Oxidative Stability of Green Tea Extract-Enriched Rice Bran Oil During Storage
2013-12-13AnggiHayuHapsariSungHeeLeeandJongBangEun
Anggi Hayu Hapsari,Sung-Hee Leeand Jong-Bang Eun*
1.Department of Food Science and Technology and Functional Food Research Center,Chonnam National University,South Korea.
2.Department of Food Nutrition and Culinary,Chosun College of Science and Technology,Gwangju,South Korea.
1.Introduction
Lipid oxidation generally involves degradation of polyunsaturated fatty acids and production of secondary decomposition products including carbonyls and hydrocarbon compounds[1].The first product formed during the mechanism is hydroperoxide which are produced from free radicals and the photo-oxidation mechanism.Oxidation proceeds slowly and uniformly during the primary phase.The primary phase product is usually expressed as a peroxide value(PV).Lipid hydroperoxides are very unstable and undergo cleavage to produce free alkoxy radicals,which decompose to give mainly aldehydes but also ketones and hydrocarbons.The oxidative process proceeds quickly during the secondary phase[2]. Malonaldehyde(MDA)is a secondary decomposition product of polyunsaturated fatty acids with three or more double bonds.Aldehyde products of lipid oxidation other than MDA may also react with TBA to produce yellow(λmax=455 nm),orange(λmax=495 nm),and red(λmax=532 nm)pigments[3,4].Besides aldehydes,other substances such as ketones,ketosteroids,acids,esters,sugars,imides,and amides(urea),amino acids,oxidized proteins,pyridines,and pyrimidines react with TBA and are called TBARS(substances that react with TBA)[2].The acid value is an important indicator of vegetable oil quality.
Rice bran oil generally contains high unsaturated fatty acid content such as oleic(38%-46%),linoleic(33%-40%),and linolenic(0.2%-3.9%)acids[5].High polyunsaturated fatty acid oils are commonly known as healthy oils,in contrast to their oxidative stability,as unsaturated oils are more susceptible to oxidation.Green tea extract is added to improve oxidative stability of rice bran oil(RBO).The beneficial effects of green tea are attributed to the antioxidative polyphenols present,which are collectively known as catechins.Scavenging activity of catechins against the ROO*radical is performed by blocking the radical chain reaction and thus preventing the peroxidation process[6].Catechin derivatives in green tea include(-)epicatechin(EC),(-)epigallocatechins(EGC),(-)epicatechin gallate(ECG)and(-)epigallocatechin gallate(EGCG).Green tea catechins delay the autoxidation reaction by inhibiting formation of free radicals or interrupting propagation of the free radical chain reaction.A previous study reported that ethanol extracts of green tea and white tea inhibit lipid oxidation of canola oil more strongly than butylated hydroxytoluene,whereas ethanol extracts from black tea and dark green tea show no or little antioxidative activity[7].The antioxidative activity of green tea extracts was further evidenced by its inhibition of lipid oxidation in canola oil with the antioxidative activity of individual epicatechin derivatives ranked as:EGC>EGCG>EC>ECG[8].It could be assumed that green tea extracts could improve oxidative stability of RBO,which has high unsaturated fatty acid content.
Therefore,the oxidative stability of green tea extract-enriched rice bran oil was investigated during storage at 60℃ and compared to canola oil(CN),extra virgin olive oil(OL),and grape-seed(GS)oil.
2.Materials and methods
2.1.Materials
Rice bran oil(RAON)and green tea extract-enriched rice bran oil(RBOG)were obtained from Boseong-Nonghyeop,whereas the CN,OL and GS oil were purchased from a local market in Gwangju,South Korea.The RBOG was prepared by adding green tea extract after refining the RBO.
2.2.Methods
The five oils were stored in brown bottles with caps(25 ml)at 60℃for 15 days in an incubator(DASOL SCIENTIFIC CO.LTD.SEOUL,KOREA).Acid,peroxide,and TBARS values were determined every 3 days during storage for 15 days.Each sample was prepared in two replications.
The saponification,acid and PVs were determined by the titermetric method[9].Total phenolic contents were determined by spectrophotometry,using gallic acid as the standard[10].TBARS was determined with the direct method[11].A 50-200 mg aliquot of oil or lipid extract sample was weighed into a 25 ml volumetric flask.The samples were dissolved in a small amount of 1-butanol and brought to volume with 1-butanol,then mixed thoroughly.The 5.0 ml sample solutions were transferred to a dry screw-cap glass test tube and 5.0 ml of 0.2%TBA in 1-butanol was added.A reagent blank was prepared.Then,the solutions were vortexed and incubated for 2 hr in a 95℃ water bath.The tubes were removed from the bath and cooled under running tap water for 10 min,and absorbance was measured with spectrophotometer at a 532 nm wavelength.The TBARS were obtained from a standard curve of 0.2 mM TMP(1,1,3,3-tetramethoxypropane)in 1-butanol with concentrations ranging from 1 ×10-6-1×10-5M MDA.
3.Results and discussion
3.1.Characteristics of the oils
Table1 shows the characteristics of the RBOG,RBO,CN,OL and GS before storage.The lowest refractive index was found in OL and the highest one was in GS.Increasing refractive index values suggest decreasing clarity.The refractive index values of the RBO,CN,OL,and GS were 1.460-1.473,1.465-1.467,1.4677-1.4705,and 1.467-1.477,respectively[12].Almost all oils,except OL,showed higher refractive index values.The highest refractive index was found for GS.The refractive index is a characteristic of oil that identifies the presence of unsaturated,conjugated,hydroxyl-substituted,or uniquely structured fatty acid compounds.Increases in molecular weight and the degree of unsaturation cause an increase in refractive index[13],suggesting that the degree of unsaturation was high and some compounds from grape seed may have remained or formed from its components.One study reported that repeated heating of oil at high temperature and over the long term causes dimerization and polymerization[14].Heating applied during oil processing may cause polymerization,which increases the refractive index.
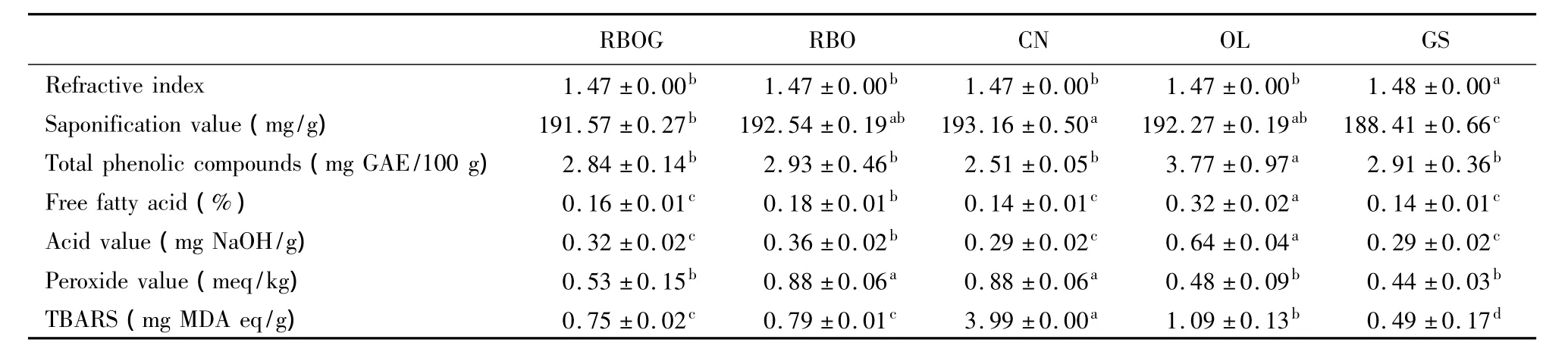
Table1.Characteristics of the fresh oils.
The saponification number indicates the molecular weight of fatty acids.Oils composed of short carbon-chain fatty acids have a lower molecular weight and a higher saponification number.In contrast,oils composed of long carbon-chain fatty acids have a higher molecular weight and a lower saponification number.The results were in agreement with Codex Standards,which define the saponification numbers of RBO,CN,GS,and OL as follows:180-199,182-193,188-194,and 184-196,respectively[12,15].The RBOG also agreed with the standard range of RBO.Based on Table1,it can be concluded that CN,RBO,and OL had a high number of shorter carbon-chain fatty acids compared to that of GS.
Phenolic compounds are present in all vegetable oils,which is very important for the oxidative stability of the polyunsaturated fatty acids in these oils.Factors influencing the antioxidant activity of phenolic compounds include position and number of hydroxyl groups and the polarity,solubility,and stability of phenolic compounds during processing.The highest total phenolic compound content was found in OL(3.77 mg GAE/100 g).This result was due to the refining process,which causes loss of polyphenolic content.
The highest PVs were found in CN and RBO and the highest TBARS was found in CN.OL showed the highest free fatty acid and acid values because it was not purified.
3.2.Changes in the acid values of the oils during storage
Figure 1 shows that there was no significant change in the acid values of the oils during storage.The acid values of the four refined oil samples were not higher than 0.6 mg NaOH/g oil,except for OL(>0.6 mg NaOH/g).Free fatty acids were not released during storage because of the absence of hydrolytic breakdown.Hydrolysis can occur due to the presence of moisture or enzyme activity[16], butthere was no moisture or enzymes present in the commercial oils during storage.The high OL acid value may have been caused by the nature of the oil because extra virgin olive oil does not undergo refining.
3.3.Changes in PVs of the oils during storage
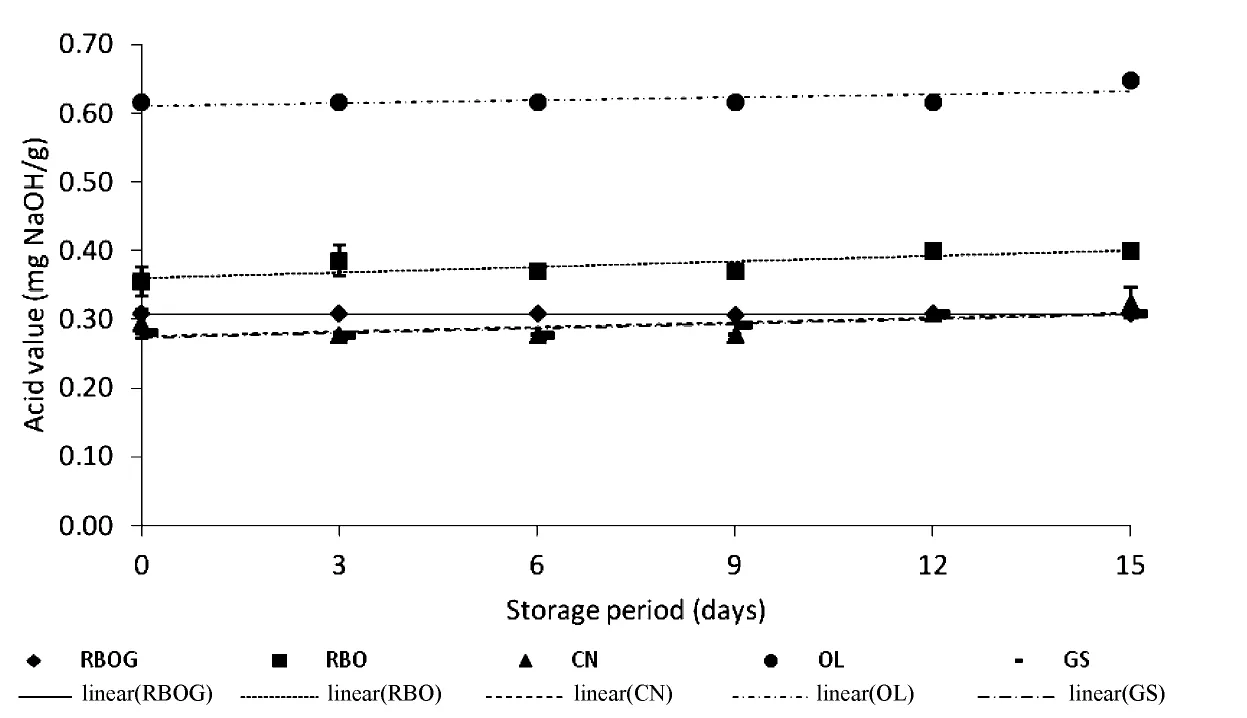
Fig.1.Changes in acid value of oils during storage at 60℃.RBOG,Rice bran oil+green tea extract;RBO,rice bran oil;CN,canola oil;OL,extra virgin olive oil;and GS,grape seed oil.Error bars represent standard deviations.
Changes in PVs of the oils are shown in Fig.2.RBOG,RBO,CN,OL,and GS showed an increasing trend in PV up to day 15 of storage.In general,the lower the PV,the better is the oil quality.However,the PV decreases as secondary oxidation products appear.The PV test is a good way to measure the amount of primary oxidation products in fresh oils.Oils with significant levels of peroxides may still be odorless if secondary oxidation hasnotstarted.If oxidation of oil occurs,the PV may be relatively low but the oil will be obviously rancid[17].Eventually,the PV will actually begin to decrease because the formation of peroxides is slower than decomposition.Hydroperoxides break down to form a wide variety of decomposition products that are further oxidized and degraded.Aldehydes are one of the groups produced and can be used as a means of tracking the progress of fat degradation[18].
The PV is highly related to oil saturation level.The oleic,linoleic,and linolenic acid contents in RBO,OL,CN(low erucic acid-rapeseed oil),and GS are as follows:RBO(38%-48%;29%-40%;0.1%-2.9%),OL(55%-83%;3.5%-21%;<1.5%),CN(51%-70%;15%-30%;5%-14%),and GS(12%-28%;58%-78%;ND-1%)[12].As reported in Table2,the highest average oxidation rate constant was found for RBO(0.282),followed by OL(0.256),CN(0.194),and GS(0.182).The lowest was found for RBOG(0.125);The R2values of all samples were≥0.8.The high oxidation rate in OL was probably caused by the presence of chlorophyll in the extra virgin olive oil that promoted and accelerated photoxidation,although the OL had high monounsaturated fatty acid content and the highest total phenolic content.The stability of CN is limited mostly by the presence of linolenic acid,chlorophyll and its decomposition products and other minor components with high chemical reactivity,such as trace amounts of fatty acids with more than three double bonds.The increasing PV of CN was also affected by the presence of phosphorus.Phosphorus in oils is mostly present in the form of phospholipids and is removed mostly through degumming procedures and other refining steps but is still present in oil.Phospholipids tend to complex heavy metals,and these complexes are a stable catalyst that can initiate and stimulate oxidation[19].Although linolenic acid is easier and more rapidly oxidized than that of linoleic acid,linolenic hydroperoxides are more unstable than those of linoleic acid[20].Linoleic acid was higher in RBO and linolenic acid was lower than that in CN,yet the average rate of change in RBO oxidation was higher than that of CN.This result may have been caused by the higher amount of linoleic acid that created the higher PV in RBO.GS showed a slight increase in PV up to 15 days,even though it contained high linoleic acid content.GS is a rich source of polyphenolic compounds and their antioxidant activity has manifested itself in the protection of β-carotene in an emulsion with linoleic acid against oxidation[21].Phenolics in grape seed exist as proanthocyanidins in the form of oligomers and polymers of polyhydroxy flavan-3-ols such as(+)-catechin and(-)-epicatechin,which can couple with gallic acid to form gallate esters or sugar molecules to form glycosides[22].Grape seed extract can reduce the initial burst of oxidation and inhibit development of lipid hydroperoxides in raw ground chicken stored at-18℃ and 4℃ during 6-month storage[23].The RBOG showed the lowest PV during storage,which was caused by phenolic compounds in it.Green tea extract had positive DPPH free radical scavenging,superoxide anion scavenging and hydrogen peroxide scavenging activity[6].Green tea extract including catechin could retard producing peroxides in rice bran oil during storage.
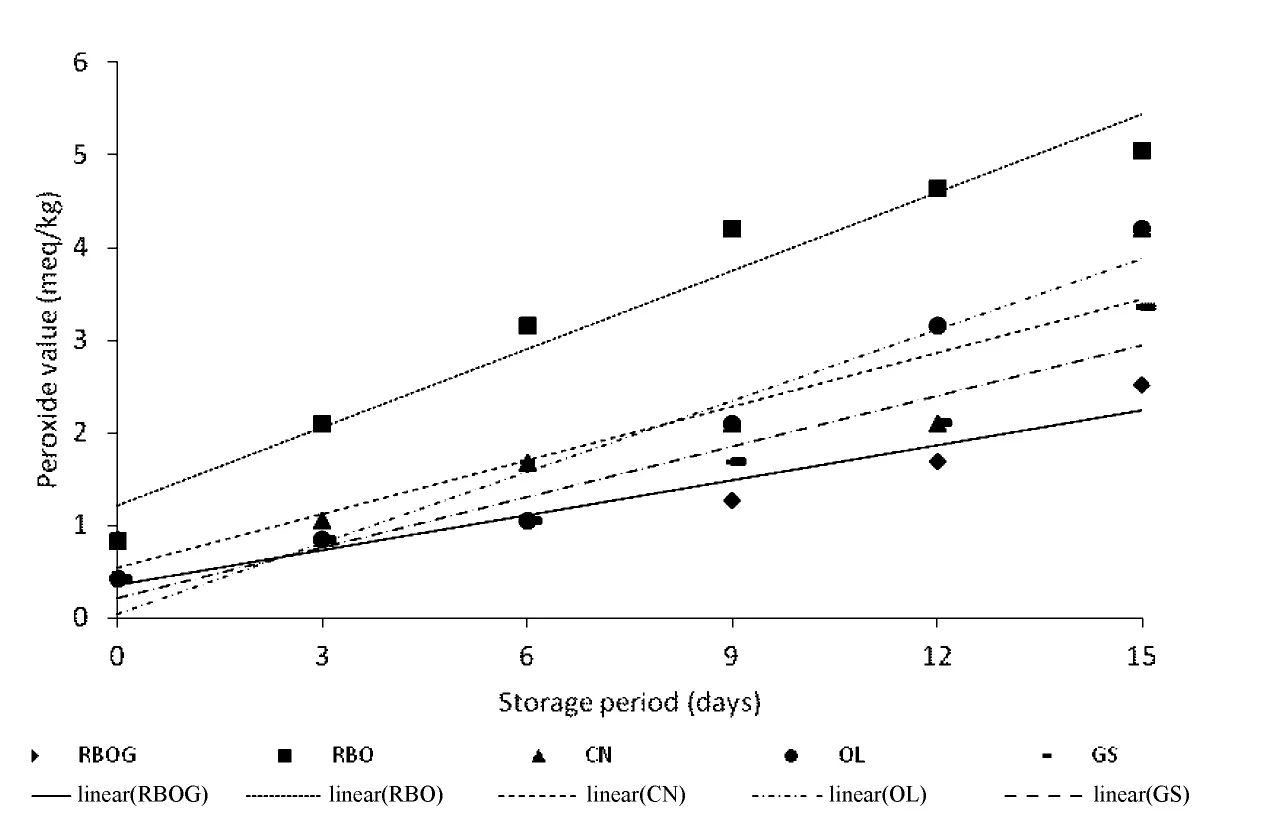
Fig.2.Changes in peroxide values of oils during storage at 60℃.RBOG,Rice bran oil+green tea extract;RBO,rice bran oil;CN,canola oil;OL,extra virgin olive oil;and GS,grape seed oil.Error bars represent standard deviations.

Table2.Equations and R2of peroxide values and TBARS.
3.4.Changes in TBARS of the oils during storage
Figure 3 shows changes in TBARS of the oils during storage.Four oil samples showedincreasingTBARS,whereas GS showed increasing TBARS up to 9-days of storage and then decreased afterward.The average rate constants of change in TBARS of RBOG,RBO,CN,OL,and GS were as follows:0.075,0.197,0.390,0.138,and 0.166,respectively,with R2of all samples≥ 0.8.The highest TBARS was found in CN and the lowest was found in RBOG.This result may have been caused by high linolenic acid content and the presence of chlorophyll in CN.Linolenate hydroperoxides are easily decomposed into a very complex mixture of secondary products.The instability of linolenate hydroperoxides can be explained by the presence of active methylene groups.These structural features promote the formation of dihydroperoxides,which,in turn,decompose into dialdehydes.TBARS are easily produced during methyl linolenate oxidation and much less easily from methyl linoleate oxidation and a higher fluorescence quantum yield of TBARS is derived from methyl linolenate oxidation products[20].The decreasing TBARS in GS may have been caused by less optimum detection of the pigment produced in oxidation process at 532 nm.Aldehyde products of lipid oxidation other than MDA may also react with TBA to produce yellow(λmax=455 nm),orange(λmax=495 nm),and red(λmax=532 nm)pigments[3,4].Some alkanals are produced from the reaction between aldehydes and TBA,which produce pigments that show maximum absorbance at 450 nm resulting in lower absorbance at 532 nm.Moreover,hexanal is formed from linoleic acid in high amounts[24],as found in GS.RBOG showed an initial slight increase in TBARS up to day 15.OL showed a low rate of increase in TBARS due to the high amount of oleic acid,whereas RBO showed higher TBARS due to the high amount of polyunsaturated fatty acids,which have two and more double bonds.Therefore,RBOG improved the oxidative stability of RBO.The oxidative stability of RBOG was related to adding the green tea extract.Similarly,adding a green tea extract significantly reduces lipid oxidation in refrigerated ground pork during storage for 5 days.Green tea extract is a potent natural antioxidant and effectively enhances raw ground pork quality[25].
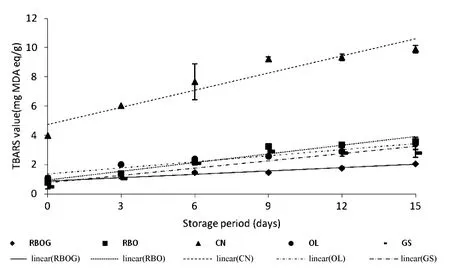
Fig.3.Changes in TBARS of oils during storage at 60℃.RBOG,Rice bran oil+green tea extract;RBO,rice bran oil;CN,canola oil;OL,extra virgin olive oil;and GS,grape seed oil.Error bars represent standard deviations.
4.Conclusions
RBOG showed the highest stability during storage,whereas CN showed the lowest stability against oxidation with respect to changes in PVs and TBARS.The green tea extract exhibited high antioxidant activity for maintaining the oxidative stability of RBOG.Adding green tea extract to rice bran oil could improve oxidative stability of the oil resulting in better stability than that of RBO,CN,OL,or GS.Based on these results,further intensive study on polyphenol activity such as epicatechin derivatives,which can optimally improve oxidative stability of rice bran oil could be done in the future.
1.Sun Q,Faustman C,Senecal A,Wilkinson AL,Furr H.Aldehyde reactivity with 2-thiobarbituric acid and TBARS in freeze-dried beef during accelerated storage.Meat Science,2001,57:55-60.
2.Sans RG,Chozas MG.The thiobarbituric acid(TBA)reaction in foods:a review.Critical Reviews in Food Science and Nutrition,1998,38(4):315-330.
3.Kosugi H,Kato T,Kikugawa K.Formation of yellow,orange,and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid.Analytical Biochemistry,1987,165:456-464.
4.Kosugi H,Kikugawa K.Reaction of thiobarbituric acid with saturated aldehydes.Lipids,1986,21,537-542.
5.Orthoefer FT.Rice bran oil.In Bailey's Industrial Oil and Fat Products(6thed.,vol.2),Shahidi F.Eds.;John Wiley & Sons,Inc.,2005,pp465-487.
6.Geetha T,Garg A,Chopra K,Kaur IP.Delineation of antimutagenic activity of catechin,epicatechin and green tea extract.Mutation Research,2004,556:65-74.
7.Chen ZY,Chan PT,Ma HM,Fung KP,Wang J.Antioxidative effect of ethanol tea extracts on oxidation of canola oil.Journal of The American Oil Chemists'Society,1996,73:375-380.
8.Chen ZY,Chan PT.Antioxidative activity of green tea catehins in canola oil.Chemistry and Physics of Lipids,1996,82:163-172.
9.Pearson D.The Chemical Analysis of Foods.Churchill Living Stone:London,1976,pp121-150.
10.Capannesi C,Palchetti I,Mascini M,Parenti A.Electrochemical sensor and biosensor for polyphenols detection in olive oils.Food Chemistry,2000,71:553-562.
11.Pegg RB.Determination of TBA reactive substances in oils or lipid extracts:direct method.In Handbook of Food Analytical Chemistry,Wrolstad RE,Acree TE,Decker EA,Penner MH,Reid DS,Schwartz SJ,Shoemaker CF,Smith DM,Sporns P Eds.;John Wiley & Sons,Inc.,2000,pp551-553.
12.Joint FAO/WHO Food Standard Programme Codex Standard for Named Vegetable Oils.Codex Stan 210-1999,pp1-16.
13.Vahcˇic'N,Hrusˇkar M.Quality and sensory evaluation of used frying oil from restaurants.Food Technology and Biotechnology,1999,37(2):107-112.
14.Nawar WW.Lipids.In Food Chemistry(3rded),Fennema,OR Eds.;Marcel Dekker,1996,pp225-319.
15.Joint FAO/WHO Food Standard Programme Codex Standard for Olive Oils and Olive Pomace Oils.Codex Stan 33-1981,pp1-8.
16.Buckle KA,Edward RA,Fleet GH,Wotton M.Course Manual in Food Science.Watson Ferguson& Co.:Brisbane,1978,pp105-107.
17.Miller M.Oxidation of food grade oils,2004,available at:http://www.oilsfats.org.nz/Oxidation%20101.pdf(accessed:January,15th2012).
18.Sewald M,& DeVries,J.Food product shelf life,2005,available at:http://www.medlabs.com/Downloads/food_product_shelf_life_web.pdf(accessed:January 21st2012).
19.Przybylski R,Eskin NAM.Phospholipid composition of canola oils during the early stages of processing as measured by TLC with flame-ionization detector,Journal of The American Oil Chemists'Society,1991,68:241-245.
20.Zamora R,Hidalgo FJ.Comparative methyl linoleate and methyl linolenate oxidation in the presence of bovine serum albumin at several lipid/protein ratios.Journal of Agricultural and Food Chemistry,2003,51:4661-4667.
21.Negro C,Tommasi L,Miceli A.Phenolic compounds and antioxidative activity from red grape marc extracts.Bioresource Technology,2003,87:431-444.
22.Weber HA,Hodges AE,Guthrie JR,O'Brien BM,Robaugh D,Calrk AP,Harris RK,Algaier JW,Smith CS.Comparison of proanthocyanidins in commercial antioxidants:grape seed and pine bark extracts.Journal of Agricultural and Food Chemistry,2007,55(1):148-156.
23.Brannan RG,Mah E.Grape seed extract inhibits lipid oxidation in muscle from different species during refrigerated and frozen storage and oxidation catalyzed by peroxynitrite and iron/ascorbate in a pyrogallol red model system.Meat Science,2007,77:540-546.
24.Shaker ES.Antioxidative effect of extracts from red grape seed and peel on lipid oxidation in oils of sunflowers.LWT-Food Science and Technology,2006,39:883-892.
25.Maneenin N,Pilasombut K,Bundit J,Sethakul J.Effect of green tea(Camellia sinensis)extract on lipid oxidation and meat quality in raw ground pork during refrigerated storage.Proceeding of The 14thAnimal Science Congress,Pingtung,Taiwan,August 23-27,2010,pp1331-1334.
杂志排行
茶叶的其它文章
- Stability of Tea Catechins and Antioxidant Properties of Green Tea Extracts as Affected by Boiling-treatment
- Effects of Tannic Acid on Active Aluminum Species Distribution in Various Tea Soils
- The 30-Day Oral Administration Studies of Liposoluble Tea Polyphenols in Rats
- Analysis of the Volatile Chemicals of Longjing Tea from Different Production Locations Using Electronic Nose
- A Study on Physiological Character of Fresh Tea Leaves in Different Cold-Resistant Varieties
- Analysis of Volatile Compounds of Jinmudan Oolong Tea by Different Wrapping-Twisting
