EFFECTS OF INHALED TiO2NANOTUBES ON LUNG TISSUE AND SERUM BIOCHEMICAL INDEXES OF MICE
2013-12-02TaoJie陶杰HePinting何娉婷ZhangYanyan张焱焱TangYuxin汤育欣WangYueqin王月勤
Tao Jie(陶杰),He Pinting(何娉婷),Zhang Yanyan(张焱焱)Tang Yuxin(汤育欣),Wang Yueqin(王月勤)
(College of Material Science and Technology,Nanjing University of Aeronautics and Astronautics,Nanjing,210016,P.R.China)
INTRODUCTION
Regarded as low toxicity[1-5],titanium dioxide(TiO2),existing in various nanostructures,such as in dots,rods and tubes,has already been a highly investigated material for a plethora of application(e.g.solar cells,self-cleaning coating,purification of water and air,and as a white pigment in the production of paper,paints,plastics and food colorant)due to its specific properties over the past several years[6-12].Especially titanates nanotubes have received a great deal of attention when prepared in a nanomaterial,in part because TiO2can exhibit a lot of important photovoltaic(solar energy conversion),photocatalytic,semiconductor,catalytic support,gas sensing properties[13-16]and so on.
However,Garabrant,et al[17]reported that 50%of workers exposed to TiO2suffered from respiratory symptoms,accompanied by impairment of pulmonary function.Trochimowicz,et al[18]completed a two-year inhalation exposure study using pigmentary titanium dioxide with 10,50and 250mg/m3.Evidence of inflammation(alveolar hyperplasia)was found at all exposure levels.Hext[3]discovered that following chronic exposures in rats to high particle concentrations(e.g.250mg/m3)leading to substantial particle overload conditions,inhaled pigment-grade TiO2particles resulted in benign lung tumors.In this regard,Bermudez,et al[2],Lee,et al[8],and Heinrich,et al[19]recorded that the results of 90-day and two-year inhalation studies with anatase/rutile ultrafine-TiO2or rutile fine-TiO2particles(average primary particle sizes were 25and 300nm,respectively)had demonstrated that inhaled ultrafine anatase/rutile TiO2particles produced a lot of pulmonary inflammation,fibrosis,or lung tumors in rats.Moreover,the work of Warheit,et al[20]showed that exposures to nanoscale TiO2rods(dlong=92—233nm,dwide=20—35nm)produced transient inflammatory and cell injury effects with 24hpostexposure (pe)when compared with a positive control particletype(α-quartz).
However,the toxicity study of TiO2nanotubes was seldom reported. Therefore,the effects of TiO2nanotubes(generated by hydrothermal treatment)on serum biochemical indexes and acute lung toxicity in mice through inhalation are studied in this paper,which are also compared with those exposed to room air.
1 MATERIALS AND METHODS
1.1 Animals
Mice used in this study are specific pathogenfree animals from the Comparative Medical Center of Yangzhou University (Yangzhou,China).The mice are approximately four weeks old at the start of the study (with mean weights of 18—22g).
1.2 Preparation and characterization of TiO2 nanotube
In a typical preparation,1.5g of TiO2powder(P25ultrafine-TiO2particles are purchased from Degussa,average primary particle size of 25nm as reported)is mixed with 140mL of 10 mol NaOH solution followed by hydrothermal treatment of the mixture at a fixed temperature in a 200mL Teflon-lined autoclave for 48h.After hydrothermal reaction,the precipitate is separated by filtration and washed with HCl solution and Milli-Q ultrapure water until pH=7.0[21-22].The washed TiO2nanotube samples are dried in a vacuum oven at 80°C for 8h,and then heat-treated at 400°C for 1h,from which we get the final product(following referred to as N-1).
P25and N-1TiO2powders are characterized using X-ray diffraction(XRD),transmission elec-tron microscopy(TEM),and Brunauer-Emmett-Teller(BET)specific surface area analysis.XRD patterns are collected using a BRUKER D8ADVANCE with a Cu Kαsource.TEM measurements(Hitach-800)are taken on the sample in solution:Ethanol solution.BET (Micromeritics ASAP 2010),to measure specific surface area,is taken on the sample in its dry state under nitrogen.
1.3 Inhalation study
The focus of this study are the following:(1)Time-course intensity of serum enzyme activities,(2)Time-course intensity of pulmonary inflammation,and (3)Histopathological evaluation of lung tissue.
Groups of mice (eight mice per group per time point),approximately 30dold,are exposed to test atmospheres(250mg/m3)3h/d for 7,14 and 28d,respectively.Another eight mice are exposed to room air only and served as controls.
Mice are exposed to N-1TiO2aerosols in a 0.24m3intoxication chamber constructed of organic glass.There is a hole on the cover as the inlet,through which TiO2nanotubes are put into the chamber.In addition,the chamber is divided into two parts with a wire mesh,and the mice are put on the wire mesh while a fan is working below the mesh,as a result,the dust nanotubes (250 mg/m3)are dispersed during the inhalation progress.
1.4 Clinical chemistry
The animals are fasted overnight and all mice are killed by exsanguinations following sodium pentobarbital injection,and blood samples are collected from the orbital plexus.
Blood for clinical chemistry studies is collected into tubes containing no anticoagulant,allowed to clot,and centrifuged to obtain serum.An O-lympus AU2700Automatic Analyzer is employed to measure the following parameters:Lactate dehydrogenase(LDH),alanine aminotransferase(ALT),aspartate aminotransferase(AST),alkaline phosphatase(ALP),creatine kinase(CK),glucose(Glu),blood urea nitrogen(BUN),creat-inine(Cr),total bile acid(TBA),and angiotensin converting enzyme(ACE).
The lungs are lavaged with a warmed physiological saline solution,and the bronchia alveolus lavage fluid(BALF)is collected.After being centrifuged,the supernatant is removed for biochemical studies,and the deposit cells are counted.All biochemical assays are performed on BALF using reagents purchased from Jiancheng Bioengineering Institute(Nanjing,China).Moreover,lactate dehydrogenase(LDH),alkaline phosphatase(ALP),malondialdehyde(MDA),and total protein(TP)are measured respectively.
1.5 Morphology
The lungs of mice exposed to TiO2nanotubes or room air are fixed with 10%formalin solution for tissue pathological examination.Sagittal sections of the lungs are made with a razor blade.Tissue blocks are dissected from left,right upper,and right lower regions of the lung and are subsequently prepared for examination by light microscopy (paraffin embedded,sectioned,and hematoxylin-eosin stained).
1.6 Statistical analysis
For analysis,each of the experimental values is compared with their corresponding control values for each time point.The differences between the groups are examined using the standard oneway analysis of variance(ANOVA).SPSS for Windows 13.0software package is used.P <0.05indicates statistical significance.
2 EXPERIMENTAL RESULTS
2.1 N-1TiO2nanotube types
XRD patterns for the N-1TiO2nanotubes and P25particles confirm their phase composition(Fig.1).P25particles are mixed-phase with anatase/rutile.The phase structure of TiO2nanotubes(N-1)is anatase at 400°C.
A TEM image of nano-TiO2after suspending in ethanol and sonicating for a period of time is shown in Fig.2.N-1TiO2nanotubes possess an average size of dlong=80nm and dwide= 10nm with a surface area of 349.76m2/g.

Fig.1 XRD patterns of P25and N-1nanotubes

Fig.2 TEM image of N-1TiO2nanotubes
2.2 Clinical chemistry
ALT and AST,as serum biochemical indexes,widely exist in liver,heart and other organs.Companied with serum TBA,they may indicate the degree of liver damage.In this study,their levels in mice consuming ultrafine TiO2nanotubes with 28dexposure are significantly different from control groups(P<0.05),which suggests that TiO2nanotube inhalation could cause damage to the liver.However,the damage does not appear during the first two periods(7dand 14d).The index values are shown in Table 1.
The level of serum Glu can indicate the state of the pancreas.In this study,Glu values only with 7dexposure are greatly higher than those of the control group(P<0.05).Thus it can be concluded that inhalation of TiO2nanotube probably has an impact on the pancreas in the initial stage of test(7d).
CK is the most sensitive indicator of muscle damage,and increased levels of CK indicate either an acute muscle necrosis or a sudden change in the permeability of the sarcolemma .Serum CKvalues are obviously higher than those of the control group(P<0.05)with 28dexposure,which demonstrate that it is possible for TiO2nanotube to cause injuries on muscle.

Table 1 Summary of statistically significant clinical chemistry indexes(mean value±standard deviation)
There are no obvious changes observed in other serum biochemical indexes among the control and experimental groups(Table 1).
Analyses of serum biochemical indexes indicate that exposures to TiO2nanotubes produce time-dependent responses characterized by ALT and AST activities,Glu and TBA values,and CK levels.The extent of these time-dependent changes is deteriorated with the exposure time prolonged.Also,it is easily found that there is an obvious difference between the control group and the 28dexposure group.
2.3 Pulmonary bioassay study
The numbers of cells recovered by bronchoalveolar lavage from the lungs of TiO2nanotube exposed groups are greatly different from controls with 14dand 28dexposure(P<0.05).
Lactate dehydrogenase(LDH),a kind of intracellular enzyme,is used as an indicator of cell injury.Transient significant increase compared with room air controls LDH value in BALF is measured in the lungs of TiO2-nanotube exposed mice only with 14dexposure,but are not sustained throughout the whole 28dexposure(Table 2).
Malondialdehyde(MDA)is an index for lipid peroxidation and free radical generation.Levels of MDA in all groups consuming TiO2-nanotube greatly differ from those of control group (Table 2).
Increase of protein concentrations in BALF generally is consistent with enhanced permeability of vascular proteins into the alveolar regions,indicating a breakdown in the integrity of the alveolar-capillary barrier. Transient significant increase compared with controls total protein(TP)value in BALF is measured in the lungs of TiO2-nanotube exposed mice with 14dexposure,but is not sustained to 28d(Table 2).
ALP activity is a measure of Type II alveolar epithelial cell secretory activity,and increased ALP activity in BALF is considered to be an indicator of Type II lung epithelial cell toxicity.No significant increase of alkaline phosphatase(ALP)values is measured in BALF among any of the nanotube-exposed groups when compared with room air control(data not shown).
2.4 Lung morphology
Histopathological analyses of lung tissues reveal that pulmonary exposures to TiO2nanotubes in mice produce some adverse effects when compared with control with 7d,14d,and 28dexposure.The micrograph for 7dillustrates the fusion of pulmonary alveoli along with some occasionaltissue thickening(Fig.3(a),with a magnification of 10),and just few TiO2nanotubes can be found in the interstitium of pulmonary alveoli(Fig.3(b),with a magnification of 40).Also,the micrograph for 14d illustrates the fusion of pulmonary alveoli along with some occasional tissue thickening (Fig.4(a),with a magnification of 10),but there are more TiO2nanotubes found in the interstitium of pulmonary alveoli(Fig.4(b),with a magnification of 40).The fusion of pulmonary alveoli is evident in Fig.5(a)(with a magnification of 10),but the tissue thickening is relaxed compared with the former(Fig.3(a)and Fig.4(a)),meanwhile,it is easily found that many TiO2nanotubes accumulated in the interstitium of pulmonary alveoli(Fig.5(b),with a magnification of 40).

Table 2 Numbers of cells recovered in BALF,LDH,MDA,and TP values for mice exposed to TiO2nanotubes and corresponding controls for 7,14,28d
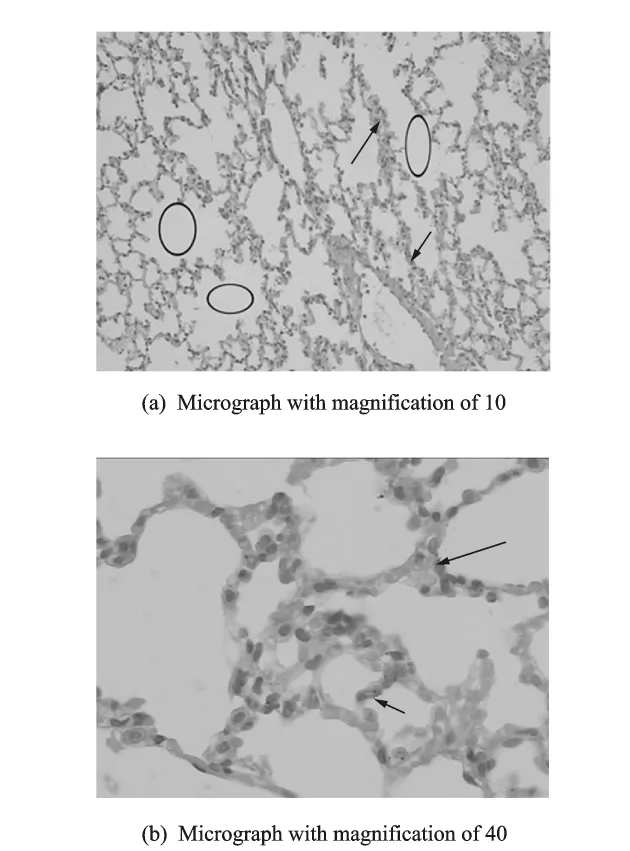
Fig.3 Light micrograph of lung tissue from a mouse exposed to TiO2nanotubes for 7d
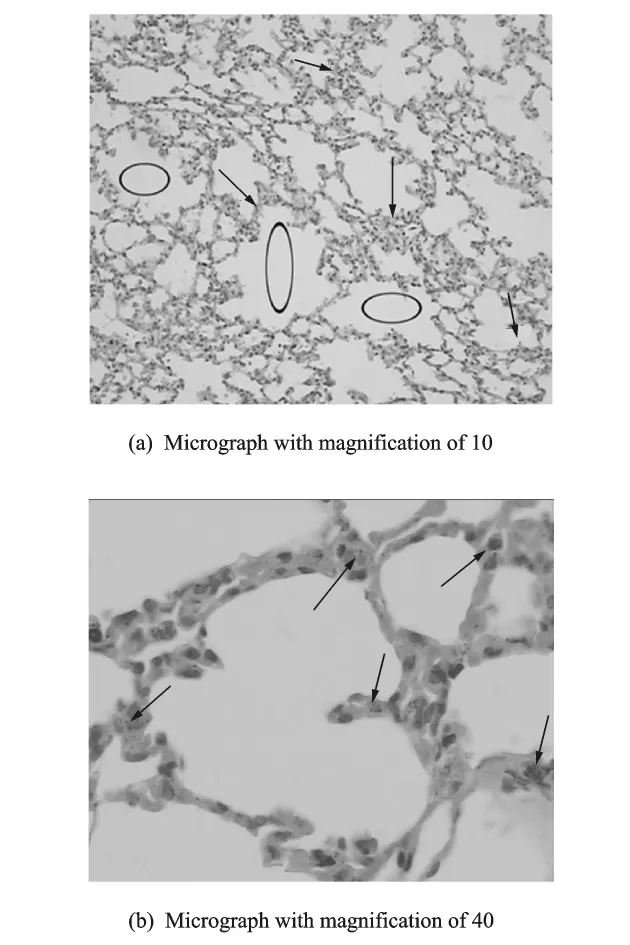
Fig.4 Light micrograph of lung tissue from a mouse exposed to TiO2nanotubes for 14d


Fig.5 Light micrograph of lung tissue from a mouse exposed to TiO2nanotubes for 28d
3 DISCUSSIONS
It is known that nanotube is different from nanoparticle in some ways,such as size,surface area(inner surface and outer surface),accumulation and other physical and chemical properties.The objective of this study is to assess the acute lung toxicity and the effect on serum biochemical indexes of inhaled TiO2nanotubes in mice.Using pulmonary and serum bioassay methodology,the toxicity and inhaled TiO2nanotubes are compared with room air control.
Analyses of serum biochemical indexes indicate that exposures to TiO2nanotubes produce time-dependent responses,for the changes of the indexes are deteriorated with the experimental time prolonged.Also,the analyses of BAL fluid demonstrate that the pulmonary exposures to TiO2nanotubes produce reverse lung responses to the mice,but as the experimental time prolonged to 28d,the indexes including LDH and TP are not obviously different from those of the control.
Accordingly,based on the results of the two kinds of analyses,it can be concluded that longterm exposure to TiO2nanotubes produce a more severe influence to serum biochemical indexes,but a moderate reverse response to pulmonary inflammation compared with short-term exposure and room air exposure control.
In previous studies[23-26],mouse models of inhalation were established that groups of mice were exposed to the test atmospheres for a fixed period.Instead of being killed immediately after the exposure,the mice were still kept alive for several different days as recovery periods,for example,24h,1week,1or/and 3months.In order to determine the exposure-time/response intensity,another model is conducted in this study.Mice are exposed to TiO2aerosols and room air continuously over 7,14,28d,and killed at each time point without recovery periods.
However,the model difference is not the only variable in these studies,it can be noted that the form of the TiO2(i.e.nanoparticles,nanorods and nanotubes)and the preparation methods in these studies are also different.
Earlier inhalation studies on TiO2ultrafine particles[23,26]focused on pulmonary inflammation through analyses in BAL fluid and Lung morphology.This study emphasizes on effects of inhaled TiO2nanotubes on serum biochemical indexes as well as pulmonary toxicity.The results show that the values of ALT,AST,TBA and CK increase with the prolonging of time,which indicates that the inhalation of TiO2nanotubes has caused damage to the heart,liver,and other organs with 28 d exposure.From the histopathological analyses,it is clearly found that many inhaled TiO2nanotubes accumulate in the interstitium and largely gain access to the pulmonary interstitium on the 28th d exposure.
4 CONCLUSION
In this papaer,using apulmonary bioassay and analysis in serum biochemical indexes,the acute lung toxicity and effects on serum biochemical indexes of inhaled TiO2nanotubes are compared with control group,room air exposure.TiO2nanotubes are used with an average size of dlong=80nm,dwide= 10nm and a surface area of 349.76m2/g.The experimental results presented herein indicate that mice exposed to these TiO2nanotubes show little response to the 7th day inhalation exposure,as the exposure time prolonged,several serum indexes,like ALT,AST,TBA and CK,are all changed greatly compared with those of the control group,and mice exposed for 28dshow a little more serious adverse effects.However,from the pulmonary bioassay,it seems that inhaled TiO2nanotubes produce less damage to the mice exposed for 28d,whereas it is unclear why the results analyzed by the two methods differ from each other with the experimental time prolonged.In this regard,we still presume that if the people engaging in the work concerning TiO2nanotubes (or TiO2nanoparticles)could have a day more for holiday in a week,which could relax the responses of TiO2nanotubes(or particles)produced to them.
[1] Donaldson K.Nonneoplastic lung responses induced in experimental animals by exposure to poorly soluble nonfibrous particles [J].Inhalation Toxicology,2000,12(1/2):121-139.
[2] Bermudez E,Mangum J B,Asgharian B,et al.Longterm pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles [J].Toxicological Sciences,2002,70(1):86-97.
[3] Hext P M.Current perspectives on particulate induced pulmonary tumours [J].Human & Experimental Toxicology,1994,13(10):700-715.
[4] Paul M H,John A T,Peter T.Titanium dioxide:Inhalation toxicology and epidemiology[J].The Annals of Occupational Hygiene,2005,49(6):461-472.
[5] Tessa M S,Ronny V A,Blair D J,et al.High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function[J].Toxicological Sciences,2009,109(2):372-380.
[6] Fujishima A,Honda K.Electrochemical photolysis of water at a semiconductor electrode[J].Nature,1972,238:37-38.
[7] Gelis C,Girard S,Mavon A,et al.Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models[J].Photodermatology,Photoimmunology &Photomedicine,2003,19(5):242-253.
[8] Lee K P,Trochimowicz H J,Reinhardt C F.Pulmonary response of rats exposed to titanium dioxide(TiO2)by inhalation for two years[J].Toxicology and Applied Pharmacology,1985,79(2):179-192.
[9] Lomer M C,Thompson R P,Powell J J.Fine and ultrafine particles of the diet:Influence on the mucosal immune response and association with Crohn′s disease[J].The Proceedings of the Nutrition Society,2002,61(1):123-130.
[10]O′Regan B,Grätzel M.A low-cost,high-efficiency solar cell based on dye-sensitized colloidal TiO2films[J].Nature,1991,353:737-740.
[11]Parida K M,Nruparaj S.Visible light induced photocatalytic activity of rare earth titania nanocomposites[J].Journal of Molecular Catalysis A:Chemical,2008,287(1/2):151-158.
[12] Wang Y P,Wang L J,Peng P Y.Photocatalytic degradation of L-acid by TiO2supported on the activated carbon[J].Journal of Environmental Sciences,2006,18(3):562-566.
[13]Jing D W,Tang W D,Xing C J,et al.Study on photocatalytic hydrogen production in simulated organic pollutants over cadmium sulfide composite photocatalyst[J].Journal of Fuel Chemistry and Technology,2011,39(2):135-139.
[14]Teles F S.Biosensors and rapid diagnostic tests on the frontier between analytical and clinical chemistry for biomolecular diagnosis of dengue disease:A review [J].Analytica Chimica Acta,2011,687(1):28-42.
[15]Li X D,Zhang D W,Sun Z,et al.Metal-free indoline-dye-sensitized TiO2nanotube solar cells[J].Microelectronics Journal,2009,40(1):108-114.
[16]Qamar M,Yoon C R,Oh H J,et al.Preparation and photocatalytic activity of nanotubes obtained from titanium dioxide[J].Catalysis Today,2008,131(1/4):3-14.
[17]Garabrant D H,Fine L J,Oliver C,et al.Abnormalities of pulmonary function and pleural disease among titanium metal production workers.[J].Scandinavian Journal of Work,Environment & Health,1987,13(1):47-51.
[18]Trochimowicz H J,Lee K P,Reinhardt C F.Chronic inhalation exposure of rats to titanium dioxide dust[J].Journal of Applied Toxicology,1988,8(6):383-385.
[19]Heinrich U,Fuhst R,Rittinghausen S,et al.Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust,carbon black,and titanium dioxide[J].Inhalation toxicology,1995,7(4):533-556.
[20]Warheit D B,Webb T R,Sayes C M,et al.Pulmonary instillation studies with nanoscale TiO2rods and dots in rats:Toxicity is not dependent upon particle size and surface area [J].Toxicological Sciences,2006,91(1):227-236.
[21]Tomoko Kasuga, Masayoshi Hiramatsu,Akihiko Hoson,et al.Formation of titanium oxide nanotube[J].Langmuir,1998,14(12):3160-3163.
[22]Wang Qin,Tao Jie,Weng Lüqian,et al.Mechanism of hydrothermal preparation of TiO2nanotube[J].Journal of Nanjing University of Aeronautics & Astronautics,2005,37(1):130-134.(in Chinese)
[23]Bermudez E,Mangum J B,Wong B A,et al.Pulmonary responses of mice,rats,and hamsters to subchronic inhalation of ultrafine titanium dioxide particles[J].Toxicological Sciences,2004,77(2):347-357.
[24]Grassian V H,O′Shaughnessy P T,Adamcakova-Dodd A,et al.Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5nm [J].Environmental Health Perspectives,2007,115(3):397-402.
[25]Liu H T,Ma L N,Zhao J F,et al.Biochemical toxicity of nanoanatase TiO2particles in mice[J].Biological Trace Element Research,2009,129(1/3):170-180.
[26]Warheit D B,Brock W J,Lee K P,et al.Comparative pulmonary toxicity inhalation and instillation studies with different TiO2particle formulations:Impact of surface treatments on particle toxicity[J].Toxicological Sciences,2005,88(2):514-524.
杂志排行
Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- ENERGY-SAVING MATCHING STRATEGY AND EXPERIMENTAL STUDY OF PUMPING SYSTEM FOR TRUCK-MOUNTED CONCRETE PUMP
- NOVEL HIGH-SPEED FPGA-BASED FFT PROCESSOR
- SPATIAL REGULARIZATION OF CANONICAL CORRELATION ANALYSIS FOR LOW-RESOLUTION FACE RECOGNITION
- SOFT IMAGE SEGMENTATION BASED ON CENTER-FREE FUZZY CLUSTERING
- PREDICTION OF SURFACE ROUGHNESS FOR END MILLING TITANIUM ALLOY USING MODIFIED PARTICLE SWARM OPTIMIZATION LS-SVM
- EXPERIMENTAL INVESTIGATION ON R134AAIRBORNE VAPOR-COMPRESSION REFRIGERATION SYSTEM
