酰胺型配体的镍ギ和锌ギ配合物的合成、晶体结构及荧光性质
2013-10-17吴伟娜李飞飞董幼青2
吴伟娜 王 元*, 李飞飞 董幼青2
(1河南理工大学物理化学学院,焦作 454000)
(2温州大学化学与材料工程学院,温州 325027)
The amide type ligands have nitrogen and oxygen as electron donor atoms,and they are effective chelating agents that can form complexes with different metal ions.Usually,such ligands have strong antenna effect to Euバ or Tbバ ion due to their flexible structure which could shield the encapsulated ion from interaction with the surroundings[1-3].Recently,we have also found that some acetamide ligands bearing quinolinyloxy unit could transfer energy to Znギion and enhance the fluorescence emission intensity effectively[4].Thus,in this work,nickelギ and zincギcomplexes containing an amide type ligand were synthesized and characterized by X-ray diffraction.In addition,the fluorescence spectra of the compounds in CH3CN solution were investigated.
1 Experimental
1.1 Physical measurement
Solvents and starting materials for synthesis were purchased commercially and used as received.Elemental analyses were carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1)were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.The UV spectra were recorded on a Shimadzu UV-240 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipsespectrophotometer.In the measurements of emission and excitation spectra the pass width is 2.5 nm.
1.2 Synthesis of the complexes
The ligand nbqa[5](0.293 3 g,1 mmol)was dissolved in acetonitrile (10 mL),then an acetonitrile solution(10 mL)containing Ni(NO3)2·6H2O(0.291 1 g,1 mmol)was added dropwise at room temperature.After stirring for 2 h,the mixture was filtered and set aside to crystallize at room temperature.The product 1,which was collected by filtration,washed with Et2O and dried in air.Some of the obtained crystals were suitable for a single crystal X-ray analysis.The synthesis of 2 is similar to that of 1.1:Green Crystals.Yield 37%.Anal.calcd.for C18H20N4O10Ni(%):C,42.30;H,3.94;N,10.96.Found(%):C,42.58;H,3.67;N,11.20.2:Colorless Crystals.Yield 53% .Anal.calcd.for C18H20N4O10Zn(%):C,41.76;H,3.89;N,10.82.Found(%):C,41.89;H,3.57;N,10.90.
1.3 X-ray crystallography
The crystals were put on Bruker SMART APEXⅡ CCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[6].The structure was solved by direct methods and refined by fullmatrix least-square on F2using the SHELXTL-97 program[7].H atoms for the water molecules are located from difference Fourier map and refined with restraints in bond length and thermal parameters.All the other H atoms were positioned geometrically and refined using a riding model.A summary of crystal data and details of the structure refinements are listed in Table 1.
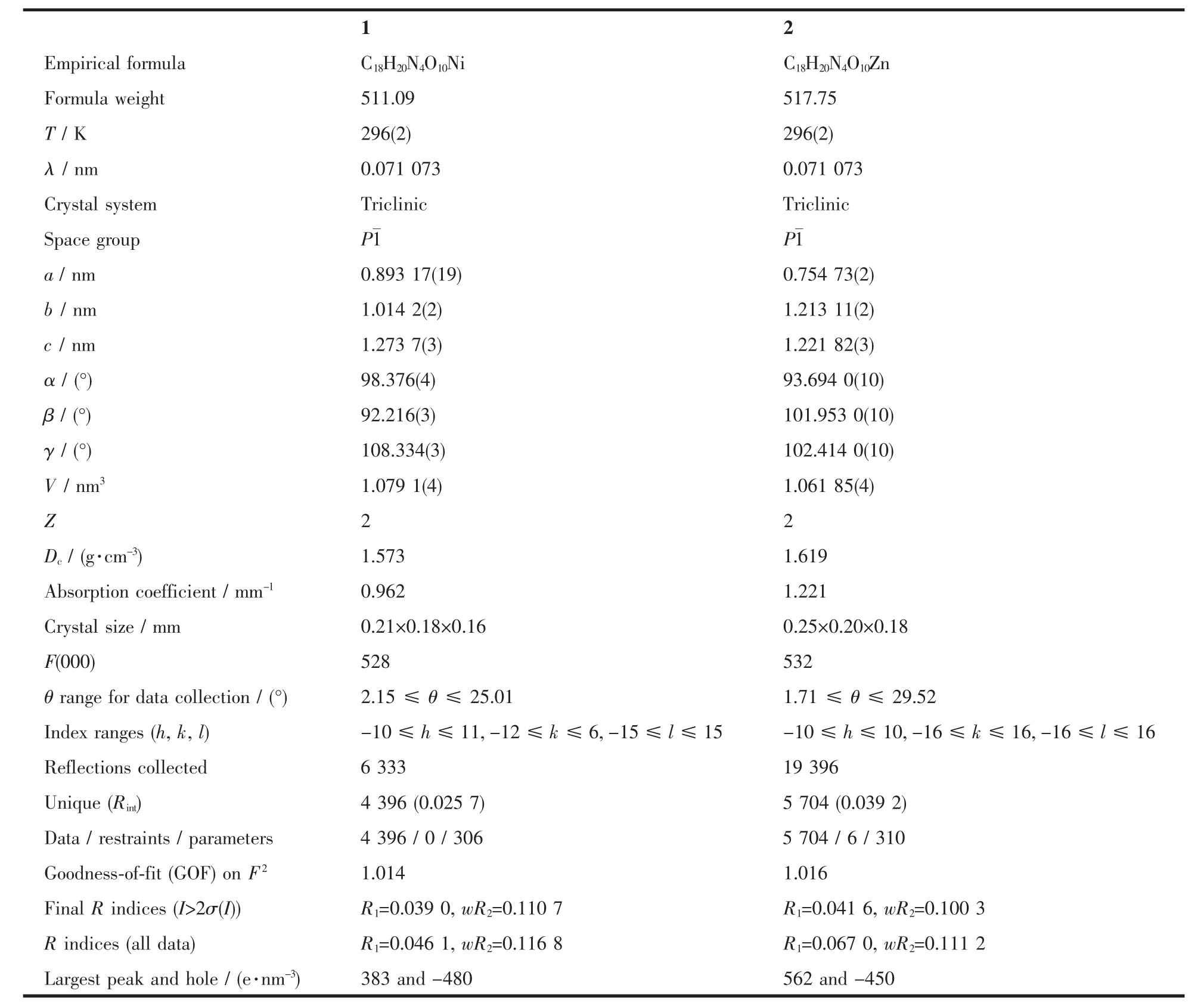
Table 1 Crystal data and structure refinement for the complexes
CCDC:838270,1;838271,2.
2 Results and discussion
2.1 Crystal structure of the complexes
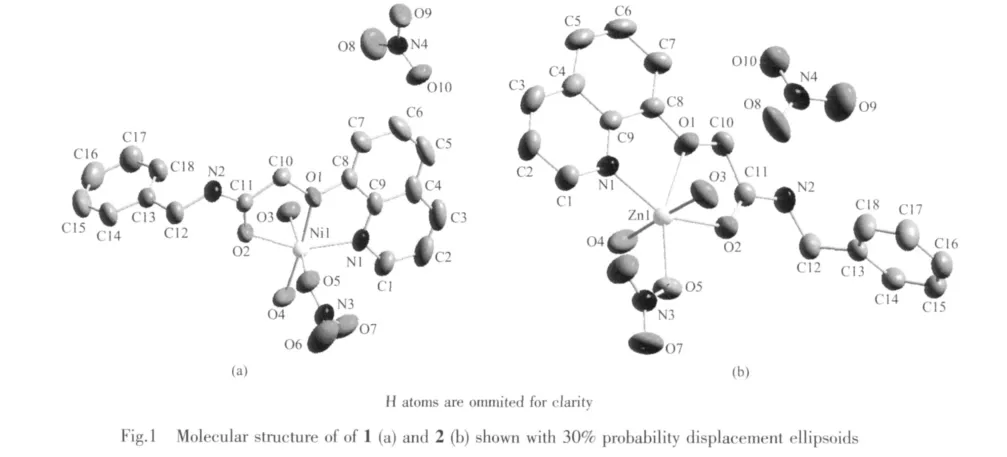
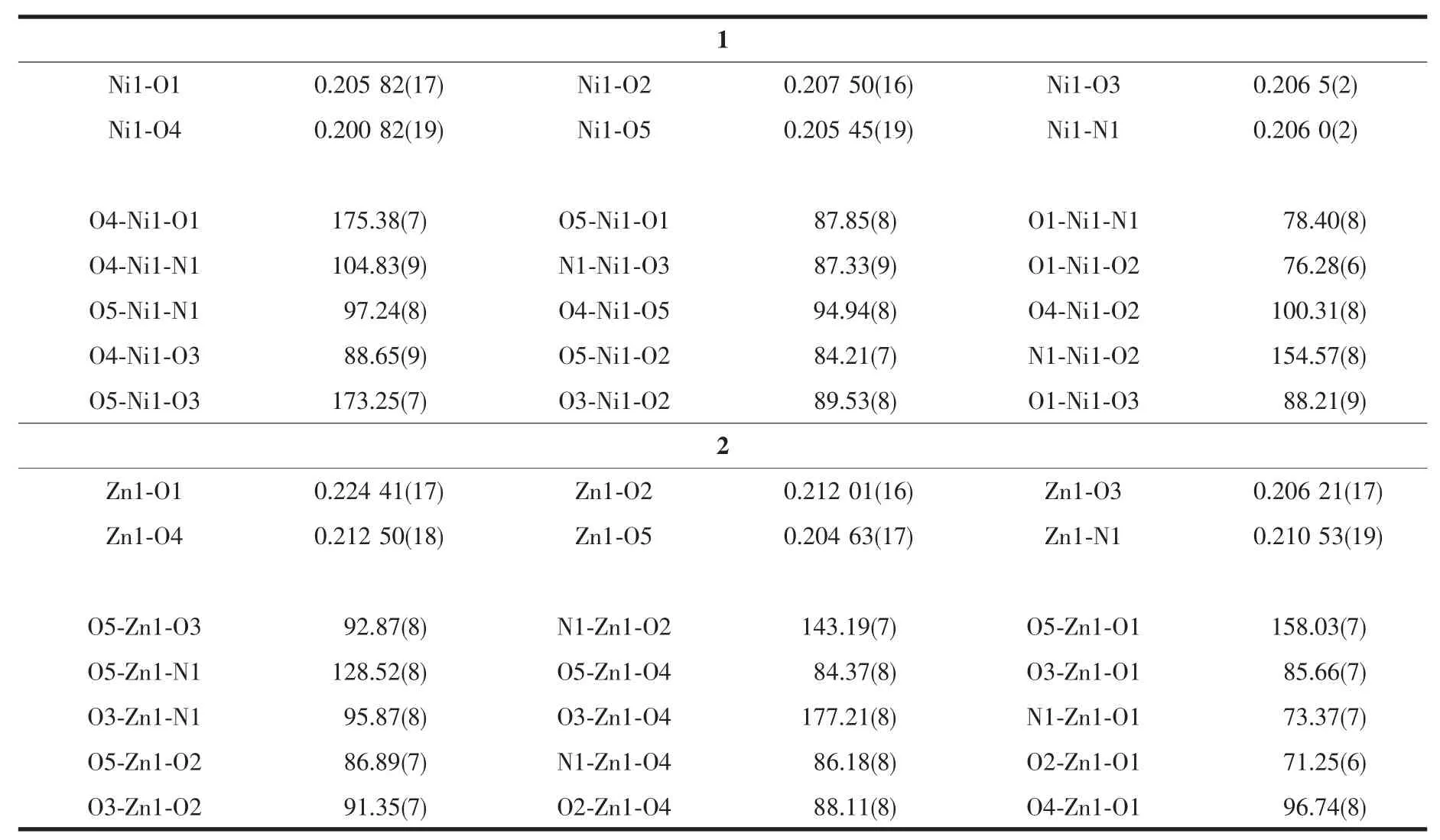
Table 2 Selected bond lengths(nm)and angles(°)in the complexes

Table 3 Selected hydrogen bonding parameters in 1 and 2
As shown in Fig.1,the asymmetric unit of each complex consists of a coordination cation and a free nitrate anion.The coordination behaviors of the metal ions in 1 and 2 are similar.The six-coordinated metal ion is in a distorted octahedral geometry with the donor centers of two O atoms and one N atom from theligand nbqa,twoO atomsfrom twowater molecules and one O atom from one nitrate anion.Selected bond lengths and angles are summarized in Table 2.The bond angles of O4-Ni1-O1,O5-Ni1-O3,O3-Zn1-O4 and O5-Zn1-O1 are 175.38(7)°,173.25(7)°,177.21(8)°and 158.03(7)°,respectively;confirming that in each complex,the four atoms O1 (from the ligand),O3,O4 (from two water molecules)and O5(from nitrate group)are in the basal plane.The axial positions are occupied with N1 atom and O2 atom from the ligand nbqa.In both complexes,most bond angles are highly deviated from those of ideal octahedral geometry.Although the coordination behavior of the organic ligands is similar,the structures of 1 and 2 are different from those of[ZnL1(NO3)2]·CH3CN(L1=N-(naphthalen-1-yl)-2-(quinolin-8-yloxy)acetamide)[4]and[ZnL2(NO3)2(H2O)](L2=N-phenyl-2-(quinolin-8-yloxy)acetamide)[8].In the literature complexes,there are two monodentate nitrate anions.In addition,the Zn atom isfive-coordinated in the formerone and sixcoordinated in the latterone (additionalwater molecular taking part to the coordination).
In the crystals of 1 and 2,O-H…O hydrogen bonds between the coordinated water molecules and nitrate O atoms,and N-H…O hydrogen bond between the ligand and nitrate O atom are helpfulto consolidate the three-dimensional network(Table 3).
2.2 IR spectra
The major IR data of the compounds are shown in Table 4.Comparing with the ligand nbqa[5],the ν(C=O),ν(C=N)and ν(Ar-O-C)of complexes 1 and 2 shift by 1 and 5,39 and 19,35 and 33 cm-1,respectively;thus indicating that carbonyl,ethereal oxygen atom and quinoline nitrogen atom take part in coordination to the metal ions[4-5,9-11].The small shift in ν(C=O)is mainly due to the fact that the ligand nbqa has large sterically hindered effect,which prevents carbonyl oxygen atom from tight coordinating metal ions[4-5].For complexes 1 and 2,the aqueous ν(OH)bands appear at 3 354 and 3 370 cm-1,ρ(OH)bands at 622 and 615 cm-1respectively;showing that there are coordinative water molecules in the complexes.Additionally,bands at about 1 384 cm-1indicate that free nitrate groups (D3h)exist.The general pattern of the IR spectroscopy supports the normal coordinationof the monodentate nitrate group[12].It is in accordance with the result of the crystal structure study.

Table 4 Major IR data of the compounds(cm-1)
2.3 UV spectra
The UV spectra of the compounds in CH3CN solution (concentration:1 ×10-5mol·L-1)were measured at room temperature (Fig.2).The spectra of the ligand nbqa features one main band located around 296,two shoulders around 269 and 314 nm.The bands could be assigned to characteristic π-π*transitions centered on benzene,quinoline ring and the acetamide unit respectively[13].The complexes show similar spectra as that of the ligand nbqa.However,the changes of the absorbance intensity indicate that the ligand nbqa takes part in the coordination in the complexes.

2.4 Fluorescence spectra

The fluorescence intensity of1 in CH3CN solution is much too weak,it is not discussed in this work.The fluorescence spectra of the ligand nbqa and 2 in CH3CN solution (concentration:1×10-5mol·L-1)were measured at room temperature.The excitation wavelengths are at 332 and 344 nm,respectively.The emission peak of 2 is at 430 nm,but that of the ligand nbqa is at 390 nm (Fig.3).It also can be seen that the emission intensity of 2 is much higher than that of the ligand nbqa.This is probably due to the energy transferring from the ligand nbqa to the Znギion[14].The behavior of Zn2+coordinated to the ligand is in general as that of emissive species leading to a CHEF effect (chelation enhancement of the fluorescence emission).
[1]Song X Q,Wen X G,Liu W S,et al.J.Solid State Chem.,2010,183:1-9
[2]Sharma A K,Biswas S,Barman S K,et al.Inorg.Chim.Acta,2010,363:2720-2727
[3]Munjal M,Kumar S,Sharma S K,et al.Inorg.Chim.Acta,2011,377:144-154
[4]WU Wei-Na(吴伟娜),WANG Yuan(王元),TANG Ning(唐宁).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:425-428
[5]Wu W N,Yuan W B,Tang N,et al.Spectrochim.Acta A,2006,65:912-918
[6]Sheldrick G M.SADABS,University of Göttingen,Germany,1996.
[7]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[8]Wu W N,Wang Y,Zhang A Y,et al.Acta Cryst.E,2010,66:m288
[9]HU Xiao-Li(胡晓黎),FAN Li-Yan(范丽岩),YAN Lan(闫兰),et al.Chinese J.Applied Chem.(Yingyong Huaxue),2002,19:727-729
[10]JIANG Yi-Hua(江以桦),YANG Ru-Dong(杨汝栋),YAN Lan(闫兰),et al.J.Chin.Rare Earth Soc.(Zhongguo Xitu Xuebao),2002,20:474-477
[11]ZHOU Yu-Ping(周毓萍),YANG Zheng-Yin(杨正银),YU Hong-Jun(于红娟),et al.Chin.J.Applied Chem.(Yingyong Huaxue),1999,16:37-41
[12]Nakamato K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New York:John Wiley,1978:227
[13]Song X Q,Zang Z P,Liu W S,et al.J.Solid State Chem.,2009,182:841-848
[14]Wang Y,Yang Z Y.J.Lumin.,2008,128:373-376