H,s),1.06(3H,s),1.02(3H,s),1.00(3H,s),0.96(3H,s),0.87(3H,s);13C NMR(CDCl3,125 MHz)δ:39.7(t,C-1),34.0(t,C-2),217.9(s,C-3),47.7(s,C-4),55.2(d,C-5),19.6(t,C-6),34.0(t,C-7),39.5(s,C-8),49.3(d,C-9),36.7(s,C-10),30.9(t,C-11),70.4(d,C-12),48.6(d,C-13),51.9(s,C-14),31.5(t,C-15),26.2(t,C-16),49.9(d,C-17),15.9(q,C-18),15.3(q,C-19),74.5(s,C-20),21.7(q,C-21),30.9(t,C-22),21.9(t,C-23),124.5(d,C-24),132.0(s,C-25),25.7(q,C-26),17.8(q,C-27),26.6(q,C-28),20.9(q,C-29),16.9(q,C-30)。以上数据与文献[10]报道一致,故该化合物鉴定为12β,20(S)-dihydroxydammar-24-en-3-one。
化合物2 无色针状结晶(MeOH),mp.205~206 ℃;ESI-MS(negative):m/z 605 [M-1]-;分子式C39H58O5;1H NMR(DMSO-d6,400 MHz)δ:9.58(1H,d,J=4.1 Hz,3'-OH),9.15(1H,d,J=6.9 Hz,4'-OH),7.43(1H,d,J=15.9 Hz,H-8'),7.02(1H,s,H-2'),6.98(1H,d,J=8.2 Hz,H-6'),6.73(1H,d,J=8.2 Hz,H-5'),6.22(1H,d,J=15.9 Hz,H-7'),5.05(1H,t,J=7.2 Hz,H-24),4.47(1H,dd,J=10.6,4.1 Hz,H-3),4.65(1H,d,J=11.8 Hz,H-1),3.86(1H,s,20-OH),1.60(3H,s,H-26),1.51(3H,s,H-27),1.00(3H,s),0.91(3H,s),0.87(3H,s),0.84(3H,s),0.83(3H,s),0.81(3H,s);13C NMR(DMSO-d6,100 MHz)δ:38.9(t,C-1),24.3(t,C-2),79.8(d,C-3),38.1(s,C-4),55.2(d,C-5),17.8(t,C-6),35.2(t,C-7),39.9(s,C-8),50.0(d,C-9),36.6(s,C-10),21.9(t,C-11),24.9(t,C-12),41.6(d,C-13),49.9(s,C-14),31.3(t,C-15),27.3(t,C-16),49.9(d,C-17),16.4(q,C-18),16.1(q,C-19),73.0(s,C-20),25.3(q,C-21),41.3(t,C-22),22.3(t,C-23),125.3(d,C-24),130.1(s,C-25),25.6(q,C-26),17.5(q,C-27),27.8(q,C-28),15.3(q,C-29),16.6(q,C-30),125.5(s,C-1'),114.4(d,C-2'),144.9(s,C-3'),148.4(s,C-4'),114.8(d,C-5'),121.3(d,C-6'),145.6(d,C-7'),115.7(d,C-8'),166.4(s,C-9')。以上数据与文献[11]报道一致,故该化合物鉴定为dammarendiol II 3-O-caffeate。
化合物3 片状结晶(MeOH),mp.110~112℃;EI-MS:m/z 438[M]+;分子式C31H50O;1H NMR(CDCl3,500 MHz)δ:4.71(1H,br s,H-31),4.65(1H,br s,H-31),1.09(3H,s,H-30),1.03(3H,s,H-29),1.02(3H,d,J=6.5 Hz,H-27),1.02(3H,d,J=6.5 Hz,H-26),1.00(3H,s,H-18),0.98(3H,s,H-28),0.89(3H,d,J=6.0 Hz,H-21);13C NMR(CDCl3,100 MHz)δ:33.4(t,C-1),37.4(t,C-2),216.5(s,C-3),50.2(s,C-4),48.4(d,C-5),21.5(t,C-6),28.1(t,C-7),47.8(d,C-8),21.0(s,C-9),25.9(s,C-10),25.8(t,C-11),35.5(t,C-12),45.3(s,C-13),48.7(s,C-14),32.7(t,C-15),26.7(t,C-16),52.2(d,C-17),18.3(q,C-18),29.5(q,C-19),36.1(d,C-20),18.0(q,C-21),34.9(t,C-22),31.2(t,C-23),156.7(s,C-24),33.7(d,C-25),21.9(q,C-26),21.8(q,C-27),19.3(q,C-28),22.1(q,C-29),20.7(q,C-30),105.9(t,C-31)。以上数据与文献[12]报道一致,故该化合物鉴定为24-methylenecycloartenone。
化合物4 无色粉末,FAB-MS(negative):m/z 709[M-1]-;分子式C41H58O10;1H NMR(CDCl3,500 MHz)δ:8.04(2H,d,J=7.7 Hz,H-3' and H-7'),7.52(1H,m,H-5'),7.38(2H,t,J=7.5 Hz,H-2'and H-6'),5.30(1H,br.d,J=2.1 Hz,H-15),5.13(1H,br.s,H-7),4.83(1H,s,H-3),4.65(1H,d,J=11.8 Hz,H-1),3.92(br.s,W1/2,J=3.5 Hz),3.54(1H,m,H-21),2.12(3H,s,COCH3),1.60(3H,s,COCH3),1.35(3H,s,H-27),1.24(3H,s,H-26),1.08(3H,s,H-30),1.06(3H,s,H-29),0.96(3H,s,H-18),0.95(3H,s,H-19),0.86(3H,s,H-28);13C NMR(CDCl3,125 MHz)δ:72.6(d,C-1),25.3(t,C-2),77.0(d,C-3),36.4(s,C-4),37.3(d,C-5),23.0(t,C-6),75.4(d,C-7),41.9(s,C-8),35.2(d,C-9),40.1(s,C-10),16.0(t,C-11),34.6(t,C-12),46.3(s,C-13),158.8(s,C-14),119.2(d,C-15),33.7(t,C-16),56.8(d,C-17),19.9(q,C-18),16.1(q,C-19),29.7(d,C-20),65.2(t,C-21),32.6(t,C-22),67.4(d,C-23),96.5(s,C-24),76.3(s,C-25),23.1(q,C-26),24.1(q,C-27),27.9(q,C-28),21.3(q,C-29),26.7(q,C-30),165.2(s,C-1'),130.5(s,C-2'),129.4(d,C-3'),128.2(d,C-4'),132.9(d,C-5'),128.2(d,C-6'),129.4(d,C-7'),169.7,170.1(s,COCH3),20.9,21.3(q,COCH3)。以上数据与文献[13]报道一致,故该化合物鉴定为meliavolin。
化合物5 无色针状结晶(MeOH),mp.215~217 ℃;FAB-MS(positive):m/z 733[M +1]+;分子式C37H48O15;1H NMR(CDCl3,400 MHz)δ:6.91(1H,dd,J=13.7,6.3 Hz,H-3'),6.40(1H,d,J=3.0 Hz,H-23),5.65(1H,s,H-21),5.38(1H,d,J=2.9 Hz,H-22),4.89(1H,s,H-3),4.75(1H,s,H-1),4.23(1H,d,J=2.3 Hz,H-7),4.14 and 3.84(2H,d,J=9.1 Hz,H-19),4.11(1H,s,H-15),3.93(1H,dd,J=12.9,2.7 Hz,H-6),3.68(3H,s,12-OMe),3.54 and 3.46(2H,d,J=2.5 Hz,H-28),3.34(3H,s,11-OMe),3.03(1H,d,J=12.8 Hz,H-5),2.91(1H,d,J=4.5 Hz,H-17),2.10(3H,s,OCOCH3),1.92(3H,s,OCOCH3),1.48(3H,s,H-30),1.34(3H,s,H-18),0.98(3H,s,H-29);13C NMR(CDCl3,100 MHz)δ:70.1(d,C-1),28.1(t,C-2),70.7(d,C-3),42.2(s,C-4),34.9(d,C-5),71.7(d,C-6),82.9(d,C-7),51.9(s,C-8),48.1(d,C-9),49.8(s,C-10),106.6(s,C-11),169.0(s,C-12),93.5(s,C-13),92.8(s,C-14),81.9(d,C-15),28.8(t,C-16),48.1(d,C-17),25.8(q,C-18),70.5(t,C-19),91.7(s,C-20),105.8(d,C-21),105.4(d,C-22),146.7(d,C-23),76.0(t,C-28),18.1(q,C-29),18.0(q,C-30),166.6(s,C-1'),128.2(s,C-2'),138.1(d,C-3'),14.3(q,C-4'),12.0(q,C-5'),170.2,171.4(s,COCH3),20.9,21.4(q,COCH3),52.3(q,C11-OCH3),53.1(q,C12-OCH3)。以上数据与文献[14]报道一致,故该化合物鉴定为3,20-diacetyl-11-methoxy-1-tigloylmeliacarpinin。
化合物6 无色针状结晶(MeOH),mp.145~147 ℃;EI-MS:m/z 210[M]+,分子式C10H10O5;1H NMR(CDCl3,400 MHz)δ:12.89,(1H,s,4-OH),12.42(1H,s,2-OH),10.34(1H,s,CHO),6.29(1H,s,H-5),3.96(3H,s,OCH3),2.53(3H,s,CH3);13C NMR(CDCl3,100 MHz)δ:193.9(s,CHO),172.0(s,C-1'),168.3(s,C-4),166.6(s,C-2),152.3(s,C-6),112.1(d,C-5),108.4(s,C-3),103.8(s,C-1),52.3(q,OCH3),25.2(CH3,q)。以上数据与文献[15]报道一致,故该化合物鉴定为methyl 3-formyl-2,4-dihydroxy-6-methyl benzoate。
化合物7 浅黄色针状结晶(MeOH),mp.203~204 ℃;FAB-MS(negative):m/z 343[M-1]-;分子式为C18H16O7;1H NMR(CDCl3,500 MHz)δ:13.3(1H,s,7-OH),11.0(1H,s,3-OH),5.98(1H,s,H-4),2.68(3H,s,H-14),2.66(3H,s,H-12),2.11(3H,s,H-15),1.76(3H,s,H-10);13C NMR(CDCl3,125 MHz)δ:201.8(s,C-1),103.9(s,C-2),191.7(s,C-3),98.3(d,C-4),101.5(s,C-6),163.9(s,C-7),109.3(s,C-8),157.5(s,C-9),32.1(q,C-10),198.0(s,C-11),27.9(q,C-12),200.4(s,C-13),31.3(q,C-14),7.6(q,C-15),179.4(s,C-4a),155.2(s,C-6a),105.2(s,C-9a),59.1(s,C-9b)。以上数据与文献[16,17]报道一致,故该化合物鉴定为usnic acid。
化合物8 浅黄色针状结晶(丙酮),mp.241~243 ℃;FAB-MS(negative):m/z 289[M-1]-;分子式为C15H14O6;1H NMR(CD3OD,400 MHz)δ:6.82,(1H,d,J=1.5 Hz,H-2'),6.75(1H,d,J=8.1 Hz,H-5'),6.70(1H,dd,J=8.1,1.6Hz,H-6'),5.91(1H,d,J=2.2 Hz,H-6),5.84(1H,d,J=2.2 Hz,H-8),4.55(1H,d,J=7.5 Hz,H-2),3.96(1H,dd,J=13.8,7.7 Hz,H-3),2.83(1H,dd,J=116.1,5.4 Hz,H-3),2.48(1H,dd,J=16.1,8.1 Hz,H-3);13C NMR(CD3OD,100 MHz)δ:82.8(d,C-2),68.8(d,C-3),28.5(t,C-4),100.8(s,C-4a),157.8(s,C-5),96.3(d,C-6),157.6(s,C-7),95.5(d,C-8),156.9(s,C-8a),132.2(s,C-1'),115.2(d,C-2'),146.2(s,C-3' and C-4'),116.1(d,C-5'),120.2(d,C-6')。以上数据与文献[18,19]报道一致,故该化合物鉴定为epi-catechin。
3.2 化合物2~5 抗糖尿病活性
3.2.1 GK 体外活性筛选
化合物2~5 对人GK 激动作用筛选实验重复3次,结果表明,1 μmol/L 的阳性对照PSN-GK1 可使GK 活性增加至溶剂对照组的2.63 倍,而化合物2~5 在浓度为10 μmol/L 时,仅使其活性增加至对照组的0.79~1.11 倍,因此,受试化合物无明显的GK 激动活性。
3.2.2 化合物对人SIRT1 激动剂体外活性筛选
上述化合物对人SIRT1 的激动作用筛选试验重复2 次,结果表明,200 μmol/L 的阳性对照RSV 可使SIRT1 活性增加至溶剂对照组的10.51 倍,而上述化合物在相同浓度时,仅使其活性增加至对照组的0.82~1.00 倍,因此,受试化合物均不具有显著的SIRT1 激动活性。
3.2.3 化合物体外抑制DPPIV 活性筛选
测定上述化合物对DPPIV 的抑制作用,结果表明,0.1 μmol/L 的阳性对照MK0431 有很强的抑制作用,其比活力值为25.2%;受试化合物浓度为10 μmol/L 时,其比活力值为97.67~103.96%,表明上述化合物对DPPIV 均无激动活性。
3.2.4 化合物体外抑制11β-HSD 活性筛选
测定上述化合物对11β-羟基类固醇脱氢酶(11β-HSD)的抑制活性,结果见表1。

表1 化合物对小鼠和人抑制11β-HSD1 型酶抑制率Table 1 Inhibitory activity of compounds 2-5 against 11β-HSD1
由表1 可知,化合物2 对人11β-HSD1 有明显的抑制作用,进一步测定该化合物对11β-HSD1 抑制作用的IC50值和对人11β-HSD2 的抑制率,结果见表2。
经测定,化合物2 对人11β-HSD1 的IC50为94.15 nmol/L。从表2 可知,该化合物对人11β-HSD1 具有显著的抑制作用,当其剂量为1 mmol/L时对人11β-HSD2 的抑制率低于50%,提示该化合物对人11β-HSD2 有良好的选择性。
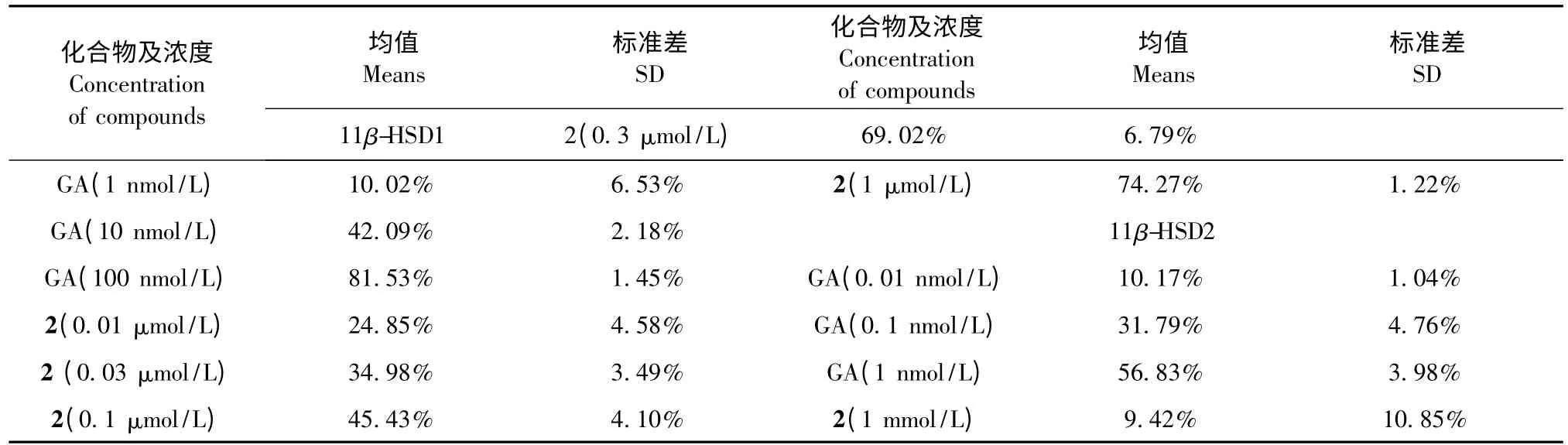
表2 化合物2 对人11β-HSD1 和11β-HSD2 的抑制率(X ± SD,n=2)Table 2 The inhibition activity of compounds 2 against human 11β-HSD1 and 11β-HSD2(X ± SD,n=2)
致谢:本实验体外抗糖尿病活性测试由中国科学院上海药物研究所内分泌组完成。
1 Chinese Pharmacopoeia Commission(国家药典委员会).Pharmacopoeia of the People’s Republic of China(中华人民共和国药典).Beijing:China Medical Science and Technology Press,2010.Vol I,141.
2 Vijayanand S,Wesely EG.Evaluation of antidiabetic activity of Melia azadirach on alloxan induced diabetic rats.Int J Curr Pharm Res,2011,3:37-40.
3 Wang JH(汪建辉),Ou Y(欧瑜).Glucokinase and diabetes.Pharm Biotechnol(药物生物技术),2012,19:552-556.
4 Milne JC,Lambert PD,Schenk S,et al.Small molecule activatiors of SIRT1 as therapeutics for the treatment of type 2 diabetes.Nature,2007,450:712-716.
5 Li WB(李文斌),Cui MY(崔美玉),Xu DM(许冬梅),et al.Correlation of dipeptidyl peptidase IV enzyme activity with diabetic nephropathy.J Shangdong Univ,Health Sci(山东大学学报,医学版),2010,48:12-14.
6 Morton NM,Holmes MC,Fiévet C,et al.Improved lipid and lipoprotein profile,hepatic insulin sensitivity,and glucose tolerance in 11β-hydroxysteroid dehydrogenase type 1 null mice.J Biol Chem,2001,276:41293-41300.
7 Wu CF(吴酬飞).Establishment of a hypoglycemic agents screening method based on human pancreatic glucokinase.Nanchang:Nanchang University(南昌大学),MSc.2009.
8 Jiang HX(江慧贤),Luo C(罗超),Lu JX(卢钧雄),et al.Construction of screening model for dipeptidyl peptidase IV in vitro and active inhibitory estimation of related compounds.China J Exp Tradit Med Formulae(中国实验方剂学杂志),2012,18:210-214.
9 Daniela S,Evelyne MM,Christian L,et al.The discovery of new 11β-hydroxysteroid dehydrogenase type 1 inhibitors by common feature pharmacophore modeling and virtual screening.J Med Chem,2006,49:3454-3466.
10 Zhao CC(赵春超),Wang JH(王金辉),Li W(李文),et al.Studies on the chemical constituents of fructus Ailanthi altissimae.Chin J Med Chem(中国药物化学杂志),2003,13:211-214.
11 Fuchino H,Satoh T,Tanaka N.Chemical evaluation of Betula species in Japan.I.Constituents of Betula ermanii.Chem Pharm Bull,1995,43:1937-1942.
12 Jayasinghe ULB,Vithana HSK,Wannigama GP,et al.24-Methylenecycloartenone from Bhesa nitidissima.Fitoterapia.2001,72:594-595.
13 Zeng L,Gu ZM,Fang XP,et al.Two new bioactive triterpenoids from Melia volkensii(Meliaceae).Tetrahedron,1995,51:2477-2488.
14 Takeya K,Qiao ZS,Hirobe C,et al.Cytotoxic azadirachtintype limonoids from Melia azedarach.Phytochemistry,1996,42:709-712.
15 Kouam SF,Ngadjui BT,Krohn K,et al.Prenylated anthronoid antioxidants from the stem bark of Harungana madagascariensis.Phytochemistry,2005,66:1174-1179.
16 Bazin MA,Lamer ACL,Delcros JG,et al.Synthesis and cytotoxic activities of usnic acid derivatives.Bioorg Med Chem,2008,16:6860-6866.
17 Behrens U,Hencken G,Kopf J.Usnic acid,C18H16O7.Cryst Struct Commun,1976,5:51-56.
18 Waterman PG,Faulkner DF.(-)-Epiafzelechin from the root bark of Cassia sieberiana.Planta Med,1979,37:178-179.
19 Hwang BY,Kim HS,Lee JH,et al.Antioxidant benzoylated flavan-3-ol glycoside from Celastrus orbiculatus.J Nat Prod,2001,64:82-84.
