人面果叶子、根部、果实提取物体外抗糖尿病活性研究
2014-01-11杨光忠
付 蒙,胡 鑫,徐 婧,陈 玉,杨光忠*
1 中南民族大学药学院,武汉 430074;2 湖北省食品药品监督检验研究院,武汉 430064;3中南民族大学化学与材料科学学院,武汉 430074
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and insufficient secretion or action of endogenous insulin[1].As yet,there are no effective therapies to cure diabetes[2].Many hypoglycemic agents such as the biguanides and sulfonylureas are used to treat this disease,but causing serious side effects[3].In recent years,there has been an increased interest in the use of natural products for pharmacological purposes,as a form of complementary or replacement therapy.Published reports have shown that numerous extracts obtained from plants are effective in reducing glycemia,causing fewer side effects than the usual antidiabetic agents[4].Most of the plants used in popular medicine for the treatment of diabetes possess biologically active compounds such as triterpenes,flavonoids and polyphenols[5].Oxidative stress is known to promote the development and progression of diabetes and its complications,and polyphenols with antioxidant properties exert beneficial antidiabetic effects by correcting the disturbed oxidative milieu in diabetic conditions.
Garcinia xanthochymus is a tropical plant which can grow up to 20 m.It is grown for its edible fruit,and a preparation of the fruit is given to treat bilious conditions,diarrhea,and dysentery.The immature leaf is also a cheap vegetable.Previous phytochemical studies on G.xanthochymus resulted in the isolation of benzophenones,flavonoids,triterpenes and xanthones from the wood,fresh leaves and fruit.The DPPH radical scavenging activities of compounds from the bark of G.Xanthochymus were evaluated by Chen[6].In the light of these findings,this study was carried out to evaluate the in vitro antidiabetic activities of the different fractions obtained from the root,leaf and fruit of G.xanthochymus.
Materials and Methods
Materials
Leaves,roots and fruit of G.xanthochymus were obtained from Xishuangbanna prefecture,Yunnan province,P.R.China,and identified by the Xishuangbanna Prefecture National Medicine Research Institute.
α-Glucosidase from baker’s yeast,4-nitrophenyl-α-Dglucopyranoside,acarbose,α-amylase from porcine pancreas,starch azure were purchased from Sigma-Aldrich(USA).Trypsine,RPMI 1640 medium and DMEM medium were obtained from Gibco(USA).All chemicals used were of analytical grade.
Preparation of G.xanthochymus extracts
The dried leaves,roots and fruit of G.xanthochymus were powdered and extracted with 95% ethanol for 3 h at 60-70 °C.The ethanol extracts were concentrated in a rotary vacuum evaporator to obtain the dried crude extracts,which were further fractionated through solvent-solvent partitioning.Petroleum ether(PFr),ethyl acetate(EFr),n-butanol(BFr)and water fractions(WFr)were obtained from each plant part,and stored in a vacuum dry cabinet until analysis.Samples were dissolved in DMSO for the assay.
Determination of α-glucosidase inhibitory activity
α-Glucosidase inhibitory activity was determined using 4-nitrophenyl-α-D-glucopyranoside substrate,based on a reported method[7].10 μL of sample extract in DMSO was added in 80 μL of sodium phosphate buffer(0.1 M,pH 7.0)in a 96-well plate,and pre-incubated with 30 μL of 0.3 U/mL baker's yeast α-glucosidase at 37 ℃for 5 min.Afterwards,30 μL of 4-nitrophenyl-α-D-glucopyranoside(3 mM in phosphate buffer)was added,and the enzymatic reaction was carried out at 37 ℃for 15 min.The reaction system without sample was used as blank test and the system without α-glucosidase was used as background test.The phosphate buffer(pH 7.0)was used as zero-setting solution to determine the release of 4-nitrophenol,at 405 nm.The α-glucosidase inhibitory activity of each fraction was calculated as follows:I%=[(1-(A1-A2)/A0]×100,where A0is the absorbance of the blank,A1is the absorbance of the sample at different concentrations and A2is the background absorbance.Acarbose was used as a positive control.
Determination of porcine pancreatic α-amylase inhibitory activity
Porcine pancreatic α-amylase inhibitory activities of the different fractions were determined using an established method[8,9].4 mg of starch azure was suspended in 1.1 mL of 0.05 M Tris-HCl buffer(pH 6.9)containing 0.01 M CaCl2,soaked in boiling water for 5 min and pre-incubated at 37 ℃for 5 min.For each assay,0.2 mL of sample extract in DMSO and 0.2 mL of porcine pancreatic α-amylase solution(2.0 U/mL)were used.The reaction was carried out at 37 ℃for 10 min and stopped by adding 0.5 mL of 50% acetic acid.The reaction mixture was then centrifuged at 4500 rpm for 5 min.The absorbance of the resulting supernatant was recorded at 595 nm.The reaction system without sample was used as blank test and the system without αamylase was used as background test.The system without either sample or α-amylase was used as zero-setting solution.The α-amylase inhibitory activity of each fraction was calculated as follows:I%=[(1-(A1-A2)/A0]×100,where A0is the absorbance of the blank,A1is the absorbance of the sample at different concentrations and A2is the background absorbance.Acarbose was used as a positive control.
Glucose consumption assay
HepG2 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum,100 units/mL penicillin and 100 μg/mL streptomycin.Cells were incubated in a humidified atmosphere of 5% CO2at 37 ℃and passaged every 3 days by trypsinization.HepG2 cells were incubated in a 96-well plate with 10% fetal bovine serum,grown to 80% confluence,and kept overnight in DMEM medium with 2% fetal bovine serum.Cell viability was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay,and the tested concentration of the sample was selected to ensure that cells had over 90% viability.In addition,cells were treated with the different fractions at several tested concentrations for 24 h,and the concentration of glucose in DMEM was determined to assess glucose consumption.
Statistical analysis
All experimental data were presented as means±SD.Statistical analysis(SigmaPlot.v10.0)was performed using the T-test,to assess whether the groups are significantly different.A value of P<0.05 was considered to be significant.
Results and Discussion
α-Glucosidase and porcine pancreatic α-amylase inhibitory activities
The treatment of diabetes mainly focuses on reducing fluctuations of blood sugar and associated complications.One of the therapeutic approaches was to decrease the postprandial hyperglycemia by retarding glucose absorption through the inhibition of carbohydratehydrolyzing enzymes,such as α-amylase and α-glucosidase[10].α-Glucosidase inhibitors can retard the liberation of the D-glucose of oligosaccharides and disaccharides from dietary complex carbohydrates and delay glucose absorption,reducing postprandial plasma glucose levels and suppressing postprandial hyperglycemia.α-Amylase inhibitors can slow down the digestion rate of starch and restrain the increase of post-meal blood sugar.They are among the most promising oral hypoglycemic agents for diabetics.
Fig.1 and 2 showed the α-glucosidase and α-amylase inhibitory activities of each G.xanthochymus fraction.Seven fractions from G.xanthochymus had a dose-dependent inhibitory activity.The root EFr and BFr showed the highest α-glucosidase inhibitory activity of all samples(ca.90%,even at a concentration of 600 μg/mL).The root EFr and BFr also reached about 90% α-amylase inhibitory activity at a concentration of 1.5 mg/mL.Five extracts(leaf-WFr,leaf-PFr,root-WFr,fruit-BFr and fruit-WFr;not shown in Fig.1 and 2)showed no α-glucosidase or α-amylase inhibitory activities,which may be due to the carbohydrate composition of the extracts.In general,the ethyl acetate and n-butanol fractions showed higher α-glucosidase inhibitory activity than the other fractions.Hence ethyl acetate and n-butanol can be used as solvents for the extraction of bioactive substances(mainly triterpenes,flavonoids and polyphenols)with α-glucosidase inhibitory activity.

Fig.1 α-Glucosidase inhibitory activity of G.xanthochymus extracts

Fig.2 α-Amylase inhibitory activity of G.xanthochymus extracts
As shown in Table 1,the α-glucosidase inhibitory activity decreased in the following order:root-BFr>root-EFr>fruit-EFr>leaf-EFr>root-PFr>fruit-PFr>leaf-BFr,while α-amylase inhibitory activity decreased as follows:root-PFr>root-EFr>root-BFr>fruit-PFr>leaf-EFr>fruit-EFr(leaf-BFr).Moreover,the inhibitory activities of extracts against α-amylase are all lower than those against α-glucosidase.This might be due to the different mechanism of action between α-glucosidase and α-amylase.

Table 1 IC50of α-glucosidase and α-amylase inhibitory activities of G.xanthochymus extracts
Glucose consumption assay
HepG2 cells are a suitable in vitro model system for the study of glucose consumption due to their role as targeting cells in carbohydrate metabolism.Four fractions(root-BFr,root-EFr,fruit-EFr,leaf-EFr),which showed α-glucosidase and α-amylase inhibitory activities,were assessed at a concentration of 250 mg/mL,incubating for 24 h.Cell viabilities after treatment with each of the four fractions,determined by MTT assay,were 56.82%,52.65%,57.95% and 56.82%,respectively.This result shows that high concentrations of these extracts will inhibit the growth of HepG2 cells,and the hypoglycemic effect may not be due to the increase in the number of cells.At a level of 90% cell viability,the most effective concentrations of root-BFr,root-EFr,fruit-EFr and leaf-EFr were 100,30,30 and 60,respectively.
As shown in Table 2,glucose consumption increased after treatment with the four fractions for 24 h(P<0.05).In particular,fruit-EFr.at a concentration of 7.5-30 mg/mL significantly promoted glucose consumption(P<0.001).The glucose consumption rates of cells treated with leaf-EFr,root-BFr and root-EFr were 3.08,3.12 and 1.93,respectively,being slightly lower than the glucose consumption rate of cells treated with fruit-EFr.(3.91).
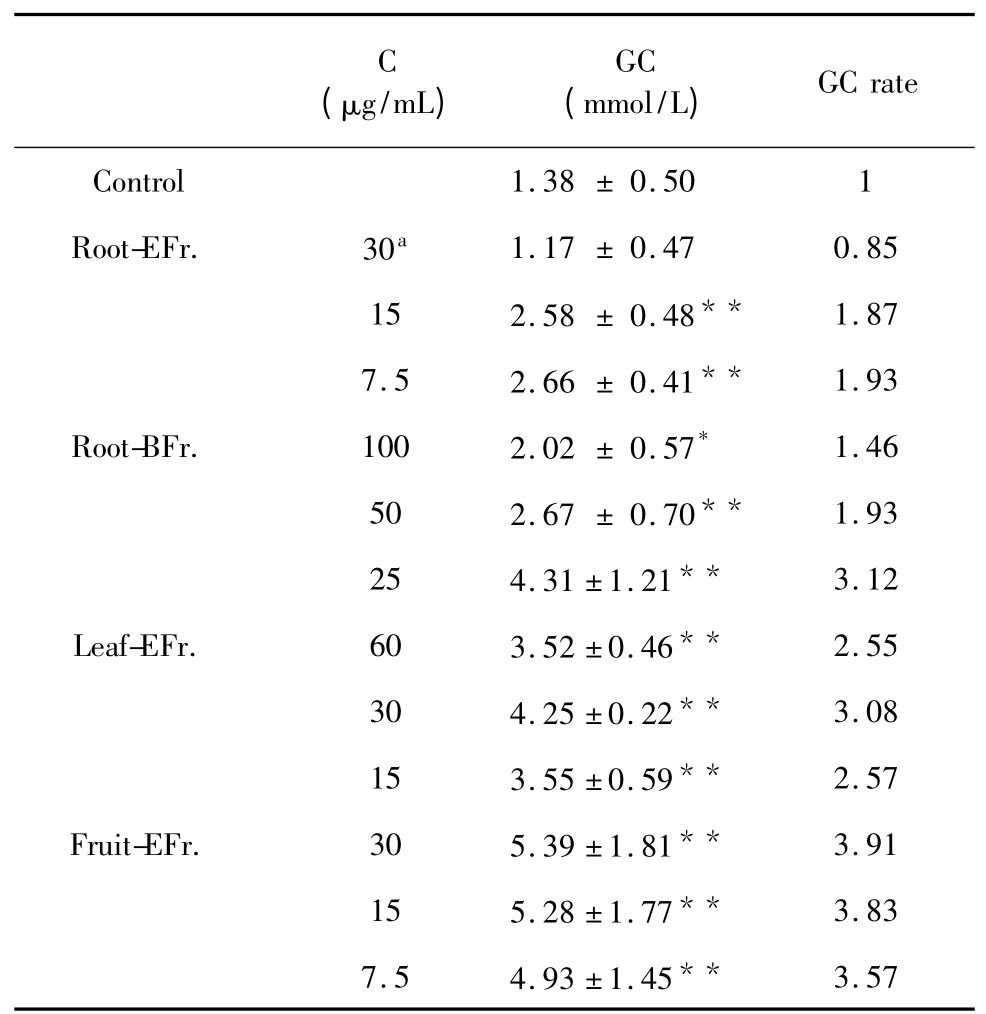
Table 2 Effects of treatment with G.xanthochymus extracts on HepG2 cells’glucose consumption
Conclusion
The results presented in this study showed that fruit-EFr,leaf-EFr,root-EFr and root-BFr had high α-glucosidase and α-amylase inhibitory activity,and are effective on promoting glucose consumption.This study gave basic scientific support for the use of G.xanthochymus as a treatment of diabetes,and therefore can help to develop medicinal preparations or nutraceutical and functional foods for diabetics.
1 Gupta RK,Kesari AN,Murthy PS,et al.Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L.in experimental animals.J Ethnopharmacol,2005,99:75-81.
2 Maiti R,Jana D,Das UK,et al.Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin induced diabetic rats.J Ethnopharmacol,2004,92:85-91.
3 Hwang HJ,Kim SW,Lim JM,et al.Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin induced diabetic rats.Life Sci,2005,76:3069-3080.
4 Pushparaj P,Tan CH,Tan BKH.Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin diabetic rats.J Ethnopharmacol,2000,72:69-76.
5 Marles RJ,Farnsworth NR.Antidiabetic plants and their active constituents.Phytomedicine,1995,2:137-189.
6 Chen Y,Fan H,Yang GZ,et al.Prenylated xanthones from the bark of Garcinia xanthochymus and their 1,1’-diphenyl-2-picrylhydrazyl(DPPH)radical scavenging activities.Molecules,2010,15:7438-7449.
7 Matsui T,Yoshimoto C,Osajima K,et al.In vitro survey of αglucosidase inhibitory food components.Biosci Biotechno Biochem,1996,60:2019-2022.
8 Gao H,Huang YN,Gao B,et al.α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L..Food Chem,2008,106:1195-1201.
9 Wang H,Du YJ,Song HC.α-Glucosidase and α-amylase inhibitory activities of guava leaves.Food Chem,2010,123:6-13.
10 Bhandari MR,Nilubon JA,Gao H,et al.α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herbPakhanbhed(Bergenia ciliata Haw.).Food Chem,2008,106:247-252.
