恒容吸收系统中水和稀硝酸对NO2的吸收过程研究
2013-10-17王晓旭张志炳
张 锋 王晓旭 袁 刚 耿 皎*,,2 张志炳,2
(1南京大学化学化工学院,南京 210093)
(2国家有机毒物控制与资源化工程技术研究中心,南京 210093)
The removal of NOxin industrial exhaust gas from nitric acid towers,nitration reactors and devices for fuel combustion has drawn extensive attention due to a stringent body of legislation for air pollution control and reduction[1-2].Nowadays,the technologies for the NOxremoval from exhaust gas mainly include reduction methods:SCR (Selective Catalytic Reduction),SNCR(Selective Non-Catalytic Reduction)and NSCR(Non-Selective Catalytic Reduction).These methods usually convert NOxto N2,while with the absorption methods,NOxcan be absorbed to produce nitric acid,nitrate or nitrite[2].Generally,the absorption of nitrogen oxides by water is very attractive,since no chemicals but water and air are required.Moreover,the obtained nitric acid can compensate for the process cost[3].The NOx absorption in water consists of complex mass-heat transfer and numerous reactions in both gas and liquid phases,thus systematic study is necessary for design and operation ofindustrialabsorption processes.Furthermore,since absorption of NO2in water is also an important step in the production of nitric acid which is one of the top bulk chemicals,various aspects of the NOx-water system have been studied extensively to explore the absorption mechanism of NOxin water[4].
A number of investigations on NOxabsorption in water,alkaline or nitric acid aqueous solutions have been performed in commonly used mass transfer devices[5-7].Based on the two-film theory for NOxabsorption with chemical reaction, the overall absorption rate of species J,which is enhanced by chemical reaction,is expressed with overall kinetic parameters(OKP):

OKP for N2O4has been summarized by Patwardhan and Joshi[4].It was found that HP for N2O4decreases with the increasing of nitric acid concentration,while alkali concentration has little influence on the value of HP for N2O4.
Dekker et al.[3]has investigated the mechanism of the absorption of NO2-N2O4from nitrogen into water with a wetting wall column and measured the absorption rate of NO2in water.Lee et al.[8-9]studied absorption of NOxin packed column and derived a mathematical model which matches well with the experimental results.Based on the double-film theory,Yu et al.[10]developed a mathematic model,by which the removal rate,outlet gas concentration,oxidation degree of NOx,outlet acid concentration,and liquid temperature could be calculated in structured packing columns under an adiabatic condition.In 2003,a unified model for NOxabsorption in aqueous alkaline and dilute acidic solutions(over a wide range of pH value 3~14)was derived and compared with data from patent on Ca(NO2)2manufacture[4].Chien et al.[11]studied the absorption kinetics of nitrogen oxides in twin spray columns where insoluble NO was first oxidized underacidic conditions,then NO2was removed in alkaline NaClO2solution.Alongside the investigation of mechanism or model for the common devices,some works were focused on the new-type of absorption equipment.In 2011,Yasuda et al.[12]carried out high-efficiency NOxabsorption in water using equipment packed with a glass fiber filter.
Besides the above works on the absorption of NOxin the column,detailed studies on the kinetics and mechanism have been performed for the uptake of NOxby water.Heterogeneous interactions of NO2with aqueous surfaces were studied in both the droplet train and bubble train flow reactors by Cheung et al.[13],and the values of Henry′s law coefficient for NO2and the second-order hydrolysis reaction rate coefficient were estimated.
In the actual industrial production,the largest emissions of nitrogen oxides present in the form of nitric oxide and nitrogen dioxide.Since nitric oxide can easily react with oxygen to produce nitrogen dioxide,nitrogen oxides will mainly be nitrogen dioxide if a certain amount of oxygen exists in the exhaust gas.It has been proved that the absorption rate is controlled by the chemical reaction between N2O4and water in the liquid phase,since one N2O4molecule can hit one water molecule more easily than two NO2molecules do[3].
The absorption of NOxis one of the most complex gas liquid operations with numerous reactions between several species,thus strict and exact mathematics models for the absorption process are hard to be obtained.Though the absorption of NOxin water has been studied for a quite long time,the reactions and the interface mass transfer are still unclear.With the aid of modern measuring apparatusand technique,the temperature,pressure and composition of two phases during the absorption can be determined rapidly.In the present work,the absorption of NOxin water and nitric acid solution is carried out in a constant-volume absorption system.The pressure variations of gas phase are continuously measured to indicate the absorption degree.Once the compositions of the solution and the gas phase are determined,the influences of nitric acid concentration,absorbent weight and the initial pressure on the absorption performance and liquid product can be discussed to find suitable condition for NOxremoval.Moreover,a calculation relationship based on the nitrogen balance and reactions was also suggested to estimate the gas composition from gas pressure and liquid composition.
1 Experimental
The absorption of NO2was carried out with a constant-volume absorption system widely used in the researches on the absorption of acid gases(e.g.CO2)[14].Fig.1 shows the schematic diagram of gas absorption apparatus.The experimental procedure was as follows:absorbent of a known weight was placed in the absorption vessel(39.6 mL)and degassed by vacuum pump for 2 h;then close valve 1 and valve 4,open valve 2 and transfer gas into the storage vessel(120.0 mL)until the pressure reached a scheduled value;close valve 2 and valve 3,open valve 4 to transfer gas from storage vessel to absorption vessel where the absorbent wasstirred magnetically.In the experiment,the temperature of the storage vessel and absorption vessel was maintained at a certain value with a large thermostatic water bath,thus the absorption was performed thermostatically with the quick removal of the reaction heat.The gas pipe was maintained at 298 K to keep NO2gaseous.NO2of different pressure was absorbed in water with different weights to explore the effect of water amount and gas pressure on the absorption efficiency and absorption product.3wt% ~15wt%nitric acid aqueous solutions were also used as absorbents to evaluate the influence of nitric acid concentration on theremovalofNOx.Afterthe absorption,liquid samples were taken to measure the amount of HNO3by photometry,and the sum of HNO2and HNO3was determined by chemical titration.

2 Results and discussion
2.1 Reactions
The absorption of NOxin pure water or nitric acid is a kinetically controlled process in which complex reactions are combined with multi-components mass and heat transfer in both gas and liquid phases[7].In gas phase,the following reactions may occur:

Since there is no O2in the system,NO cannot be oxidized.The equilibrium relationships for Equation(2)and(3)could be expressed as follows:

where,the equilibrium constants has been reported by Joshi et al.[15]:

According to Equation(6)and(7),the values of K1and K2are 0.064 and 0.005 1 at 298 K,respectively.Since NO concentration is quite small in the gas phase and very little N2O3can be produced under the experimental condition,N2O3could be neglected in the gas phase.Thus,the essential chemical reactions accompanied the absorption of NO2in water are as follows:


The overall reaction can be represented as:

And the solubility of gas in the solutions can be described as:

where,i=NO,NO2,N2O4and N2O3.piand cistand for the partial pressure and the liquid concentration of the species i,respectively.The whole pressure of the gas is given as:

Since the pressure in the present work is under normal atmospheric pressure,the state equation of ideal gas can be used to describe the gases:

where Vgrepresents the volume of gas and nithe amount of the species i.The liquid concentration cican be calculated by Equation(15):

Substituting Equation(14)and(15)into Equation(12)gives:

where,m is a parameter relative to absorption condition.
The whole nitrogen balance is as follows:
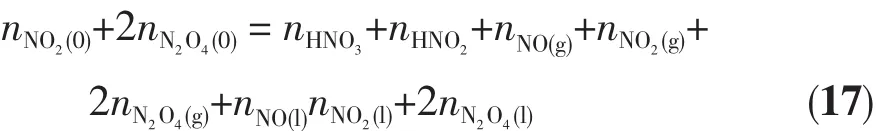
Taking into account Equation(16),Equation(17)can be rewritten as:

Setting the consumed amount of N2O4as s and the decomposing fraction of HNO2as a,there are the following relationships:

The initial pressure of the whole gases,P0,is calculated by the following equation:
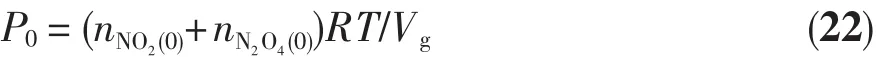
Then,Equation(4)can be rewritten as:

By solving Equation(22)and(23),the value nNO2(0)and nN2O4(0)could be obtained.Then,since nHNO3and nHNO2could be measured by photometer and titration,the value of nN2O4(g),nNO2(g)and nNO(g)could be obtained by solving Equation(18)~(23).
2.2 Absorption of NO2in water
The absorption of NO2in water is the primary process for NOxabsorption in aqueous solutions.For the design of removal process device,the influences of NO2partial pressure and water weight on the absorption performance are required. Furthermore, the compositions of absorbed solution underdifferent absorption conditions need to be measured to choose the optimal operation condition for nitric acid production.In the presentwork,the absorption performance of NO2in water would be analyzed by measuring gas pressure variation and solution compositions.
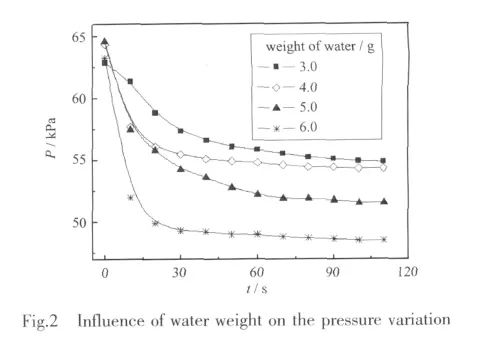
The effect of water amount on the variations of the gas pressure is illustrated in Fig.2.The gas pressure drops quickly at the beginning,then the pressure drop becomes slowdown and the pressure eventually approaches constant.It should be noted that with the augment of water weight,the absorption rate at the beginning of the absorption and the absorbed amount of the gases are all increased,while the equilibrium time is shortened.Moreover,the pressure in the constantvolume system rises a little,since the reactions between NOxand water are exothermic and the increase of the liquid temperature will cause a rise in gas temperature eventually.Eventually,the temperature of liquid and gas drops to the scheduled temperature due to the recycling constant temperature system.It should be noted that the gas phase consists of NO2,N2O4,NO and very little N2O3,thus the pressure drop indicates the amount changes of these three species.
According to the total amount of nitrogen element in the initial gas phase and the nitrogen amount in the liquid obtained by the titration,the absorption efficiency is defined by Equation(24):

where nlstands for the total amount of nitrogen element absorbed in the liquid.Obviously,n0g=nNO2(0)+2nN2O4(0).As shown in Fig.3,the absorption efficiency η rises with an increase of water weight.The nitric acid concentration of obtained solution increases first(when water weight is less than 4 g)and then drops with the further increase in water weight,showing a conflict impact of water weight on the absorption product.Obviously,η is less than 40%for the absorption of pure NO2,showing limited removal of NOxby water.On the other hand,nitric acid concentration of the solution cannot exceed 3%when P0is less than 65 kPa.

According to the reactions of water and NOx,the absorbed solution is usually made of nitric acid,nitrous acid and dissolved NOx,and the sum of them is the amount of total N absorbed.As displayed in Fig.4,the amount of total N absorbed andboth increase with a rise in water weight,and the difference between them also increases,indicating that more nitrous acid exists in the dilute nitric acid aqueous solutions.On the hand,the amount of NO3-rises slightly when water weight is higher than 4 g,thus nitric acid concentration drops as shown in Fig.3.

The absorption of NO2in 3 g water with P0ranging from 60 to 140 kPa is illustrated in Fig.5.The pressure decreases rapidly at the beginning of the absorption,and then keeps constant when time is over 1 min.It can be seen that the time required for the absorption equilibrium is higher for smaller P0.It can be concluded from Fig.2 and Fig.5 that the equilibrium time is less than 1 min for the absorption of NO2in water when P0is larger than 60 kPa.


The variations of η and nitric acid concentration(cnitric)with P0have the similar trend.As shown in Fig.6,in 3 g water,η and cnitricrise dramatically with the increase of P0when P0<100 kPa.With a further increase of P0,the increase of η and cnitricbecomes moderate,and variation of η is smaller when P0>100 kPa.Noticeably,cnitricreaches 6%when P0approaching 140 kPa.It can be seen in Fig.7 that the amount of total N absorbed andboth increases linearly with a rise in P0.The amount of total N raises faster than,indicating that more nitrous acid and NOxare dissolved in the nitric acid aqueous solutions under higher initial pressure.As shown in Fig.6 and Fig.7,increasing the partial pressure of NOxgenerally leads to a higher concentration of HNO3and also the increase of impurity content(nitrous acid and NOx).In that sense,increase in P0alone cannot obtain a qualified nitric acid for the industrial use.
2.3 Absorption of NO2in HNO3aqueous solutions
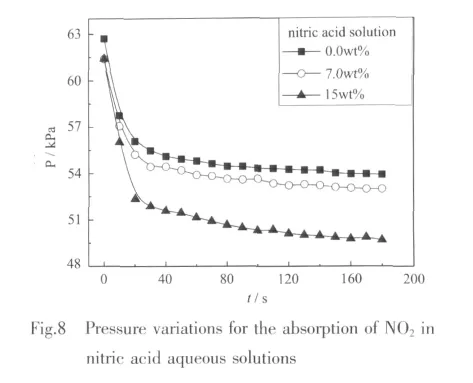
In general,solubility of NOxin nitric acid aqueous solutions is higher than that in water[16],thus HNO3aqueous solutions are also used toabsorb NOx,especially for the case of exhaust gas with high concentration of NOx.The absorption performances of NO2in 4 g water and nitric acid solutions(7.0wt%and 15.0wt%)are compared in Fig.8.The pressure drops rapidly with the absorption going on when t is less than 40 s,then with the time going on pressure drop becomes slowdown and the pressure approaches constant ultimately during the absorption of NO2in nitric acid solutions.Obviously,more NO2could be absorbed in the solution with higher concentration of nitric acid.
Absorption of NO2in 4 g nitric acid aqueous solutions under different initial pressure P0is depicted in Fig.9.Similar to the case of the absorption in water,for 3%,7%and 15%nitric acid solutions,the amount of total N absorbed andboth increase with a rise in P0.Furthermore,the higher P0is,the larger the difference between the amounts of total N and.It can be found in Fig.9 that the amount of total N absorbed andis higher for aqueous solution of nitric acid with higher concentration,while the gap between amounts of total N absorbed andnarrows due to the decomposition of HNO2.It should be pointed out that the original amount of nitric acid in the aqueous solution has been eliminated from the amount of

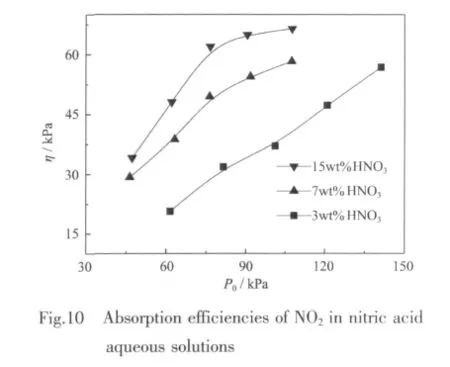
As shown in Fig.10,the absorption efficiencies of NO2in three nitric acid aqueous solutions all increase with a rise in P0.For the same P0,the higher nitric acid concentration inducesthe higher η.Forhigher percentage of nitric acid concentrations,the absorption efficiency η increases with P0linearly when P0is less than 70 kPa.When P0increases further,η gradually rises and the curves tend to go flat.Comparison between Fig.6 and Fig.10 shows that the absorption efficiency of NOxin nitric acid solutions is much higher than that in water under the same initial pressure.
2.4 Calculation of the absorption performance of NO2in water
By solving Equation(18)~(23),nN2O4(g),nNO2(g),nNO(g),and the decomposed fraction of HNO2could be obtained to evaluate the absorption performance.The decomposed fraction of HNO2is illustrated in Fig.11 for absorption of NO2in 3 g water under different P0.The decomposed fraction of HNO2slightly varies when P0<90 kPa,and with a further increase of P0,the decomposed fraction drops rapidly from 0.8 to 0.55 until P0of 120 kPa,then the decomposed fraction of HNO2is unchangeable when P0>120 kPa.This means that more HNO2exists in the liquid product.Fig.12 shows that the calculated data is a little higher than the experimental data for the equilibrium pressure Pe.The deviationsbetween the calculated data and the experimental one decrease with a rise in P0.Noticeably,Pe,the absorption equilibrium pressure can be easily measured,and thus the gas composition can be determined by the calculation when the liquid composition is measured.

When P0is set to 64 kPa with water weight ranging from 3~6 g,it can be seen in Fig.13 that the amount of N2O4consumed gradually rises with an increase in water weight.The variation for the decomposed fraction of HNO2is quite different.With the increase in water weight,the decomposed fraction of HNO2significantly raises until water weight of 5 g.Then with a further increase in water weight,the decomposed fraction of HNO2drops dramatically.

3 Conclusions
Conversion of NOxinto nitric acid is a feasible way to solve the serious environmental pollution problems caused by exhaust NOxgases from industrial processes.For the absorption of NO2in water,with the increasing of water amount,the absorption efficiency increases,and the concentration of nitric acid obtained rises first and then drops.On the other hand,with a rise in P0,the absorption efficiency and the concentration of nitric acid both increase fast when P0<100 kPa and then the increase becomes moderate with a further increase of P0.Increasing the partial pressure of NOxgenerally leads to a higher concentration of HNO3and also the increase of impurity content(nitrous acid and NOx).The absorption of NO2in dilute nitric acid was also studied.It is found that more NO2could be absorbed in the solution with higher concentration of nitric acid.Moreover,larger P0and higher concentration of nitric acid both lead to a significant rise of the absorption efficiency.Through solving the equations,nN2O4(g),nNO2(g),nNO(g),and the decomposed fraction of HNO2could be obtained.The deviations between the calculated Peand experimental one decrease with a rise in P0.This means that the gas phase composition could be calculated through measuring total pressure and liquid phase compostipon.
Acknowledgement:The authors are grateful for the financial support from:National Natural Science Foundation of China(No.20906046)and Natural Science Foundation of Jiangsu Province(No.BK2009043).
Nomenclature:
Roman
cnitirc:mass percent of nitric acid
H:Henry constant,mol·L-1·Pa-1
K1:equilibrium constant for Equation(2)
K2:equilibrium constant for Equation(3)
OKP:the overall kinetic parameter
P:pressure,Pa
RJ:rate of mass transfer of component J,accompanied by chemical reaction,kmol·m-2·s-1
HJ:Henrys constant for component J,mol·L-1·Pa-1
K:equilibrium constant
DJ:diffusivity of component J in the liquid phase,m-2·s-1
PJI:partial pressure of component J at the interface,Pa
Greek symbols
η:absorption efficiency
Subscripts
0:initial
g:gas
l:liquid
J:of component J
water:water
nitric:nitric acid
[1]Decanini E,Nardini G,Paglianti A.Ind.Eng.Chem.Res.,2000,39(12):5003-5011
[2]Li Y,Liu Y,Zhang L,et al.Chinese J.Chem.Eng.,2010,18(2):244-248
[3]Dekker W A,Snoeck E,Kramers H.Chem.Eng.Sci.,1959,11(1):61-71
[4]Patwardhan J A,Joshi J B.AIChE J.,2003,49(11):2728-2748
[5]Jethani K R,Suchak N J,Joshi J B.Comp.Chem.Eng.,1992,16(1):11-25
[6]Suchak N J,Jethani K R,Joshi J B.AIChE J.,1991,37(3):323-339
[7]Hupen B,Kenig E Y.Chem.Eng.Sci.,2005,60(22):6462-6471
[8]Lee H K,Chun K S,Park H S,et al.Korean J.Chem.Eng.,1989,6(4):294-299
[9]Lee H K,Jeong M S,Park J W,et al.Korean J.Chem.Eng.,1990,7(1):13-17
[10]YU Jin-Yang(于景阳),HAN Li-Guo(韩莉果),ZHANG Wei-Jiang(张卫江),et al.J.Tianjin University(Tianjin Daxue Xuebao),2005,38(9):780-785
[11]Chien T W,Chu H,Li Y C.Water Air Soil Pollu.,2005,166(1/2/3/4):237-250
[12]Yasuda M,Tsugita N,Ito K,et al.Environ.Sci.Technol.,2011,45(5):1840-1846
[13]Cheung J L,Li Y Q,Boniface J,et al.J.Phys.Chem.A,2000,104(12):2655-2662
[14]Zhang F,Fang C G,Wu Y T,et al.Chem.Eng.J.,2010,160(2):691-697
[15]Joshi J B,Mahajani V V,Juvekar V A.Chem.Eng.Commun.,1985,33(1/2/3/4):1-92
[16]LI Yu-Ping(李玉平),GUO Chun-Mei(郭春梅),SANG Xiao-Hong(桑小红).Acta Armamemtarii(Binggong Xuebao),2000,21(2):158-160