The 30-Day Oral Administration Studies of Liposoluble Tea Polyphenols in Rats
2013-12-13LiangPengLinDaSunYueFeiWangDaWeiLiShiKangZhangYueJinZhuPuMingHe
Liang-Peng Lin,Da Sun,Yue-Fei Wang,Da-Wei Li,Shi-Kang Zhang,Yue-Jin Zhu*,Pu-Ming He*
1.Department of Tea Science,Zhejiang University,Hangzhou 310029,China.
2.Hangzhou Tea Research Institute,China COOP,Hangzhou 310016,China.
1.Introduction
Tea,originally came from China,is one of the most popular beverages in the world.The drinking of green tea made from camellia sinensis leaves has long value of promoting human health.Tea contains many compounds,caffeine,theanine,especially tea polyphenols(TP),and many studies show that polyphenolic compounds is a kind of main active components in green tea with a variety of functions.Many epidemiological studies have shown that regular consumption of tea can reduce risks for various cancers,such as skin cancer,lung cancer,liver cancer and so on[1].Further studies demonstrated TP has anti-aging,anti-radiation,lowering blood sugar,hypolipidemic,anti-bacterial and anti-oxidative activities[2-4].Because of TP with strong free radical scavenging ability,TP is considered as one of the best natural antioxidants.In fact,TP has been employed as a anti-oxidant in the food industries of many countries[5].
In most cases,however,the biological activities of TP are dependent on in vitro experiments.As we all known,drinking of tea has many potential health benefits including the prevention of cancer.But the major problem is that the concentrations of TP required to observe these biological effects in vitro exceed the concentrations achievable in blood and tissues by 10 to 100-fold[6].Joshua D.Lambert pointed that studies in animal models and with cell lines have demonstrated cancer preventive activity,but the epidemiological data remain mixed.So he held that results on the bioavailability and biotransformation of TP help to explain some of the differences[7].Moreover,many in vivo studies demonstrated the limited bioavailability of TP limits its biological activity.For example,the in vitro testing of a pooled plasma sample showed that a plasma polyphenol concentration of 7.5 mol/L was necessary to cause an increase in the antioxidant activity,but the mean maximal plasma concentration of total flavanols(1.2 mol/L)after green tea extract supplements consumption was below the plasma concentration necessary to expect an increase in the antioxidant activity[8].This findings confirmed by S.M.Henning et al.[9],who also found the maximum plasma concentration of total tea flavanols of 3.2 mol/L was not high enough to cause an increase in the antioxidant capacity.A recent study by D.D.Rio et al.researched the absorption of catechins in human volunteers after ingestion of a commercial ready-to-drink tea.The bioavailability of TP was calculated as a ratio between total excretion and total intake of flavan-3-ols and gallic acid.The results,a total flavan-3-ol excretion equal to 7.2%of intake with tea and excretion of gallic acid reaching 4.5%of the amount ingested,confirmed previous many studies[10].The low bioavailability of TP may be in part explained by Lipinski's Rule of five[11].This rule states that if a compound has a molecular weight of>500 and contains five or more hydrogen-bond donors and/or ten of more hydrogen-bond acceptors,it is likely to be poorly bioavailable.So it is expected that TP will have a large polar surface area due to the numerous hydrogen bond donors and acceptors.Because of interaction with water molecules,these groups maybe result in the formation of a large hydration shell,which make them less likely to pass through transient pores form in the lipid bilayer by rotation of the acyl chains of phospholipids[7].Therefore,from the structural characteristics of TP,we can predict that TP soluble in water and have low bioavailability.
Due to the weak liposolubility and stability as well as low bioavailability of TP,more and more researchers prepared the liposoluble tea polyphenols(LTP)through the structural modification in order to improve its application. Thereare many methodsforTP modification, such assolvent, emulsion and molecular modification[12-14].Hara et al.[15]using the ethanol solvent as a carrier of TP added to the oils and Li QL et al.[16]prepared the fat-soluble synergic tea polyphenols solution by using mixed nonionic surfactants as solvent.However,the main method of TP modification is molecular modification at present,which is to introduce some groups(acyl group,methyl and glycoside)into the molecular structure of TP.Several studies have shown that the incorporation of fatty acid hydrocarbon chains into compounds can increase their propensity to interact with lipid bilayers and increase or maintain the biological activity.So researchers synthesized acylated derivatives of TP with various chain lengths[17-21]and methylated catechins[22-23].Because of LTP with lipophilic characteristics and excellent antioxidant activity,it can be used as the edible-oil antioxidant instead of butyl hydroxy anisd(BHA)or butylated hydroxytoluene(BHT).But from a safety perspective,very few studies have explored the toxicity potentials of LTP.Therefore,the objective of this study was to synthesis LTP by acylation and to investigate the potential toxicity of the sample.The synthesis method of LTP according to Sun D et al.[21]and the 30-days feeding test were based on the standards in"Toxic Chemical Identified Technical Specification"(The Ministry of Health Superintend made【2005】No.272).
2.Materials and methods
2.1.Preparation of liposoluble tea polyphenols
2.1.1.Materials
Tea polyphenols was purchased from Zhejiang Orient Tea Development Co.,Ltd.(TP>95%,EGCG >38%);Ethyl Gallate was purchased from Chendu XiYa Chemical Technology Co.,Ltd;Fatty acid chloride was purchased from Zhejiang Quzhou Mingfeng Chemical Co.,Ltd.(> 95%);Ethyl Acetate(Analytical Reagent,AR),Trichloromethane(AR),Ethyl Alcohol Absolute(AR),Acetic Acid(AR),Hydrochloric Acid(AR),Triethanolamine(AR),Sodium Bicarbonate(AR),AL204 Electronic Balance,DK-S26-type Electric Heated Water Bath,ZX98-1 Rotary Evaporator,SHB-D Water Circulating Multipurpose Vacuum Pump,UV-2102PC UV/Vis Spectrophotometer.
2.1.2.Preparation methods
The method described by Sun D et al.[21]used to prepare LTP.A certain amount of refined TP and ethyl acetate put in the three-necked flask,connecting the reflux condenser and the tail gas recovery unit,which nitrogen used as protection,putting sodium bicarbonate as catalyst and fatty acid chloride when the temperature reached the specified temperature with the magnetic stirring.After reaction the mixture was rinsed with hydrochloric acid and distilled water to remove the catalyst and the unreacted TP as cooling to room temperature.Next,the dehydrating agent was added to dry the organic phase.Then,the organic phase was concentrated with a rotary evaporator to distill off acetic ether under reduced pressure.Finally,the modified product of LTP was obtained.
2.1.3.Determination of liposoluble tea polyphenols content
In triethanolamine-aqueous solution,LTP reacted with ferrous ions to form a violet blue complex,which has a maximum absorption peak at 540 nm,so the absorbance value can be determined by spectrophotometry.Therefore,the content of LTP was calculated through comparison with the standard of ethyl gallate.
The sample of LTP(200 mg)was dissolved in ethanol and diluted to 100 mL.The LTP solution(1.0 mL)and triethanolamine-aqueous solution(1:1,1.0 mL)mixed in 25 mL colorimetric tube.Then ferrous tartrate solution(5 mL)was added and diluted with distilled water to 25 mL.As a control,equal volumes of ethanol without LTP were added.The content of LTP was calculated as follows:
LTP(%)=E ×1.5 ×100/[m(1-G)]
Where E,1.5,m and G were defined as the corresponding content of ethyl gallate from the standard curve according to the absorbance value,the conversion factor of TP and ethyl gallate,the quality of sample and the moisture content of sample,respectively.
2.2.The 30-Day oral administration test
2.2.1.Test materials and diet
Liposoluble tea polyphenols was used.It was prepared as mentioned above.Test diets were prepared by mixing 0%(control),1%(low-dose),2%(mid-dose),or 4%(high-dose)LTP to the basal diet(GB 14924-2001.purchased from Experimental Animal Center of Zhejiang Academy of Medical Sciences),and these diets were kept in a refrigerator at 4-6℃ throughout the test period.
2.2.2.Animals and treatment
Eighty(40 of each sex)Sprague-Dawley(SD)rats(2001001)were purchased from Experimental Animal Center of Zhejiang Academy of Medical Sciences(China).Following three days acclimatation period,healthy males and females were randomly selected and assigned to the treatment and control groups.Each group had ten rats of each sex.The rats were housed five per cage and the animal room had a 12-h light/dark cycle.Temperature of the animal room was 22 4℃ during the study and the humidity ranged from 50 to 60%.Feed and water were provided to animals ad libitum except for overnight fasting prior to necropsy.
2.2.3.Experimental design and observation
The study included four groups of ten rats per sex which were provided the experimental diet from the start of the study.The rats in the control group were given basal diet,and rats in the treatment groups were administed basal diet supplement with 1,2 and 4%LTP for 30 days as noted above,respectively.During the study,the animals were observed daily for abnormal clinical signs and the food intake was recorded daily.Each animal was weighed twice weekly till the end of the study.Final body weights(fasted)were recorded prior to the necropsy.At the end of experiment,all the animals were killed and blood was collected with 15 g/L EDTA-Na2as an anticoagulant after the animals were fasted overnight.All blood samples were then analyzed for haematology and clinical biochemistry.Examination of haematology parameters included:red blood cell count(RBC),white blood cell count(WBC),haemoglobin concentration(Hb)and differential leukocyte count.Serum chemistry parameters included:albumin,total protein,globulin,aspartate aminotransferase(AST),alanine aminotransferase(ALT),glucose(GLU),triglycerides(TG),cholesterol(CHO),creatinin(CREA)and urea.
Gross necropsy of all males and females included an initial examination of external surfaces and orifices as well as the thoracic and abdominal cavities and their contents.Heart,liver,kidneys spleen and testes or ovaries of all animals were weighed soon after dissection then preserved in 10% formalin for histopathological examination.Histopathological assessments were performed for organs retained from the control and high-dose animals of the toxicity study.Organs were trimmed,processed,embedded in paraffin,sectioned and stained with haematoxylin and eosin before their microscopic examination.
2.3.Statistical analysis
Data are expressed as means standard deviation.Treated and control groups were compared using a one-way analysis of variance(ANOVA)followed by Dunnett's test.P values less than 0.05(P <0.05)were considered as indicative of significance.
3.Results and discussion
3.1.The property and content of LTP
The prepared LTP are brown and viscous,and it has the smell of ethyl acetate.Because of TP with good solubility in ethyl acetate[24],this synthesis method of LTP used ethyl acetate as TP solvent.Accordingly,there is still a little residual ethyl acetate in LTP.
The standard curve of ethyl gallate was drawn at the concentration ranging from 0 to 100 mg/mL,and its linear regression equation was calculated as follow:y=0.0032x-0.0577,R2=0.9831.Good linear relationship between absorbance and concentration can be used as a standard curve.So the content of LTP reached 23.4%by measuring the absorbance.Since different reaction conditions,the content of LTP will be different[17].Shen SR et al.[25]found that the product,which two hydroxyl groups in EGCG have been esterified,had a good lipophilic.According to the pope analysis of LTP,at least one fatty acid hydrocarbon chain was incorporated into the substrate of TP[26].
3.2.Clinical observation and survival
All animals survived until they were killed at the end of the study,and any abnormal clinical sign was not observed over the duration of the toxicity test.
3.3.Body weights
There was no statistically significant difference(P >0.05)in body weight in either male or female rats between treatment and control groups except for the males of high-dose group(Fig.1).Reduced body weight gain was observed for males treated with LTP in comparison to male rats of control group(MC,Table1).The body weight gain,however,for females treated with the high-dose of LTP was a little higher than the control group(no significant difference).Probably because LTP contained long-chain fatty acids,which can increase body weight.The difference of body weight gain between males and females was from their sexual difference,and nothing to do with the effect of LTP.The final body weight of MH was a little lower than that of MC,and the difference is acceptable being with the range of 5.9%for body weight loss or gain inferior to 15%should not be considered of toxicological significance[27].
3.4.Food intake
Food intake was not significantly different(P >0.05)between treatment and control groups as shown in Table1.The food intake of males of treat groups was relatively lower than control group,while females of treat groups were opposite,which indicated that feed intake of rats was affected by the oral administration of LTP.However,there were no statistically significant inter-group differences in feed conversion efficiency.Therefore,the results indicated that it was not considered to be toxicologically significant.
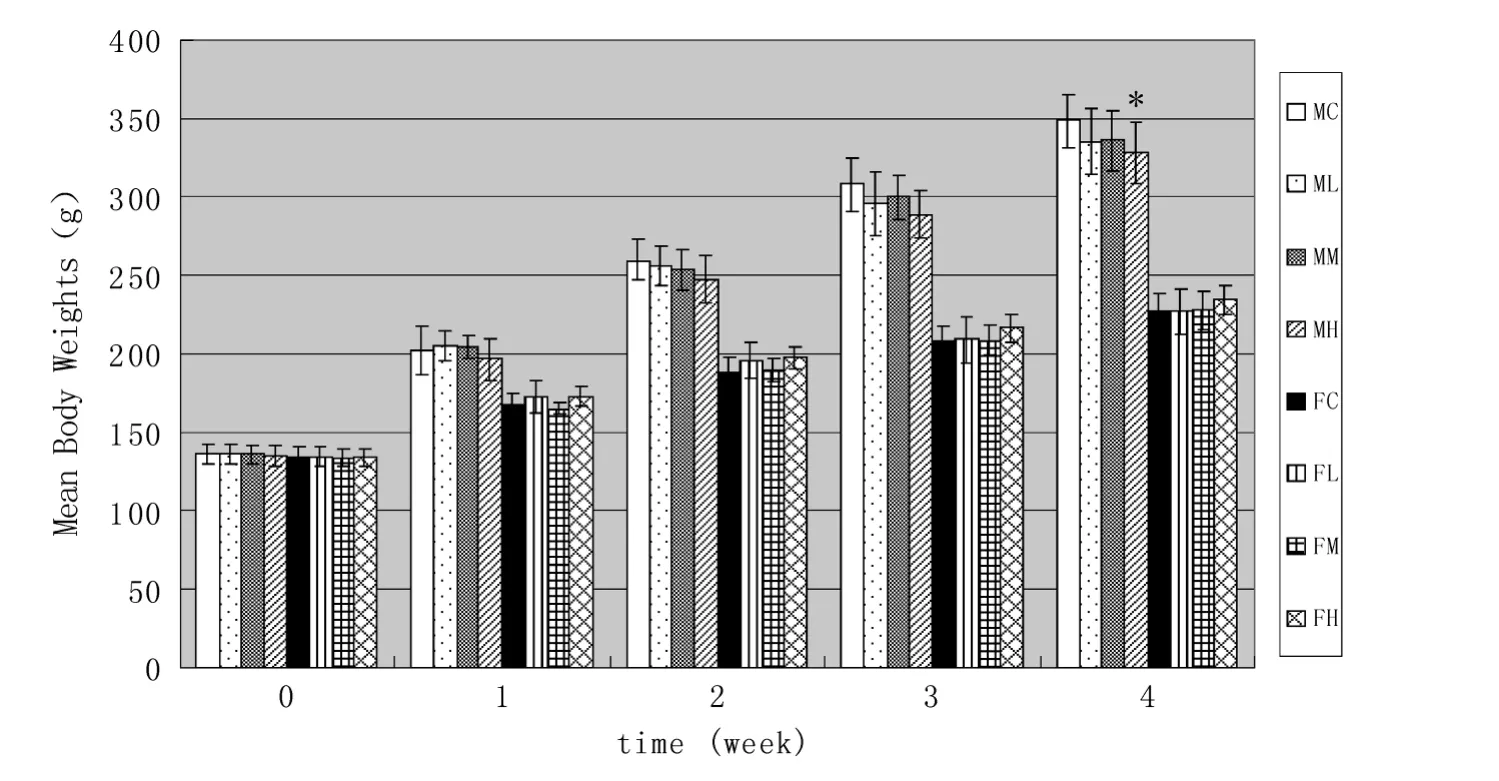
Fig.1 Effects of liposoluble tea polyphenols on body weight in rats.
3.5.Haematology and clinical chemistry
The results of haematology parameter are shown in Table2.There were no treatment-related changes in red blood cell count,total and differential white blood cell counts.Mean haemoglobin concentration was statistically significantly higher in the high-dose group of males comparing to the control group.According to Chen JG et al.[28],the mean haemoglobin concentration of this study was 147.6 within the reference values(98 ~156 g/L).The difference,the increment of haemoglobin concentration,was not observed in females,was of small magnitude and was not noted in a dose-related manner,so it was not considered toxicologic consequence.
Clinical chemistry values showed statistically significant(P <0.05)changes in the plasma levels of total protein and albumin in high-dose group of females(Table3).However,albumin/globulin ratio was no significant difference between the high-dose and control group of females.Compared to the control group,a decrease in alanine aminotransferase was observed in high-dose group of males,but the value was within the reference values[29].In addition,there was no significant difference for the other biochemical parameters measured in the present study among the male or female groups.These differences were of minimal magnitude,were no treatmentrelated and were not considered to be toxicologically meaningful.
3.6.Gross necropsy examinations
There were no treatment-related macroscopic findings at the scheduled necropsies in treatment groups(data not shown).
3.7.Organ weights and histopathology
There were no significantly different(P >0.05)organ weights and relative organ weight/body weight ratio in treatment groups compared with control groups(Table4).Microscopic examination revealed no changes attributable to the administration of LTP.The examination revealed only common background pathology findings between the control group and the high-dose group(data not shown).
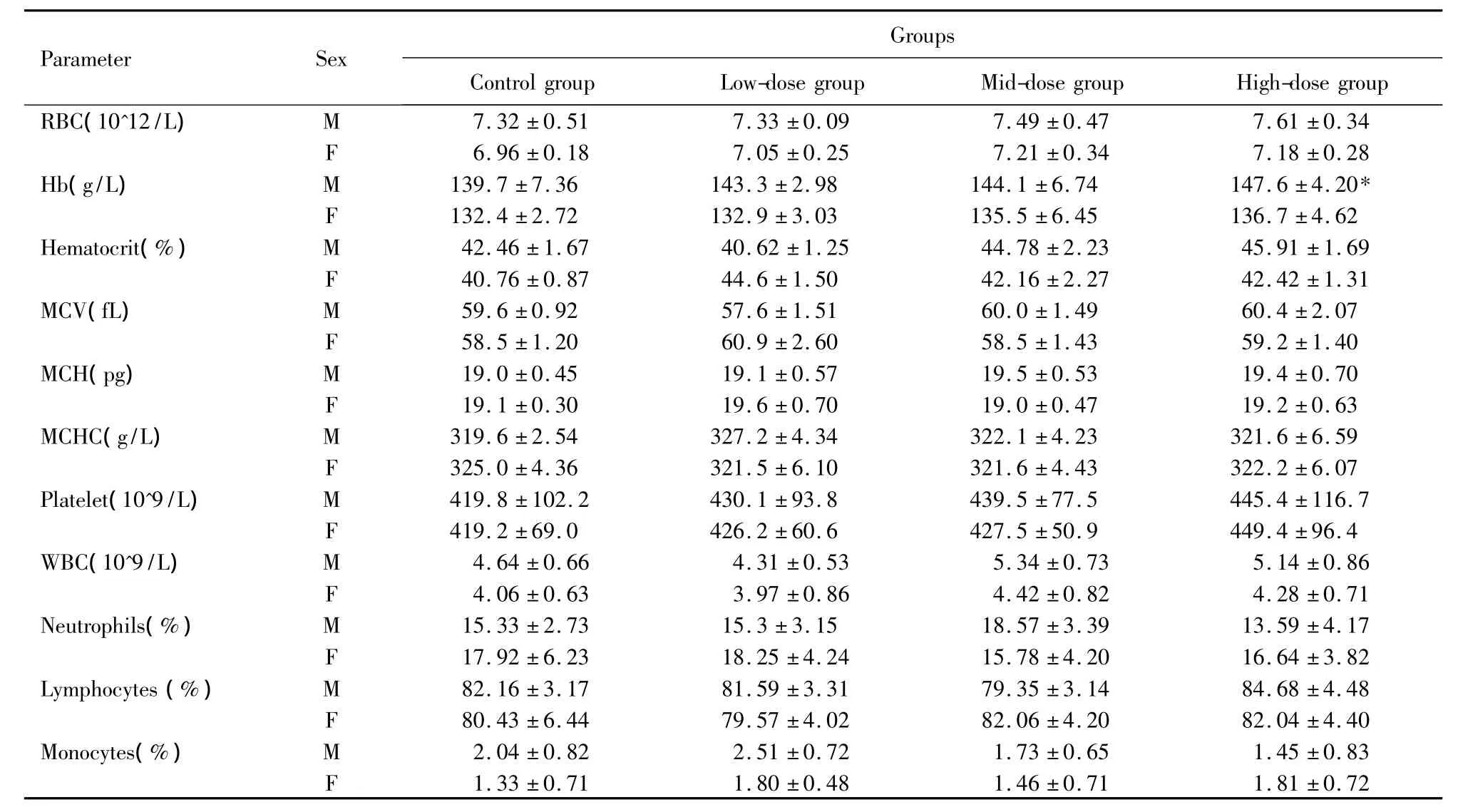
Table2.Haematology values of rats fed with LTP for 30 days.
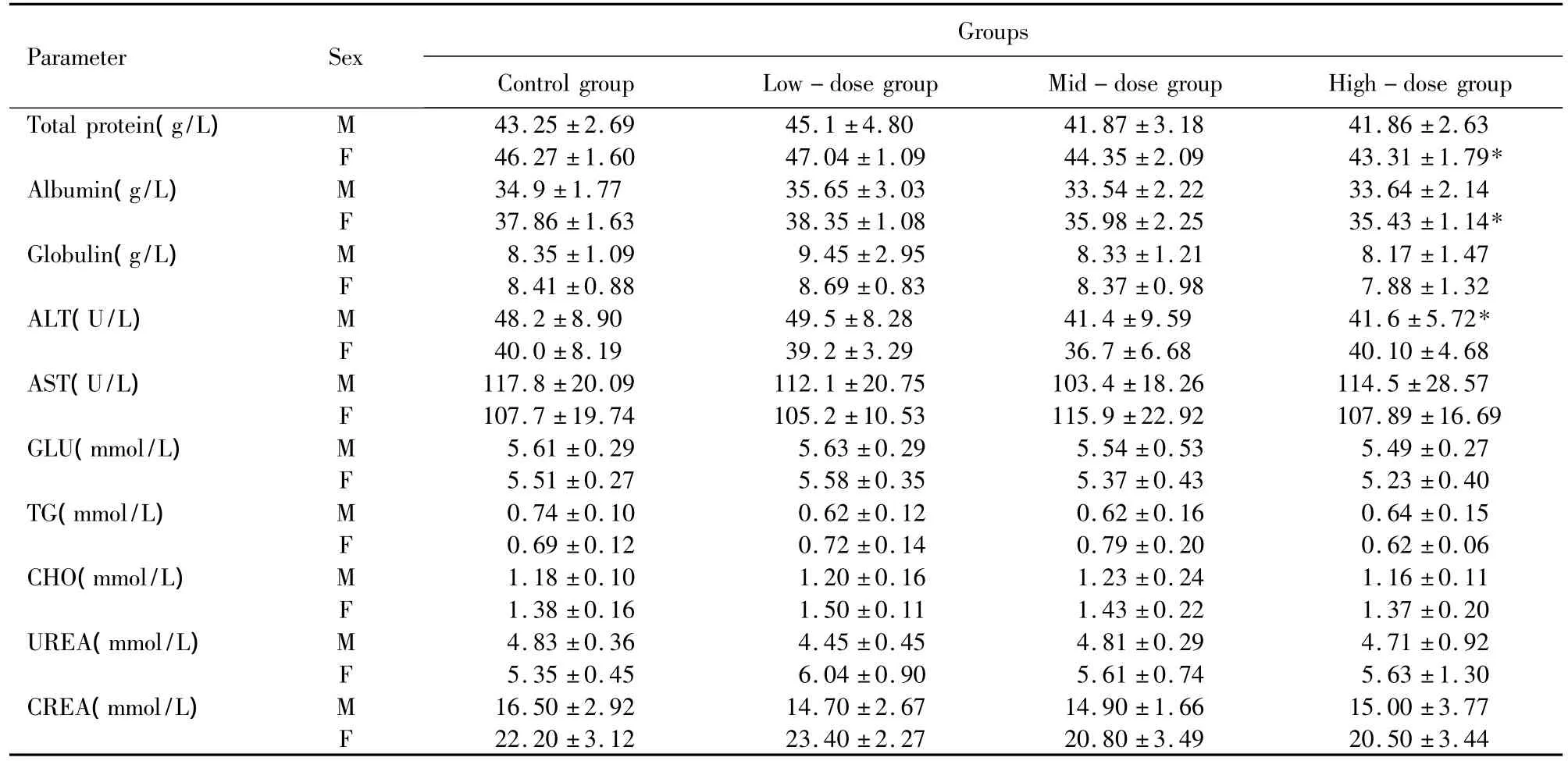
Table3 Clinical chemistry values of rats fed with LTP for 30 days.
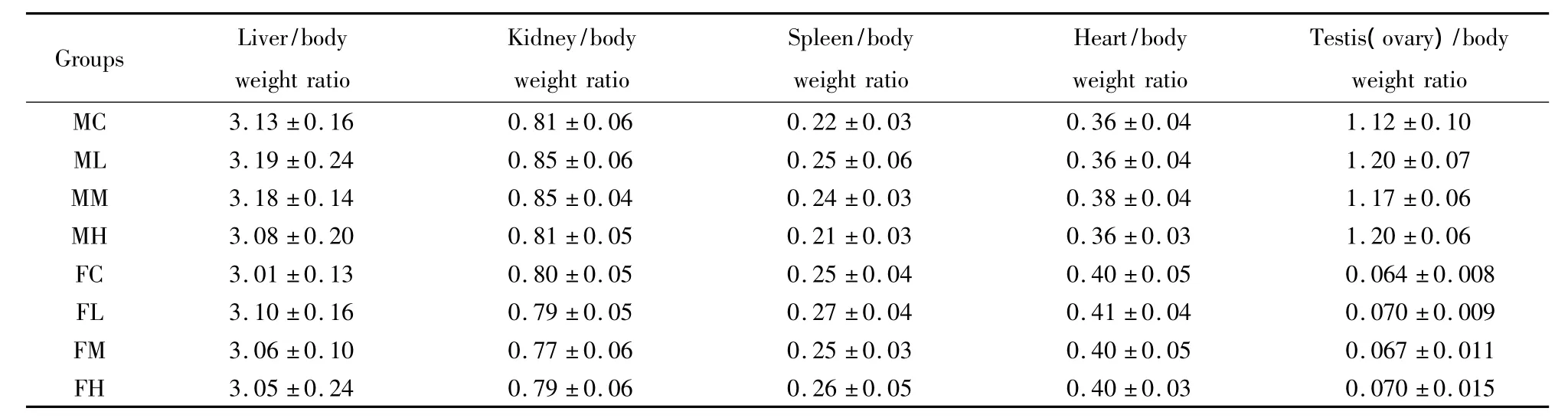
Table4 Organ to body weight ratios from rats fed with LTP for 30 days.(%)
4.Conclusions
The results of the present study demonstrate that daily oral administration of LTP at dose levels up to 4%for 30 days was safe to rats as evaluated by general condition,growth,food intake,feed conversion efficiency,haematology parameters,clinical chemistry values,organ weights,gross necropsy and histopathology.In this report,we have indicated that no dose-related toxicity effect was observed.
No mortality was observed fed with LTP.However,the results showed a few changes.Mean body weights in MH were lower than control values.The haemoglobin concentration level was significantly increased and alanine aminotransferase was decreased in MH as compared to controls,but the changes were regarded as toxicologically irrelevant because the adverse results did not appear in both sexes and/or were without dose-relation.Similar results have been reported in other studies:Hsu Yu-Wen et al.[30]reported that oral administration of green tea extract in the female mice that ingested 625mg/kg significantly decreased the serum ALT and AST levels and Almurshed[31]found that green tea extract significantly reduced the serum ALT levels.Another observation in FH was a lower total protein and albumin,which was possibly caused by the gross necropsy's order.FH was the last group,and the fasting time was the longest.However,the values in FH were in the normal range.Therefore,no toxicological significance was attached to the reduced total protein and albumin levels in FH.
In conclusion,this study showed that consumption of diets containing up to 4%LTP for 30 days was not associated with any obvious signs of toxicity in SD rats.The 4%dietary concentration was equivalent to an overall intake of 3.4 and 4.0 g LTP/kg body weight/day in male and female rats,respectively.The no observed adversed effect level(NOAEL)in male and female SD rats is considered to be greater than 3400mg/kg body weight/day.Therefore,results obtained in this study allowed us to conclude that LTP properly utilized in the oral administration could be devoid of any toxic risk.This study is first to investigate the toxicity of LTP in rats by a 30-day oral toxicity trial and indicate that there were no subacute toxicity observed.Certainly,to judge LTP safe and non-poisonous,the data from teratogenesis test is necessary.Now,this test is going on by our study group.
Acknowledgements
This work was financially supported by the National 12th Five Year Research Program of China(Project No.2012BAD36B06).
1.Khan N,Mukhtar H.Tea polyphenols for health promotion.Life Sciences,2007,81:519-533.
2.Yang XQ,Wang YE,Chen LJ.Chemistry of tea polyphenols,Shanghai Scientific and Technical Publishers:Shanghai,China,2003,pp454-455.
3.Dufresne CJ,Farnworth ER.A review of latest research findings on the health promotion properties of tea.Journal of Nutritional Biochemistry,2001,12:404-421.
4.Sharangi AB.Medical and therapeutic potentialities of tea(Camellia sinensis L.)-A review.Food Research International,2009,42:529-535.
5.He Q,Lv YP,Yao K.Effects of tea polyphenols on the activities of α-amylase,pepsin,trypsin and lipase.Food Chemistry,2006,101:1178-1182.
6.Sang S,Lambert JD,Yang CS.Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols.Journal of the Science of Food and Agriculture,2006,86:2256-2265.
7.Lambert JD,Yang CS.Cancer chemo-preventive activity and bioavailability of tea and tea polyphenols.Mutation Research,2003,523-524:201-208.
8.Henning SM,Niu YT,et al.Bioavailability and antioxidant activity of tea flavanols after consumption of green tea,black tea,or a green tea extract supplement.Am J Clin Nutr,2004,80:1558-1564.
9.Henning SM,Niu YT,et al.Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals.Journal of Nutritional Biochemistry,2005,16:610-616.
10.Rio DD,Calani L,et al.Bioavailability of catechins from ready-to-drink tea.Nutrition,2010,26:528-533.
11.Lipinski C,Lombardo F,Dominy B,Feeney P.Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.Adv.Drug Deliv.Rev.1997,23:3-25.
12.Ying L,Zhang SK,Wang YF,et al.Progress on the modification of tea polyphenols and antioxidant properties of lipid-soluble tea polyphenols.Journal of Tea Science,2010,30:511-515.
13.Bai Y,Jiang YW,Jiang HY,et al.Research advances in catechin modification.Food Science,2012,33:312-317.
14.Long D,Jiang HY,Zhang JY,et al.Progress on molecular modification for catechins and their application.Journal of Tea Science,2013,33:1-12.
15.Hara,Yukihiko.Process for the production of tea catechins.US Patent 4613672.1986,09,23.
16.Li QL,Lin XH,Preparation of synergic fat-soluble tea polyphenols solution and anti-oxidative properties in edible plant oils.Journal of Fujian Agricultural University,2001,30:244-249.
17.Zhang JX,Hu JB,et al.Study on tea polyphenols modification and its anti-oxidant activity.Cereal and Food Industry,2008,15:33-37.
18.Chen P,Sun D,Zheng XM.Preparation,structure and antioxidant activity of EGCG palmitate.Journal of Zhejiang University,2003,30:422-425.
19.Shen L,et al.Influence of stearyl chloride modification on antioxidation of tea polyphenols for oil.Science and Technology of Food Industry,2012,33:159-163,167.
20.Kuang YZ,Ma QH,Gu N.Synthesizing and token of liposoluble tea polyphenols.Food Science and Technology,2005,7:53-56.
21.Sun D,Zhang SK,Zhu YJ,et al.Study on optimization of lipid-soluble tea polyphenols synthesis technology by response surface design.Chin Tea Processing,2011,4:37-41,46.
22.Wu YJ,Wang XG,Wan XC.Present studies and research prospects on the methylated EGCG.Journal of Tea Science,2010,30:407-413.
23.Yoshiyuki A,Atsusi Y,et al.Regioselective synthesis of methylated epigallocatechin gallate via nitrobenzenesulfonyl(Ns)protecting group.Bioorganic & Medicinal Chemistry Letters,2009,19:4171-4174.
24.Wan XC et al.Tea Biochemistry.China Agriculture Press,2003,pp 8-15.
25.Shen SR,Jin CF,Yang XQ.Molecule modification of catechins.Journal of Tea,1999,25:76-79.
26.Zhang L,Zhang YJ,Tang HJ.Preparation and antioxidative activity of lipid-soluble tea polyphenols.China Oils and Fats,2004,29:54-57.
27.Saoussen T,Zeineb J et al.Preliminary safety assessment of Yarrowia lipolytica extracellular lipase:results of acute and 28-day repeated dose oral toxicity studies in rats.Food and Chemical Toxicology,2010,48:2393-2400.
28.Chen JG,Gong YJ et al.Discussion on relativity analysis of the normal value of haematology and organ to body weight ratio of SD rats.Journal of Health Toxicology,1999,13:262-265.
29.Wang Y,Lou ZQ et al.Discussion of the normal reference range of blood biochemical of SD rats.Journal of Health Toxicology,2000,14:112-113.
30.Hsu YW,Tsai CF,Chen WK,Huang CF,Yen CC.A subacute toxicity evaluation of green tea(Camellia sinensis)extract in mice.Food and Chemical Toxicology,2011,49:2642-2630.
31.Almurshed,K.S.Protective effect of black and green tea against carbon tetrachloride-induced oxidative stress in rats.Sandi Med.J.,2006,27:1804-1809.
杂志排行
茶叶的其它文章
- Stability of Tea Catechins and Antioxidant Properties of Green Tea Extracts as Affected by Boiling-treatment
- Effects of Tannic Acid on Active Aluminum Species Distribution in Various Tea Soils
- Oxidative Stability of Green Tea Extract-Enriched Rice Bran Oil During Storage
- Analysis of the Volatile Chemicals of Longjing Tea from Different Production Locations Using Electronic Nose
- A Study on Physiological Character of Fresh Tea Leaves in Different Cold-Resistant Varieties
- Analysis of Volatile Compounds of Jinmudan Oolong Tea by Different Wrapping-Twisting
