毛茛属刺果毛茛化学成分研究
2013-12-23吴碧灵覃芳敏周光雄
吴碧灵,覃芳敏,周光雄
暨南大学药学院中药及天然药物研究所,中药药效物质基础与创新药物研究重点实验室,广州510632
刺果毛茛Ranunculus muricatus Linn. 别名:野芹菜,是毛茛科毛茛属一年生草本植物,生长于潮湿田野,河沟道旁,广泛分布于亚洲、欧洲、大洋洲及北美洲。刺果毛茛在印度西北部哈里亚纳邦民间主要用于治疗扁桃体炎[1],而在我国毛茛属植物入药历史悠久,如石龙芮(Ranunculus sceleratus Linn.)、毛茛(Ranunculus japonicas Thunb.)、小毛茛(Ranunculus termatus Thunb.)等。据报道,毛茛属植物提取物多具有抗菌、抗炎、抗肿瘤、抗病毒等方面活性[2,3],特别是抗结核病方面疗效确切[4,5]。目前,关于刺果毛茛化学成分研究的报道较少,所以,我们对刺果毛茛全草乙醇提取物的醋酸乙酯萃取部位和正丁醇萃取部位进行了深入的化学成分研究。从醋酸乙酯萃取部位分离得到了7 个化合物(1~7);从正丁醇萃取部位分离得到了10 个黄酮苷(8~17)。除化合物4 外,其他均为首次从刺果毛茛中分离得到,本文报道这些化合物的提取分离和结构鉴定。
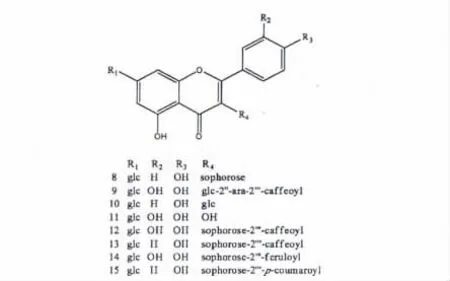
图1 黄酮苷结构式(8~15)Fig.1 Structures of flavone glycosides (8-15).
1 仪器与材料
AV-300 MHz 超导核磁共振仪(德国Bruker),氘代试剂(美国CIL),Agilent 1200 系列高效液相色谱仪(美国Agilent),LCQ Advantage MAX 质谱仪(美国Finnign),Sephadex LH-20(瑞典Pharmacia),薄层色谱用硅胶(青岛海洋化工厂),ODS(日本YMC),色谱纯甲醇(山东禹王),分析纯化学试剂(天津大茂)。
刺果毛茛全草采自江西省九江县,原植物由广东药学院刘基柱教授鉴定为毛茛属植物刺果毛茛(Ranunculus muricatus Linn.)。
2 提取与分离
刺果毛茛干燥全草粗粉90%乙醇渗漉法提取3次,合并提取液减压浓缩得到浸膏1 kg。浸膏加2倍蒸馏水混悬,依次用等体积的石油醚、醋酸乙酯、正丁醇各萃取3 次,减压浓缩,分别得到石油醚部位82 g,醋酸乙酯部位27 g,正丁醇部65 g。醋酸乙酯部位经硅胶柱梯度洗脱(石油醚∶乙酸乙酯30∶1、15∶1、10∶1、5∶1、1∶1、0∶1),得到馏分R-A~F。R-D(2 g)经硅胶柱梯度洗脱(氯仿∶甲醇40 ∶1、20 ∶1、10∶1),经Sephadex LH-20 纯化及重结晶得到化合物3、4;R-E(2.5 g)经硅胶柱梯度洗脱(氯仿∶甲醇30∶1、15∶1、10∶1),经Sephadex LH-20 纯化及重结晶得到化合物1、2、5;R-F(3 g)经硅胶柱进行梯度洗脱(氯仿∶甲醇20 ∶1、10 ∶1、5 ∶1)再经Sephadex LH-20 纯化得到化合物6、7;正丁醇部位经D101 大孔树脂梯度洗脱(30%、60%、90% 甲醇/水),得到馏分R-H1~3,其中R-H1(12 g)经反相硅胶柱色谱梯度洗脱(10%、30%、60% 甲醇/水),得到馏分RH1-1~7。R-H1-(1、2、3)经聚酰胺柱色谱层析、Sephadex LH-20 及HPLC 制备等手段分离纯化得到化合物8~15。R-H1-(4、6)经反相硅胶柱色谱梯度洗脱(10%、30%、50% 甲醇/水),再Sephadex LH-20 及HPLC 制备等手段分离纯化得到化合物16、17。
2 结构鉴定
化合物1 白色针状结晶(甲醇),2,4-二硝基苯肼反应呈阳性。ESI-MS m/z:115[M +H]+;1H NMR (300 MHz,CD3OD)δ:2.68 (2H,t,J = 5.8 Hz,H2-3),2.61 (2H,t,J = 5.8 Hz,H2-4),4.24 (2H,s,H2-6);13C NMR (75 MHz,CD3OD)δ:176.3 (C-2),33.8 (C-3),28.5 (C-4),68.7(C-5),211.0 (C-6)。以上数据与文献[6]报道一致,故鉴定化合物1 为小毛茛内酯。
化合物2 白色针状结晶(甲醇),与三氯化铁-铁氰化钾水溶液反应显蓝色。ESI-MS m/z:193[M-H]-,177 [M-OH]-;1H NMR (300 MHz,CD3OD)δ:7.60 (1H,d,J = 15.9 Hz,H-7),7.18 (1H,d,J = 1.9 Hz,H-2),7.06 (1H,dd,J= 8.2,1.9 Hz,H-6),6.81 (1H,d,J = 8.2 Hz,H-5),6.31 (1H,d,J = 15.9 Hz,H-8),4.89(3H,s,OCH3);13C NMR (75 MHz,CD3OD)δ:127.8 (C-1),116.5 (C-2),150.5 (C-3),149.4(C-4),115.9 (C-5),124.0 (C-6),146.9 (C-7),111.7 (C-8),171.0 (C-9),56.4 (OCH3)。以上数据与文献[7]报道一致,故鉴定化合物2 为阿魏酸。
化合物3 白色针状结晶(甲醇),与三氯化铁-铁氰化钾水溶液反应显蓝色。ESI-MS m/z:163[M-H]-,147 [M-OH]-;1H NMR (300 MHz,CD3OD)δ:7.59 (1H,d,J = 15.9 Hz,H-7),7.44 (2H,d,J = 8.6 Hz,H-2,6),6.80 (1H,d,J = 8.7 Hz,H-3,5),6.28 (1H,d,J = 15.9 Hz,H-8);13C NMR (75 MHz,CD3OD)δ:114.5 (C-1),129.7 (C-2),115.4 (C-3),159.7 (C-4),115.4 (C-5),129.7 (C-6),145.1 (C-7),125.9(C-8),170.0 (C-9)。以上数据与文献[8]报道一致,故鉴定化合物3 为对羟基香豆酸。
化合物4 半透明片状结晶(甲醇);ESI-MS m/z:153[M-H]-;1H NMR (300 MHz,CD3OD)δ:7.44 (1H,s,H-2),7.41 (1H,d,J = 2.0 Hz,H-6),6.80 (1H,d,J = 8.5 Hz,H-5);13C NMR (75 MHz,CD3OD)δ:123.3 (C-1),117.7 (C-2),146.0 (C-3),151.5 (C-4),115.7 (C-5),123.9(C-6),170.4 (C-7)。以上数据与文献[9]报道一致,故鉴定化合物4 为原儿茶酸。
化合物5 半透明片状结晶(甲醇),与三氯化铁-铁氰化钾水溶液反应显蓝色;ESI-MS m/z:179[M-H]-;1H NMR (300 MHz,CD3OD)δ:7.53 (1H,d,J = 15.9 Hz,H-7),7.04 (1H,d,J = 2.0 Hz,H-2),6.93 (1H,dd,J = 8.2,2.0 Hz,H-6),6.78 (1H,d,J = 8.2 Hz,H-5),6.22 (1H,d,J= 15.9 Hz,H-8);13C NMR (75 MHz,CD3OD)δ:127.8 (C-1),115.1 (C-2),147.0 (C-3),149.5(C-4),115.6 (C-5),122.8 (C-6),146.8 (C-7),116.5 (C-8),171.1 (C-9)。以上数据与文献[10]报道一致,故鉴定化合物5 为咖啡酸。
化合物6 半透明胶状物(甲醇),喷香草醛-浓硫酸并加热时显酒红色。[α]25D + 13.3(c 0.01,MeOH);ESI-MS m/z:197 [M-H]-;1H NMR (300 MHz,DMSO-d6)δ:6.63 (1H,d,J = 1.9Hz,H-2),6.61 (1H,d,J = 8.0Hz,H-6),6.46 (1H,dd,J = 8.0,1.9 Hz,H-5),4.05 (1H,dd,J =7.8,4.7 Hz,H-8),2.78 (1H,dd,J = 13.8,4.7 Hz,H-7),2.61 (1H,dd,J = 13.8,7.9 Hz,H-7');13C NMR (75 MHz,DMSO-d6)δ:120.2 (C-1),117.0 (C-2),143.7 (C-3),144.8 (C-4),115.3 (C-5),128.9 (C-6),39.7 (C-7),71.6 (C-8),175.4 (C-9)。以上数据与文献[11]报道一致,故鉴定化合物6 为丹参素。
化合物7 半透明胶状物(甲醇),喷香草醛-浓硫酸并加热时显酒红色。ESI-MS m/z:211 [MH]-;1H NMR (300 MHz,DMSO-d6)δ:6.62 (1H,d,J = 1.9 Hz,H-2),6.60 (1H,d,J = 8.0 Hz,H-6),6.43 (1H,dd,J = 8.0,1.9 Hz,H-5),4.14 (1H,dd,J = 7.8,4.7 Hz,H-8),3.59 (3H,s,OCH3),2.75 (3H,s,J = 13.8,4.7 Hz,H-7),2.65 (1H,dd,J = 13.8,7.9 Hz,H-7');13C NMR(75 MHz,DMSO-d6)δ:120.1 (C-1),116.8 (C-2),143.8 (C-3),144.8 (C-4),115.3 (C-5),128.3 (C-6),39.7 (C-7),71.7 (C-8),174.1 (C-9),51.4 (C-10)。以上数据与文献[12]报道一致,故鉴定化合物7 为丹参素甲酯。
化合物8 淡黄色粉末;ESI-MS m/z:795 [M+Na]+,449[M-324 +H]+,287[448-162 +H]+,由裂解规律可知含有槐糖、葡萄糖分子碎片;1H NMR (300 MHz,DMSO-d6)δ:8.07 (2H,d,J =8.9 Hz,H-2',6'),6.92 (2H,d,J = 8.9 Hz,H-3',5'),6.80 (1H,d,J = 2.1 Hz,H-8),6.43(1H,d,J = 2.1 Hz,H-6),5.70 (1H,d,J =7.2 Hz,H-1''),5.08 (1H,d,J = 7.5 Hz,H-1''''),4.62 (1H,d,J = 7.7 Hz,H-1''');13C NMR (75 MHz,DMSO-d6)δ:155.9 (C-2),133.2(C-3),177.6 (C-4),160.9 (C-5),99.3 (C-6),162.8 (C-7),94.5 (C-8),156.2 (C-9),105.6(C-10),120.8 (C-1'),131.1 (C-2',6'),115.4(C-3',5'),160.2 (C-4'),97.9 (C-1''),82.5 (C-2''),76.4 (C-3''),69.5 (C-4''),77.2 (C-5''),60.6 (C-6''),104.2 (C-1'''),74.5 (C-2'''),76.5 (C-3'''),69.6 (C-4'''),77.1 (C-5'''),60.7(C-6'''),99.7 (C-1''''),73.1 (C-2''''),76.6(C-3''''),69.7 (C-4''''),77.6 (C-5''''),60.9(C-6'''')。以上数据与文献[13]报道一致,故鉴定化合物8 为山萘酚-3-O-β-D-槐糖-7-O-β-D-葡萄糖苷。
化合物9 淡黄色粉末;ESI-MS m/z:943 [M+Na]+,588[M-162-132 +H]+,919[M-H]-,757[M-162-H]-,179[180-H]-,由裂解规律可知含有葡萄糖基、咖啡酰基;1H NMR (300 MHz,CD3OD)δ:7.56 (1H,d,J = 2.1 Hz,H-6'),7.51 (1H,dd,J = 8.5,2.1 Hz,H-2'),6.87 (1H,d,J =8.5 Hz,H-3'),6.53 (1H,d,J = 2.1 Hz,H-6),6.43 (1H,d,J = 2.1 Hz,H-8),5.92 (1H,d,J= 7.3 Hz,H-1''),5.15 (1H,d,J = 7.2 Hz,H-1'''),5.08 (1H,d,J = 7.2 Hz,H-1'''');13C NMR (75 MHz,CD3OD)δ:149.7 (C-2),135.1(C-3),179.4 (C-4),162.5 (C-5),100.4 (C-6),164.4 (C-7),95.5 (C-8),158.2 (C-9),107.6(C-10),123.4 (C-1'),123.2 (C-2'),146.7 (C-3'),157.7 (C-4'),116.0 (C-5'),117.3 (C-6'),98.6 (C-1''),81.2 (C-2''),76.6 (C-3''),71.4(C-4''),78.2 (C-5''),62.4 (C-6''),100.7 (C-1'''),74.7 (C-2'''),75.4 (C-3'''),70.9 (C-4'''),66.4 (C-5'''),101.5 (C-1''''),74.7 (C-2''''),77.7 (C-3''''),71.3 (C-4''''),78.2 (C-5''''),62.3 (C-6'''');咖啡酰基δH:7.38 (1H,d,J = 15.8 Hz,H-7),6.73 (1H,d,J = 1.8 Hz,H-6),6.60 (1H,m,H-2),6.56 (1H,d,J = 8.3 Hz,H-5),6.14 (1H,d,J = 15.9 Hz,H-8);δC:127.4 (C-1),115.1 (C-2),146.4 (C-3),149.1(C-4),116.2 (C-5),122.4 (C-6),145.9 (C-7),114.9 (C-8),168.6 (C-9)。以上数据与文献[14]报道一致,故鉴定化合物9 为槲皮素-3-O-(2'''-E-咖啡酰基)-α-L-阿拉伯糖-(1→2)-β-D-葡萄糖-7-O-β-D-葡萄糖苷。
化合物10 淡黄色粉末;ESI-MS m/z:633[M+Na]+,449 [M-162 + H]+,287 [M-162-162 +H]+,609 [M-H]-,由裂解规律可知含有葡萄糖基;1H NMR (400 MHz,DMSO-d6)δ:8.06 (2H,d,J = 8.8 Hz,H-2',6'),6.90 (2H,d,J = 8.8 Hz,H-3',5'),6.79 (1H,d,J = 1.9 Hz,H-8),6.44 (1H,d,J = 1.9 Hz,H-6),5.48 (1H,d,J= 7.2 Hz,H-1''),5.08 (1H,d,J = 7.3 Hz,H-1''');13C NMR (100 MHz,DMSO-d6)δ:156.0 (C-2),133.5 (C-3),177.6 (C-4),160.8 (C-5),100.7 (C-6),162.8 (C-7),94.4 (C-8),156.8(C-9),105.6 (C-10),120.8 (C-1'),131.0 (C-2',6'),115.1 (C-3',5'),160.1 (C-4'),99.3(C-1''),73.1 (C-2''),76.4 (C-3''),69.6 (C-4''),77.1 (C-5''),60.6 (C-6''), 99.7 (C-1'''),74.2 (C-2'''),76.4 (C-3'''),69.9 (C-4'''),77.5 (C-5'''),60.8 (C-6''')。以上数据与文献[15]报道一致,故鉴定化合物10 为山萘酚-3-Oβ-D-葡萄糖-7-O-β-D-葡萄糖苷。
化合物11 淡黄色粉末;ESI-MS m/z:951[2M+Na]+,303[M-162 +H]+,由裂解规律可知含有葡萄糖基;1H NMR (300 MHz,CD3OD)δ:7.75 (1H,d,J = 1.9 Hz,H-2'),7.65 (1H,dd,J = 8.5,1.9 Hz,H-6'),6.88 (1H,d,J = 8.5 Hz,H-5'),6.73 (1H,d,J = 2.0 Hz,H-8),6.45(1H,d,J = 2.0 Hz,H-6),5.06 (1H,d,J =7.5 Hz,H-1'');13C NMR (75 MHz,CD3OD)δ:148.7(C-2),137.6 (C-3),177.4 (C-4),162.1 (C-5),100.2 (C-6),164.4 (C-7),95.5 (C-8),157.7(C-9),106.2 (C-10),123.9 (C-1'),121.9 (C-2'),146.2 (C-3'),148.9 (C-4'),116.1 (C-5'),116.2 (C-6'),101.6 (C-1''),74.7 (C-2''),77.8(C-3''),71.3 (C-4''),78.3 (C-5''),62.5 (C-6'')。以上数据与文献[16]报道一致,故鉴定化合物11 为槲皮素-7-O-β-D-葡萄糖苷。
化合物12 淡黄色粉末;ESI-MS m/z:973[M+Na]+,811[M-162 +H]+,949 [M-H]-,由裂解规律可知含有葡萄糖基;1H NMR (300 MHz,CD3OD)δ:7.52 (1H,d,J = 2.0 Hz,H-2'),7.41 (1H,dd,J = 8.4,1.8 Hz,H-6'),6.86(1H,d,J = 8.4 Hz,H-5'),6.47 (1H,d,J =1.9 Hz,H-6),6.44 (1H,d,J = 2.0 Hz,H-8),6.16 (1H,d,J = 8.0 Hz,H-1''),5.27 (1H,d,J= 7.9 Hz,H-1'''),5.10 (1H,d,J = 7.2 Hz,H-1'''');13C NMR (75 MHz,CD3OD)δ:149.7 (C-2),134.9 (C-3),179.3 (C-4),162.4 (C-5),100.7 (C-6),164.3 (C-7),95.6 (C-8),157.6(C-9),107.6 (C-10),123.3 (C-1'),117.3 (C-2'),146.5 (C-3'),158.1 (C-4'),116.0 (C-5'),123.2 (C-6'),97.6 (C-1''),81.9 (C-2''),75.6(C-3''),71.2 (C-4''),78.1 (C-5''),62.3 (C-6''),98.9 (C-1'''),75.1 (C-2'''),76.0 (C-3'''),71.5 (C-4'''),78.1 (C-5'''),62.3 (C-6'''),101.4 (C-1''''),74.8 (C-2''''),77.7 (C-3''''),71.4 (C-4'''),78.3 (C-5''''),62.5 (C-6'''');咖啡酰基δH:7.28 (1H,d,J = 15.9 Hz,H-7),6.04 (1H,d,J = 15.9 Hz,H-8),6.65(1H,br s,H-5),6.48 (1H,m,H-2),6.45 (1H,m,H-6);δC:127.3 (C-1),115.1 (C-2),146.3(C-3),148.9 (C-4),116.2 (C-5),122.1 (C-6),145.8 (C-7),114.8 (C-8),168.7 (C-9)。以上数据与文献[17]报道一致,故鉴定化合物12 为槲皮素-3-O-(2'''-E-咖啡酰基)-β-D-槐糖-7-O-β-D-葡萄糖苷。
化合物13 淡黄色粉末;ESI-MS m/z:957[M+Na]+,933[M-H]-,771[M-162-H]-,由裂解规律可知含有葡萄糖基;1H NMR (300 MHz,CD3OD)δ:7.87 (2H,d,J = 8.7 Hz,H-2',6'),6.89(2H,d,J = 8.7 Hz,H-3',5'),6.51 (1H,d,J= 2.0 Hz,H-8),6.48 (1H,d,J = 2.0 Hz,H-6),6.20 (1H,d,J = 8.1 Hz,H-1''),5.31 (1H,d,J = 7.8 Hz,H-1'''),5.12 (1H,d,J = 5.9 Hz,H-1'''');13C NMR (75 MHz,CD3OD)δ:157.6(C-2),134.8 (C-3),179.3 (C-4),162.4 (C-5),110.6 (C-6),64.3 (C-7),95.7 (C-8),158.2 (C-9),107.6 (C-10),122.8(C-1'),132.2 (C-2',6'),116.0 (C-3',5'),161.3 (C-4'),97.5 (C-1''),81.9 (C-2''),75.5 (C-3''),71.2(C-4''),78.1 (C-5''),62.3 (C-6''),98.9 (C-1'''),75.0(C-2'''),76.0 (C-3'''),71.8 (C-4'''),78.0 (C-5'''),62.4 (C-6'''),101.2 (C-1''''),74.7 (C-2''''),77.7 (C-3''''),71.4(C-4''''),78.3 (C-5''''),62.5 (C-6'''');咖啡酰基δH:7.28 (1H,d,J = 15.9 Hz,H-7),6.55 (1H,br s,H-5),6.45(1H,m,H-6),6.50 (1H,m,H-2),6.03 (1H,d,J = 15.9 Hz,H-8);δC:127.3 (C-1),116.2(C-2),146.4 (C-3),148.9 (C-4),115.1 (C-5),122.0 (C-6),146.3 (C-7),114.9 (C-8),168.6(C-9)。以上数据与文献[18]报道一致,故鉴定化合物13 为山萘酚-3-O-(2'''-E-咖啡酰基)-β-D-槐糖-7-O-β-D-葡萄糖苷。
化合物14 淡黄色粉末;ESI-MS m/z:987[M+Na]+,963[M-H]-,801[M-162-H]-,由裂解规律可知含有葡萄糖基;1H NMR (300 MHz,CD3OD)δ:7.76 (1H,d,J = 2.0 Hz,H-2'),7.36 (1H,dd,J = 8.4,2.0 Hz,H-6'),6.88 (1H,d,J =8.5 Hz,H-5'),6.26 (1H,d,J = 8.2 Hz,H-1''),5.26 (1H,d,J = 7.9 Hz,H-1'''),5.11 (1H,d,J = 7.2 Hz,H-1'''');13C NMR (75 MHz,CD3OD)δ:148.4 (C-2),134.8 (C-3),179.3 (C-4),162.5 (C-5),100.8 (C-6),164.4 (C-7),95.6(C-8),157.9 (C-9),107.7 (C-10),123.1 (C-1'),116.0 (C-2'),146.3 (C-3'),157.6 (C-4'),116.2 (C-5'),122.0 (C-6'),97.3 (C-1''),82.2(C-2''),75.2 (C-3''),71.2 (C-4''),78.2 (C-5''),62.3 (C-6''),98.6 (C-1'''),75.0 (C-2'''),76.0 (C-3'''),71.4 (C-4'''),78.0 (C-5'''),62.3(C-6'''),101.5 (C-1''''),74.8 (C-2''''),77.8(C-3''''),71.7 (C-4''''),78.4 (C-5''''),62.50(C-6'''');阿魏酰基δH:7.25 (1H,d,J = 15.9 Hz,H-7),6.53 (1H,d,J = 1.8 Hz,H-5),6.42(1H,m,H-6),6.48 (1H,m,H-2),5.99 (1H,d,J = 15.9 Hz,H-8);δC:127.1 (C-1),114.7 (C-2),150.8 (C-3),149.1 (C-4),114. 3 (C-5),123.8 (C-6),146.4 (C-7),115.1 (C-8),168.4(C-9),56.8 (C-10)。以上数据与文献[18]报道一致,故鉴定化合物14 为槲皮素-3-O-(2'''-E-阿魏酰基)-β-D-槐糖-7-O-β-D-葡萄糖苷。
化合物15 淡黄色粉末;ESI-MS m/z:941[M+Na]+,917[M-H]-,1H NMR (300 MHz,DMSOd6)δ:7.98 (2H,d,J = 8.8 Hz,H-2',6'),6.90(2H,d,J = 8.7 Hz,H-3',5'),6.72 (1H,d,J= 7.8 Hz,H-1''),5.73 (1H,d,J = 7.5 Hz,H-1'''),5.07 (1H,d,J = 6.9 Hz,H-1'''');13C NMR (75 MHz,DMSO-d6)δ:155.9 (C-2),133.10(C-3),177.5 (C-4),160.9 (C-5),99.4 (C-6),162.8 (C-7),94.5 (C-8),156.4 (C-9),105.7(C-10),120.6 (C-1'),131.0 (C-2',6'),115.8(C-3',5'),159.9 (C-4'),97.2 (C-1''),79.4 (C-2''),76.5 (C-3''),70.2 (C-4''),77.2 (C-5''),60.7 (C-6''),99.3 (C-1'''),73.8 (C-2'''),74.5(C-3'''),70.3 (C-4'''),76.4 (C-5'''),60.7 (C-6'''),99.9 (C-1''''),73.2 (C-2''''),76.9 (C-3''''),69.7 (C-4''''),77.3 (C-5''''),61.0 (C-6'''');对羟基香豆酰基δH:7.47 (1H,d,J = 15.9 Hz,H-7),6.30 (1H,d,J = 15.9 Hz,H-8),7.38(2H,d,J=8.4 Hz,H-2',6'),4.69 (2H,d,J =9.0 Hz,H-3',5');δC:125.1 (C-1),130.0 (C-2),115.5 (C-3),160.6 (C-4),115.5 (C-5),130.0(C-6),144.2 (C-7),114.8 (C-8),165.9 (C-9);以上数据与文献[18]报道一致,故鉴定化合物15 为山萘酚-3-O-(2'''-E-对羟基香豆酰基)-β-D-槐糖-7-O-β-D-葡萄糖苷。
化合物16 淡黄色粉末;ESI-MS m/z:587[M+Na]+,563[M-H]-;1H NMR (300 MHz,DMSOd6)δ:8.04 (2H,d,J = 8.4 Hz,H-2',6'),6.91(2H,d,J = 8.5 Hz,H-3',5'),6.81 (1H,s,H-3);13C NMR (75 MHz,DMSO-d6)δ:164.2 (C-2),103.8 (C-3),182.4 (C-4),158.3 (C-5),108.1(C-6),161.3 (C-7),105.2 (C-8),155.2 (C-9),102.7 (C-10),121.6 (C-1'),129.1 (C-2',6'),115.9 (C-3',5'),161.0 (C-4'),[6-C-ara:74.3(C-1''),71.0 (C-2''),73.9 (C-3''),68.5 (C-4''),70.2 (C-5'')],[8-C-glc:73.4 (C-1'''),70.7 (C-2'''),78.9 (C-3'''),69.7 (C-4'''),81.9(C-5'''),61.3 (C-6''')]。以上数据与文献[20]报道一致,故鉴定化合物16 为芹菜素-8-C-α-L-阿拉伯糖-6-C-β-D-葡萄糖苷。
化合物17 淡黄色粉末;ESI-MS m/z:617[M+Na]+,1211[2M +Na]+,593[M-H]-,1H NMR(300 MHz,DMSO-d6)δ:8.03 (2H,d,J = 8.5 Hz,H-2',6'),6.90 (2H,d,J = 8.7 Hz,H-3',5'),6.82 (1H,s,H-3);13C NMR (75 MHz,DMSO-d6)δ:164.1 (C-2),103.9 (C-3),182.3 (C-4),158.6 (C-5),107.5 (C-6),161.2 (C-7),105.3 (C-8),155.1 (C-9),102.6 (C-10),121.5(C-1'),129.0 (C-2'),115.8 (C-3'),160.8 (C-4'),115.8 (C-5'),129.0 (C-6'),[6-C-glc:74.1(C-1''),70.9 (C-2''),78.9 (C-3''),70.6 (C-4''),80.9 (C-5''),59.8 (C-6'')],[8-C-glc:73.4 (C-1'''),72.0 (C-2'''),77.8 (C-3'''),69.1(C-4'''),81.9 (C-5'''),61.3 (C-6''')]。以上数据与文献[20]报道一致,故鉴定化合物17 为芹菜素-6-C-β-D-葡萄糖-8-C-β-D-葡萄糖苷。
1 Lal SD,Yadav BK. Folk medicines of kurukshetra district(Haryana),India.J.Economic Botany,1983,37:299-305.
2 Wang AW(王爱武),Wang M(王梅),Yuan JY(袁久荣),et al.The study on antitumour effects in vitro from different extraction radix Ranunculus ternatin.Nat Prod Res Dev(天然产物研究与开发),2004,16:529-531.
3 Prieto JM,Recio MC. Pharmacological approach to the proand anti-inflammatory effects of Ranunculus sceleratus L..J.Ethnopharmacol,2003,89:131-137.
4 Xie JP(谢建平),He Y(何颖),Yue J(乐军),et al.Identification of differential expression proteins of Mycobacterium tuberculosisstrain isolated from clinical species treated with radix Ranuncoli ternate extracts by comparative proteomics.Chin J Biochem Mol Biol(中国生物化学与分子生物学报),2006,22:63-69.
5 Parish T. Starvation survival response of Mycobacterium tuberculosis.J Bact Erial,2003,185:6702-6706.
6 Guo XM(郭学敏),Zhou ZL(周卓轮),Hong YF(洪永福),et al. Studies on chemical constituents of Ranunculus termatus Thunb. Acta Pharmaceutica Sinica(药学学报),1995,30:931-933.
7 Zheng XK(郑晓珂),Li Q(李钦),Feng WS(冯卫生).Studies on water solution chemical constituents of Rabdosia rubescens.Nat Prod Res Dev(天然产物研究与开发),2004,16:300-303.
8 Xiang Y(相宇),Li YB(李友宾),Zhang J(张健).Studies on chemical constituents from roots of Cudrania cochinchinensis.China J Chin Mater Med(中国中药杂志),2007,32:409-413.
9 Zheng W(郑威),Zhou CX(周长新),Zhang SL(张水利).Studies on chemical constituents of Ranunculus japonicas Thunb.China Journal of Chinese Materia Medica(中国中药杂志),2006,31:892-894.
10 Cai XY(柴兴云),Dou J(窦静),He QH(贺清辉),et al.Studies on the phenolic acid compounds from Lonicera confusa DC.Chin J Nat Med(中国天然药物),2004,2:339-341.
11 Zhang YP(张义平),Chen HY(陈鸿雁),Cheng WX(程伟贤),et al. Studies on the chemical constituents of Salvia miltiorrhiz of Lijiang. J Chin Medi Mater(中药材),2008,31:226-228.
12 Guo YS(郭雨姗),Wang GC(王国才),Wang CH(王春华),et al. Chemical constituents from Origanum vulgare.Chin Pharm J(中国药学杂志),2012,47:1109-1111.
13 Han Y,Sansei N,Yukari N,et al. Flavonol glycosides from the stems of Trigonella foenum-graecum L..Phytochemistry,2001,58:577-580.
14 Braca A,Prieto JM,Tommasi ND,et al. Furostanol saponins and quercetin glycosides from the leaves of Helleborus viridis L..Phytochemistry,2004,65:2921-2928.
15 Wu H,Slavik D,Ho C,et al.Novel acetylated flavonoid glycosides from the leaves of Allium ursinum. Food Chemistry,2009,115:592-595.
16 Chen QZ(陈秋竹),Lin RC(林瑞超),Wang GL(王刚力),et al.Studies on chemical constituents of the extract of Lonicera japonica.J Chin Medil Mater(中药材),2010,33:920-923.
17 Gluchoff-Fiasson K,Fiasson JL,Waton H. Queretin glycosides from European aquatic Ranunculus species of Subgenus Bayrachium.Phytochemistry,1997,45:1063-1067.
18 Nielsen JK,Olsen CE,Petersen MK.Acylated flavonol glycosides from cabbage leaves. Phytochemistry,1993,34:539-544.
19 Gluchoff-Fiasson K,Fiasson JL,Favre-Bonvina J. Quercetin glycosides from antarctic Ranunculus species. Phytochemistry,1994,37:1629-1633.
20 Xu JF,Studies on chemical constitutents and bioactivities of Mallotua apelta & petenlla anserine.Journal of Chinese peting Union Medical College(中国协和医科大学学报),2008.
