油气生产中的冲刷腐蚀(一)
2013-10-16BaotongLu
Baotong Lu
(美国西南研究院材料工程部)
1 Background
Wit h a few exceptions,most metals owe t heir corrosion resistance to a protective surface fil m.E-rosive fl uids can da mage t he pr otective fil m,and re move s mall pieces of material as well,leading to a significant increase in penetration rate.For instance,carbon steel pipe carrying water is usually protected by a fil m of rust and its corrosion rates are typically <1 mm/y (or 40 mils/y).The removal of the fil m by erosive slurry gives corrosion rates of t he or der of 10 mm/y(400 mils/y)in addition to t he any er osion of underl ying metal[1]The damage to the pr otect fil m may be t he results of the fl uid-induced mechanical f orces or flowing-enhanced dissolution[2-3].Meanwhile,the corrosion can cause degradation in surface pr operties and promote the mechanical erosion under action of the mechanical f orces[4].This conjoint action of er osion and corrosion is kno wn as erosion-corr osion[5].er osion-corrosion enco mpasses a wide range of flow-induced corr osion[6].It is also regar ded as a subject within the broader area of tribo-corrosion which covers all aspects of tribol ogically(mainl y mechanically)induced interactions wit h electr ochemical pr ocesses[7].As su mmarized by Postlet hwaite and Nesic[6],t he sources of t he various mechanical f orces that cause erosion-corrosion include:
(1)Tur bulent flow,fl uct uating shear stress and pressure i mpacts.
(2)Impact of suspended solid particles.
(3)Impacts of suspended liquid dr oplets in high-speed gas flow.
(4)Impact of suspended gas bubbles in aqueous flow.
(5)The violent collapse of vapor bubbles f oll owing cavitation.
The five mechanical force sources mentioned above can be f ound in oil and gas pr oduction.The fl uids to induce erosion-corr osion may be single phase like the portable water or multiphase flows such as various combinations of gas,oil,water and solid particles in petr oleu m industry[8].It is well known t hat the t ur bulent fl ow,fl uct uating shear stress and pressure i mpacts are sources of flow accelerated corrosion in pipelines transporting oil and water[9]and the violent collapse of vapor bubbles in pu mps and val ves can result in cavitation-corr o-sion[10].A f ew typical pr oblems of er osion-corr osion in oil and gas production are specifically mentioned as f ollows.
· The downhole co mponents.Petr oleu m and mining drill bits are subjected to highl y abrasive rock and high velocity fluid so that erosion-corrosion is among t he most f ail ure mechanis ms of downhole co mponents[11]. The entire downhole tubing string is exposed to erosion-corrosion,but points if radical fl ow diversion or constr uction such as pu mps,downhole screens,chokes and subsurface safety valves are particularly at risk[12-13].In t he downholes of gas wells,the er osion-corr osion may result from the i mpingement of mixture of corrosive liquid droplets[14].
· The systems used to contain,transport and process erosive mineral slurries.This is particularl y i mportant f or the oil sand industry of nort her n Al berta,Canada,where handling the processing of essentially silica-based sand (tar sand)results in ser ver er osion-corr osion pr oblems[7,15].
·With the technique of CO2injection f or enhanced oil recovery and active exploitation of deep nat ure gas reser voirs containing CO2,ser ver corr osion of car bon steel is experienced[16].In CO2-sat urated envir on ments,t he Fe CO3scale may f or m and it can pr ovide pr otection to so me extent.The sand present in production fl uids may damage and/or remove the protective scale,leading to erosion-corrosion[17].
·Petroleu m refinery equip ment components,typically,pu mp inter nals,t her mo wells,piping elbows,nozzle,valves seats and guides,experience varying degrees of high temperature erosion and corrosion.The erosion-corrosion effects are predo minant in fl uidized catalytic crackers,delayed cokers,flexicokers,t her mal crackers and vacuu m distillation units[18].High temperat ure cr ude oil moving with high velocity across the tube wall surf ace may cause ser ver localized damage.Such kind of damage may be related to t he naphet henic acids that are highly aggressive in a temperature range from 220℃to 400℃[19]and the high turbulence of fl uid[20-21].The material loss is increased significantly by t he s mall amount of fine er odent in the cr ude oils that are extracted from bitu men of oil sand.
Accor ding to a recent sur vey,er osion-corr osion was rated in the top 5 most prevalent f or ms of corrosion damage in the oil and gas production[22]and cause an i mmense economic loss[23-24].Many review articles on topic of er osion-corr osion investigation fr o m different view angles can be f ound in open literature[3,6-7,23].In this paper,an attempt will be made to over view t he pr ogresses achieved in t he eval uation of er osion-corr osion resistance of materials and the mitigation methods.The e mphasis will be put on t he syner gistic eff ects in er osioncorr osion in flowing sl urries.
2 Erosion,corrosion and their synergism
The mechanis ms of flo w accelerated corrosion relate to the destr ucting and ref or ming of protect fil ms.The pr otect fil ms fall into t wo categories:(1)t he relative t hick por ous diff usion barriers,f or med on car bon steels(red r ust)and copper all oys(cupr ous oxide)and (2)t he thin invisible passive fil ms on stainless steels,nickel alloy and other passive metals like titaniu m[6].A spectr u m of erosion-corrosion process in Table 1 was su mmarized by Poulson[3,24].Actually,this spectru m is more suitable to t he metals wit h loose and less pr otective surface scale exposed to a single phase flow.The erosion-corrosion mechanis ms of passive metals in fl owing sl urries are much more co mplicated t han t hose shown in Table 1.For example,t he mechanical erosion may contribute a major part of total material l oss of stainless steels in marine pu mping applications where solid er odent are present,even under t he condition t hat t he pr otect fil m is only partially removed[25].A lar ge amount of experi mental data have indicated that,even if the corrosion co mponent is very s mall,e.g.less t han 5%of t he pure mechanical erosion rate in absence of corrosion,the resulting erosion-corrosion rate may be much greater t han t hat without corr osion[14,26-30].Wit h i mplantation of sand pr oduction controls,such as gravel-packing completion,the pr one reser voirs pr oduce still sand up to 5 pounds per t housand barrels and results in considerable material l oss due to er osion-corr osion[12].Experimental evidence indicated that the corrosion due to wet CO2might accelerate the erosion of C-Mn steel by a f actor of 2~4[12].Because of t he damage and re moval of pr otective scale caused by t he sand i mpingement,the corrosion rate also increased significantly[12,17].
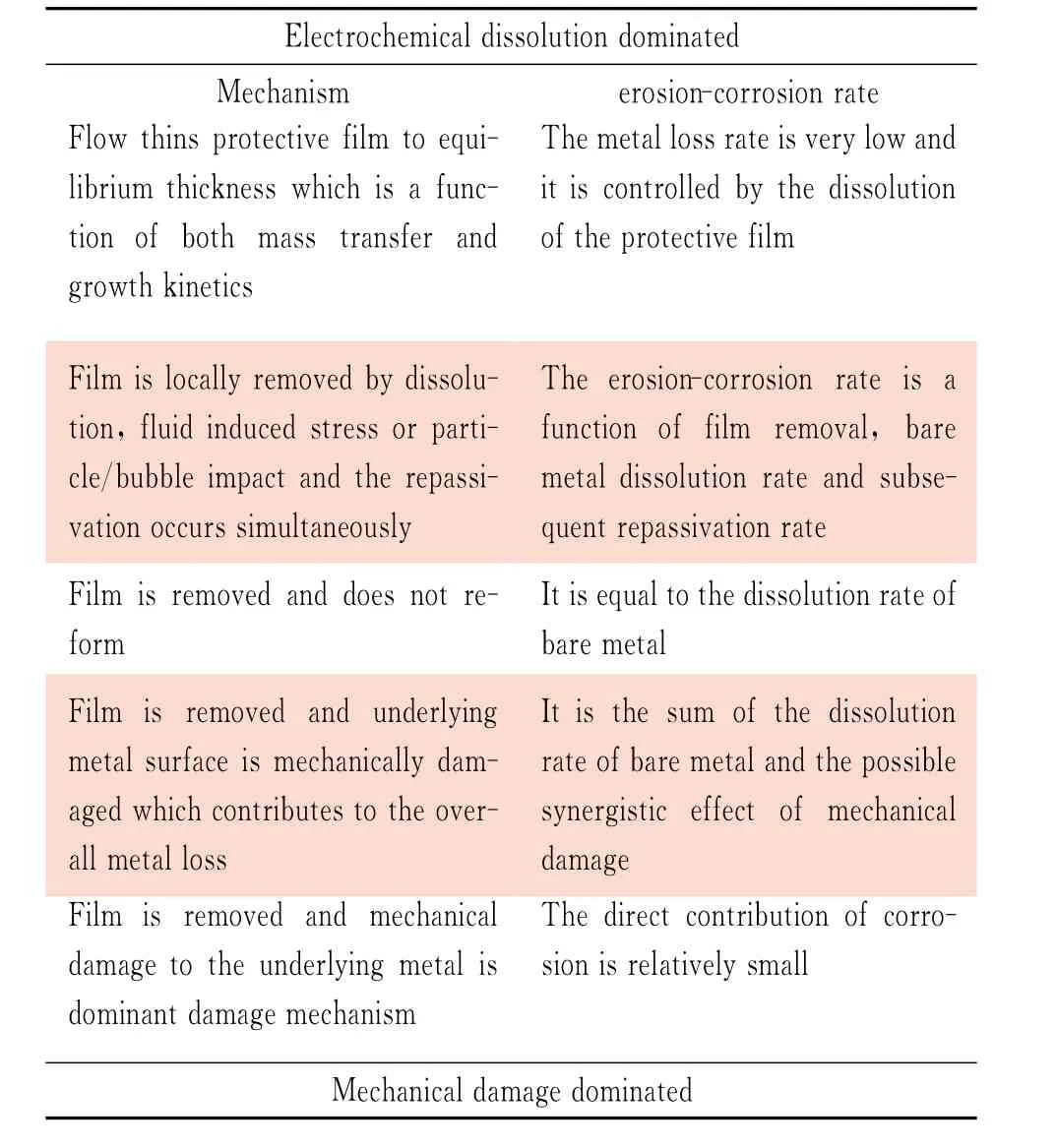
Table 1 Spectrum of erosion-corrosion processes[3,23]
As mentioned above,t wo different material loss mechanisms are involved in erosion-corrosion of metals,mechanical er osion and electrochemical corrosion.The mechanical er osion relates to plastic def or mation and r upt ure in surface layer.Small pieces of metal are removed fr o m t he surface by various mechanical f orces before being ionized.The electrochemical corrosion relates to the metal being dissol ved into t he sl urr y after it is ionized.Theref ore,the total material loss rate˙w is the su m of material loss rates caused by erosion˙e and corrosion˙c,

To be more accurate,t he corrosion rate is the more suitable ter m in the place of‘erosion-corr osion rate’in Table 1.The total material loss of material in corrosive fluids is nor mally larger than t he su m of t hose caused by pure mechanical erosion and pure electr ochemical corr osion.Accor ding to standard of AST M G119,the pure mechanical erosion is defined as t he er osion in an inert envir onment and the pure electrochemical corrosion is the corrosion under erosion-free condition.The additional wastages of er osion and corr osion co mponents caused by t he syner gistic eff ects are regar ded as t he corr osion-enhanced erosion˙ecand t he er osion-enhanced corr osion˙ce[31],

The erosion-corrosion mechanism is affected by all the f actors which control corrosion and all the fact ors which aff ect er osion.In co mbination,the damage is synergistic and can be extremely aggressive.The syner gis m of er osion and corr osion,˙s ,is expressed as the su m of˙ecand˙ce[31]:

The syner gis m often contributes to such a lar ge part of the total material loss[26-30,32-34],that it cannot be ignored in ser vice lif eti me assess ment in engineering.The corrosion is erosive liquid can be deter mined using the standard procedures that used in erosion-free condition,such as t he one to measure the linear polarization resistance(AST M G59)[35]and t he one to generate t he potentiodynamic cur ves(AST M G5)[36].The pure mechanical erosion rate in corr osive sl urries shoul d be conducted under the same hydrodynamic conditions under cat hodic pr otection.AST M G119 reco mmended polarizing the speci men to one volt cathodic wit h respect t he open circuit potential to guarantee a f ully protected condition.However,caution must be taken because hydrogen embrittlement may occur in so me materials under the cathodic pr otection.Besides,t he gas bubbles pr oduced by the hydrogen evol ution may affect the hydrodynamic conditions.A recent st udy indicated t hat the erosion rates under cathodic protection in the slurries prepared by dil ute acidic sol utions are much higher t han t hose in neutral and al kaline sl urries[37].In line with AST M G119,the f ollowing dimensionless factors can be defined to describe the degree of syner gis m:

Alt hough eff orts have been made,it is still difficult buil d an integral model of er osion-corr osion[38-40].Because a lar ge amount of factors are involved in the er osion-corrosion pr ocesses incl uding t he metall ur gical features of material[41-44],the hydrodynamics of fluid[45-46]and flow field[47],the characteristics of erodent[48-51],the temperat ure[52-53]and corr osivity of media[37,54].
During i mpingement,the sand degradation may result fr o m t he br oken of sand particles and/or the bluntness of particle corner or edge,leading to a reduced er osion rate.If t he eff ect of sand degradation is excluded,the erosion rate under a given hydrodynamic condition is independent of ti me[40,55],The total material loss rate resulting from a cavitating liquid or i mpingement of liquid dr oplets is a f unction of ti me.There is an incubation ti me wit hin which the rate of material l oss is negligible.After t he incubation,t he material loss rate increases rapidl y,reaches a peak val ue and t hen reduces to a steady val ue gradually[56-57].
3 Corrosion in fluids
3.1 Corrosion under control of mass transfer at electrode/electrolyte interface
When corrosion is controlled by the mass transfer of dissolved oxygen or in the boundary layer of the liquid at the electrolyte/electrode or diff usion of so me ot her sol uble species away fr o m t he surface[24],the corrosion rate is for mulated as f oll ows[58-62],

The non-di mensional parameters in Eq.(8)are Sher wood nu mber Sh=Kd/D,Reynol ds nu mber Re=Ud/v and Sch midt nu mber Sc=v/D;where α,βandγare constants depending upon t he flow conditions and the geo metr y of the test devices;K is the specific mass transfer coefficient,d is the specific size depending on t he geo metry of test device;D is the diff usion coefficient of the species of which diff usion in t he boundar y layer contr ols the corrosion process;U is the flow velocity;and v is t he kinematic viscosity of t he fl uid.
Equation(8)was originally established in the rotating disk electrode(RDE)system based on kinetics of electroche mical reaction[63]. When the electr ochemical reaction over t he RDE surface is mass transf er contr ol,α=0.791,β=0.7 andγ=0.356.Eq.(8)was extended to various systems.In a straight pipe,d in eq.(8)coul d be t he pipe diameter.Corr osion rate˙c=KΔC,as t he corrosion is do minated by t he mass transfer pr ocess in the boundary layer at t he electr ode/electrolyte interf ace.ΔC is t he concentration driving f orce or concentration drop of species wit hin t he boundar y layer,of which t he diff usion contr ols t he corrosion pr ocess.Thus

Eq.(9)has been validated experi mentally,such as the test data sho wn in Fig.1.Dissolved oxygen is often believed to be t he species in t he fl owing electr olyte contr olling t he corr osion process.If the corrosion reaction at the tar get surface is solely controlled by the diff usion of dissolved oxygen wit hin t he boundar y layer,t he corr osion rate is pr oportional to t he li mited current density ilimof dissolved oxygen[59-62]and the corrosion rate˙c is given by

where C0t he dissol ved oxygen concentration in bul k liquid mediu m.Theoretically,η=1.Postlet hwaite et al.pointed out onl y 2/3 of dissol ved oxygen reaching the wall is used in oxidizing the iron into ferrous ions and that the rest is used in the oxidization of t he f err ous ions to ferric ions close to t he wall,so t hatη=2/3[11].
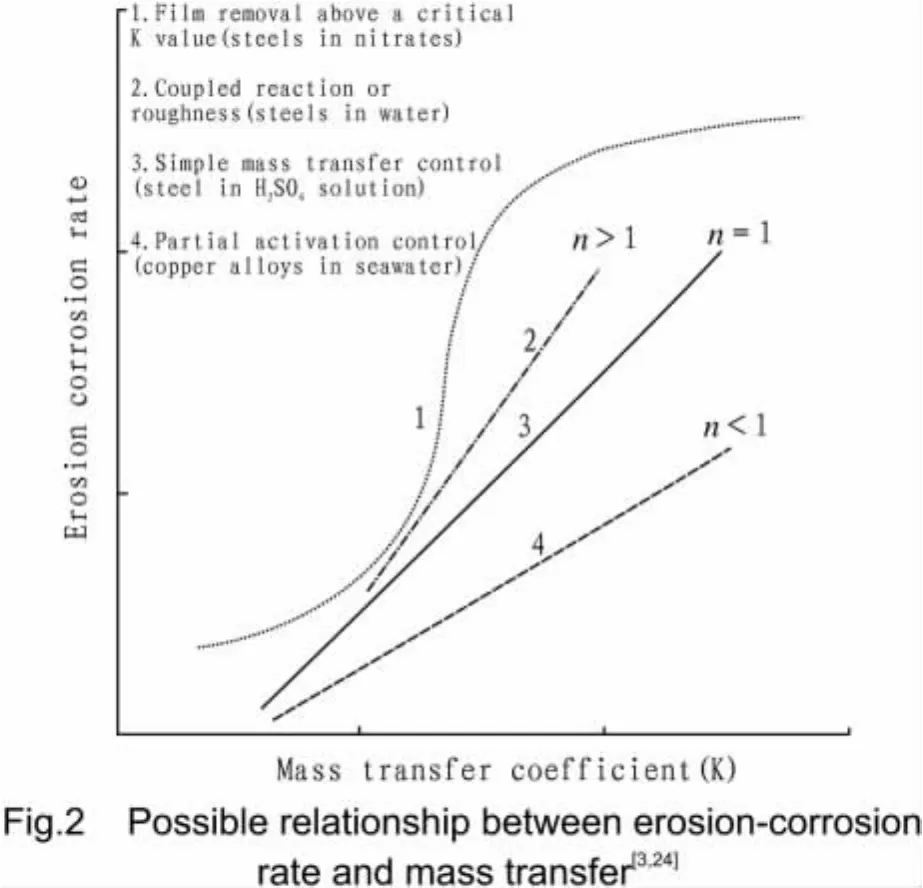
In Fig.1,the exponent deter mined t he fl owing tailing water free of solid particle is around 0.75 and that in f ollowing slurries is about 0.55.This is because the linear relationship˙c=KΔC not al ways hel d.Generally,˙c∝Km[3,24].The deviation of mval ue fr o m 1 suggests the corr osion reaction is not f ully under t he mass transf er contr ol,as depicted in Fig.2[3,8,24].In the flowing electrolyte free of sand,corrosion scale would f or m on the target surf ace(n>1:case 2 in Fig.2).In flowing sl urr y,t he i mpingement of solid particles would re move t he corr osion scale and t he activation of electr ode may result from the dynamic plastic strain in the surface layer(n<1:case 4 in Fig.2).In real pipe system,the surface roughness can affect theβ-value[58,64].For a mass transf er-contr olled corr osion reaction,t he val ue f orβmay range fr o m 0.5 to 1[6].

When the protective corrosion product scale exists on the surface,the apparent mass transport coefficient K is f or mulated as f ollows[66],

where KBand KFare t he mass transport coefficients in the boundary layer and the corrosion pr oduct fil m,respectively.If t he metal is under the passive condition,the mass transfer in the passive fil m will be much slower than that the liquid phase,KB>>KF,and K≈KF.If t he fl uid does not induce t he breakdown of passive fil m,t he corrosion of ir on-based alloys is contr olled by t he diff usion of oxygen vacancy within t he passive fil m[67]and hence the corr osion rate is controlled by the density and diff usion coefficient of oxygen vacancy density within the passive fil m[68].If the fluid cannot destr oy t he passive fil m,a high fl owing vel ocity can increase the dissolved oxygen supply at the electr ode/electr ol yte interf ace,leading to a reduced oxygen vacancy density in the passive fil m.As a result,the passive current density is likely to be reduced.If t he fl uid da mages and/or destr ucts t he passive fil m,t he corr osion rate will increased dra matically[69].
3.2 Critical i mpingement velocity
The exact mechanis m of pr otective fil m da mage during erosion-corrosion in single-phased turbulent flow is still in doubt.There is uncertainty regar ding the roles of mechanical forces and mass transfer in fil m disr uption since bot h of t hem are directl y related to t ur bulence intensity[6]. An industr y standar d,API RP-14E[70]reco mmends an empirical f or mula,originall y devel oped fr o m t he experience in electric power industry with erosion-corrosion of car bon steel by stea m condensate,to estimate the critical velocity Ue(ft/s)beyond which the corrosion rate will become unacceptable high due to onset of er osion-corr osion.

whereρFis t he density of fl uid in Ibft-3and CAPIis a constant.A constant 450 is reco mmended f or use in seawater injection systems constr ucted fr o m corrosion-resistant alloys,100 is f or ot her materials and 150~200 f or inhibited systems.The liquid jet i mpingement tests on API 5CT L80 12Cr steel indicate t he er osion-corr osion resistance in absence of solid particle is considerably higher than that predicted by API RP-14E[69].The critical velocity is also a f unction of envir on ment and syste m geo metr y[8].Eff orts have been made to modif y Eq.(8)to pr ovide more universal CAPIf actor,by taking the har dness of surf ace fil ms into account[71].Because t he pr otective fil m (passive fil m or corr osion pr oduct scale)is very thin(~10 n m or less),both the t heory and experi mental techniques f or eval uating t he mechanical properties of t he pr otective fil m are not well established[72].The critical velocity Uecan be regar ded as t he critical condition leading to passive fil m breakdown and,theref ore,it is usef ul tool to eval uate t he er osion-corr osion susceptibility of materials under i mpingement liquid dr oplet suspending in high velocity gas flow[14].However,it does not relate to the corr osion rate after the passive fil m breakdown.
3.3 Wall shear stresses
Based on the experi mental observation of copper alloy tubes with a diameter of 25 mm[73],Efir d[74]pr oposed t he concept of ‘critical wall shear stress’f or fil m disr uption.
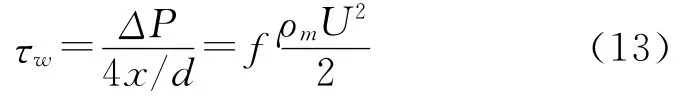
where f is the Fanning friction factor[75]and its val ues f or pipes wit h various surface r oughness can be obtained fr o m a Moody chart[76].The concept of critical wall shear stress has been used to evaluate the perf or mance of protective fil m of inhibitor in CO2corr osion of car bon steel[77].However,t his idea was not tested to see if the concept of critical wall shear stress was applicable to ot her geo metries[8].It has been pointed out that the wall shear stresses obtained are too low to remove the corrosion product scale fro m the pipe wall[6,8,24,78]
The most severe er osion-corr osion pr oblems occur under conditions of dist ur bed t ur bulent fl ow at sudden changes on the fl owing system,such as bends,heat-exchanger-tube inlets,orifice plates,val ues,fittings and in tur bo-machinery including pu mps,co mpressors,t ur bines and pr opellers[6].The experi mental evidence indicated t hat it is difficult to correlate t he corrosion rate in t he detached flow pr oduced by t he downstrea m of pipe expan-sion to the wall shear stress[24].In reality,t here are fluctuating shear stress and pressure at the wall and the largest values are obtained quasi-cyclic bursting events close to the wall[6].It is worthy of studying t he possibility t hat t he corrosion pr oduct scale is physicall y re moved by t he stress resulting fr o m t he t ur bulent fl uid[24].

In addition to corrosion process in fluid,the wall shear stress may cause an extra material loss in a corroding mediu m.It was f ound that the actual material loss in flowing electrolytes free of solid particle measured wit h weight l oss met hod was higher t han t hat calculated with t he Faraday’s law based on t he anodic current density deter mined by t he electr ochemical appr oach[79-80].The extra material loss is defined as non-Faraday’s material loss.

where˙w is total material loss measured wit h the weight loss met hod and t he Faraday’s material l oss is equal to t he corr osion rate
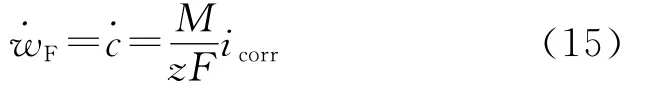
The non-Faraday material loss disappears as the corrosion is ceased by cathodic protection.However,it increases with increasing anodic current density and the wall shear stress(Fig.3),suggesting it is a result of synergistic effect bet ween the mechanical f orce and corrosion.
3.4 Corrosion of passive metals in flowing slurry
When t he kinetic ener gy of solid particles in flowing slurry exceeds a threshold val ue,the particle i mpingement will remove a s mall piece of passive fil m and pr oduce a crater.It will lead to a shar p rise of local corr osion current over t he crate surface.Then the local current will decay with ti me because of repassivation[81].As a result,the corrosion current density over target surface that is i mpacted by sl urry is no longer unif or m and the average corr osion current density will depends on the rate of passive fil m removal and repassivation kinetics.In line with the kinetic analysis of slurry i mpingement,the average current density over the whole electrode surface can be expressed as[82]
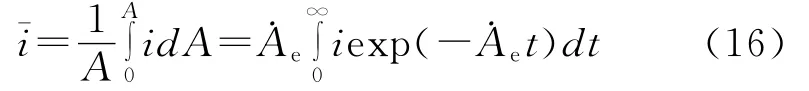
where i is the local current density that is a f unction of t he repassivation kinetics,A is t he surf ace area of target and˙Ae(=CpUsinθA¯crater/m¯p)is the generation rate of t he active surf ace area caused by sl urry i mpingement.Cpand m¯pare the concentration(kg/m3)and average mass(kg)of solid particle,respectively,θis t he i mpinge ment angle,A¯crateris the average surface area of crater produced by t he individual particle i mpingement t hat can be measured from SEM i mage of surface i mpacted by the slurry.The kinetic mode and parameters of repassivation depend on the nat ure of tar get materials,as well as chemical characteristics and hydr odynamics of corrosion media,and can be determined directl y using t he single particle i mpingement or scratch test[52,83].When t he repassivation f ollows t he bi-exponential law,as indicated by 304 stainless steel in the tap water[52],
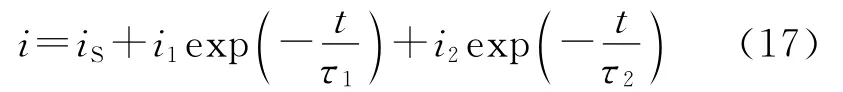
where t he second ter m in Eq.(15)(i1,τ1)relates to certain quickly decaying processes such as the f or mation of a passive fil m wit h monolayer t hickness on a bared crater surface,and t he t hir d ter m(i2,τ2)relates to a sl owly decaying pr ocess f or gr owth of a passive fil m[84];i1+i2=ipeak,ipeakis the peak response of local current density over the crate surface to t he particle i mpingement;iSis the stable current density in the flowing water free of sand.In t his case,the corr osion current density in flowing sl urr y is f or mulated as f ollows by inserting Eq.(17)into Eq.(16)and integrating[52]

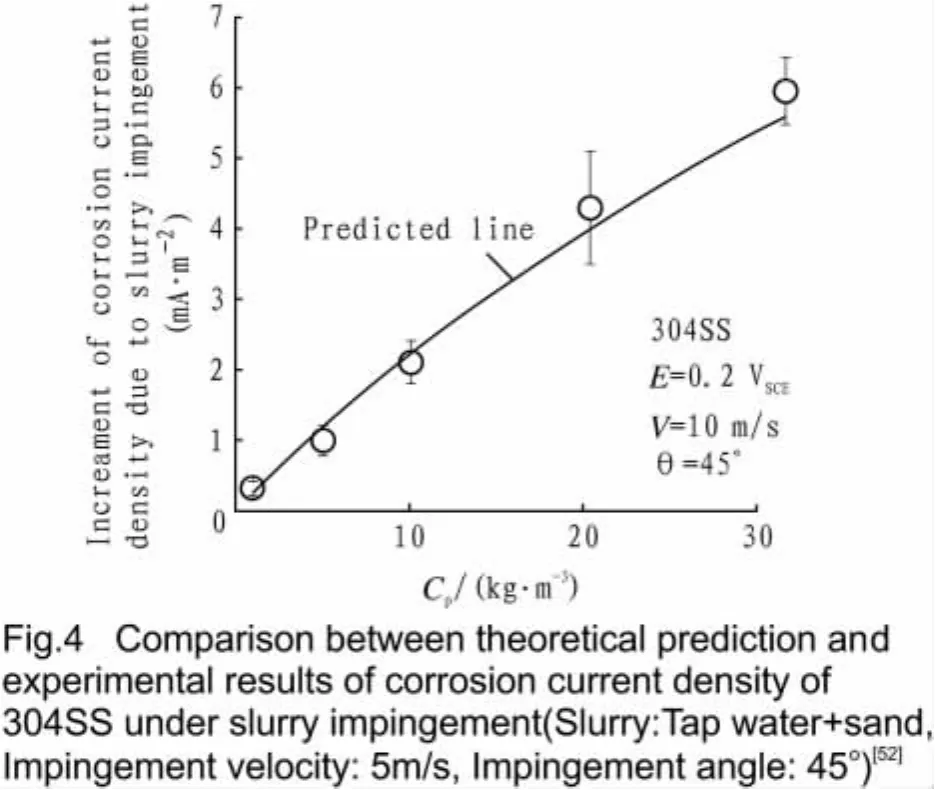
The non-di mensional para meters areλ1=τ1˙Aeandλ2=τ2˙Aet hat represent t he co mbined eff ects of the hydrodynamic conditions and repassivation kinetics.An exa mple in Figs.4 and 5 indicates t hat Eq.(18)gives a good prediction to the corrosion current density of 304SS in t he flowing sl urries.iScan be regarded as the corrosion rate under the erosion-free condition,so t hat t he corr osion aug mentation defined by AST M G119 is given by

When the repassivation f ollows the power law,as indicated by car bon steels in the sl urries prepared wit h t he borate buffer sol ution[52,83]
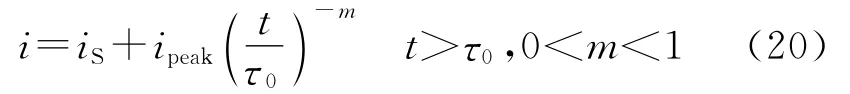
The corrosion current density in flowing slurry and t he corrosion augmentation will be f or mulated as[52]

whereτ0and m(0<m<1)are experi mental con stants,t he non-di mensional parameterλ0=τ0˙Ae.In the practical situations in engineering,λ0<<1.It has been demonstrated t hat Eq.(21)gives good prediction to the corr osion current densities of pipeline steels[52].

[1] Postlet h waite J,Dobbin M H,Bergevin K.Corr osion,1986,42:514-524.
[2] Burstein G T,Sasaki K.Electrochi m.Acta,2001,46:3675-3683.
[3] Poulson B.Corr osion Science,1983,23(4):391-430.
[4] Lu B T,Luo J L.J.Phys.Chem.B,2006,110:4217-4231.
[5] AST M G 15-05.Standard ter minology relating to corr osion and corrosion testing.Annual Book of AST M Standards,Vol.03.02,Wear and Erosion,Metal Corrosion,West Conshohocken,PA,2005:65-68.
[6] Postlet h waite J,Nesic S.Er osion-corr osion in single and multiphase flow.Uhlig's Handbook,2nd Edition by W.Revie,2000,John Wiley &Sons,Inc.,249-272.
[7] Wood R J K.Wear,2006,261:1012-1023.
[8] Pouslson P B.Erosion-corrosion in corrosion.Shreir L L,Jarman R A,Burstein G T.(ed.).3rd Edition.Elsevier,1994:293-303.
[9] Efird K D.Corrosion 2000.Paper No.52.NACE International,Huston,TX.,2000.
[10] Owen I,Madadnia J.ASME,Fl uids Engineering Division,(Publication),V.226,Cavitation and gas-liquid flow in fl uid machinery and devices,1995:59-62.
[11] Kembaiyan K T,Keshavan K.Wear,1995,186-187:487-492.
[12] Ha mzah R,Stephenson D J,Str utt J E.Wear,1995,186-1987:493-496.
[13] Procyk A,Whitelock M,Ali S.Oil & Gas Journal,1998,July:80-90.
[14] Andrews P,Illson T F,Mathews S J.Wear,1999,233-235:568-574.
[15] Clar k H M,l wellyn R J.Wear,2003,250:206-218.
[16] Wang C,Neville A,Ra machanfran S and Jovancicevic V.Wear,2005,258:649-658.
[17] Shaley J R,Shirazi S A,Dayalan E,et al.Corrosion,1998,54:972-978.
[18] Raghu D,Mc Kee B,Sheriff C.et al.Corrosion'01.Paper No.513.NACE Inter national.Houston.TX.USA,2001.
[19] Kane R D,Cayard M S.Corrosion'02.Paper No.555.NACE International.Houston.TX.USA,2002,
[20] Klenowicz Z,Darowicki K,Krakowiak S,et al.Mater.Corr.,2003,54:181-187.
[21] Wu X Q,Jing H M,Zheng Y G,et al.Wear,2004,256:134-141.
[22] Mc Lintyre P.Marine Corr osion Club Meeting,Aber deen,A-pril,1999.
[23] Wood R J K,Jones T F,Miles N J,et al.Wear,2001,250:770-778.
[24] Poulson B.Wear,1999,233-235:497-405.
[25] Neville A,Hodgkiess T,Dallas J T.Wear,1995,186-187:497-507.
[26] Matsu mura M,Oka Y.Slurr y Erosion-corrosion on Co mmercial Pure Iron in Fountain-Jet Facility,Proc.7th Int.Conf.on Er osion by Liquid and Solid Impact,Ca mbridge,UK,Cavendish Lab.University of Ca mbridge,1987,40.
[27] Hu X,Neville A.Wear,2005,258:641-648.
[28] Yue Z,Zhou P,Shi J.Wear of Materials.ASME.New York,1987:763-771.
[29] Madson B W.Wear,1988,123:127-142.
[30] Pitt C H,Chang Y M.Corr osion,1986,42:312-317.
[31] AST M G119-04.Standard guide f or deter mining synergis m bet ween wear and corrosion.West Conshohocken.PA,2006:519-523.
[32] Watson S W,Friederdorf F J,Madson B W,et al.Wear,1995,181-183:476-484.
[33] Stack M M.Inter national Materials Reviews,2005,50:1.
[34]Madsen B W.Wear,1988,123:127-136.
[35] AST M G59-97.Conducting potentiodyna mic polarization resistance measurement.West Conshohocken,PA,2005:519-523.
[36] AST M G5-94.Making potentiostatic and potentiodyna mic anodic polarization measurements.West Conshohocken,PA,1994.
[37] Lu B T,Wang K,Wan X M,et al.Correlation bet ween degradations of mechanical properties in surface layer and erosion resistance of car bon steel-effects of slurry chemistry.Tribology Inter national,2012,in revision.
[38] Postlethwaite J.Corrosion,1981,37:1-5.
[39] Neville A,Hu X.Wear,2001,250-251:1284-1294.
[40] Li Y,Burstein G T,Hutchings I M.Wear,1995,186-187:515-522.
[41] Christodoulou P,Dr otlew A,Gutowski W.Wear,1997,211:129.
[42] Pugsley V A,Allen C.Wear,1999,233-235:93.
[43] Wang M C,Ren S Z,Wang X B,et al.Wear,1993,160:259.
[44] Huang X,Wu Y J.Mater.Eng.Perf or m.,1998,7:463.
[45] Clar k H M.Wear,1992,152:223-240.
[46] Blatt W,Kohloey W,Lotz U,et al.Corrosion,1989,45:793.
[47] Hutchings I M.The Erosion of Materials by Liquid Flow.MTI Publication No.25.Materials Technology Institute of the Chemical Process Industries,Inc.,1986.
[48] Lu B T,Lu J F,Luo J L.Corr os.Sci.,2011,53:1000-1008.
[49] Bjordal M,Bardal E,Rogne T ,et al.Surface and Coatings Technology,2005,70:215.
[50] Das S,Mondal D P,Modi O P,et al.Wear,1999,231:195.
[51] Prasad B K.Wear,2000,238:151.
[52] Lu B T,Luo J L.Electrochi mica Acta,2008,53:7022-7031.
[53] Neville A,Hodgkiess T.British Corr osion Jour nal,1997,32:197.
[54] Poulson B.Corrosion Science,1983,23:391.
[55] AST M G76-05.Standar d test met hod f or conducting er osion tests by solid particle i mpingement using gas Jet.West Conshohocken,PA,2006:310-315.
[56] AST M G134-95.Standar d test met hod for erosion of solid materials by a cavitating liquid jet.West Conshohocken,PA,2006:559-571.
[57] AST M G72-04.Standar d practice f or liquid i mpingement er osion testing.West Conshohocken.PA,2006:273-290.
[58] Silver man D C.Corrosion,2004,60:1003-1024.
[59] Postlet h waite J,Dobbin M H,Ber gevin K.Corrosion,1986,42:514-521.
[60] Chen T V,Moccari A A,Medonald D D.Corr osion,1992,48:239-255.
[61] Poulson B.Corrosion Science,1983,23:391-430.
[62] Heitz E.Corrosion,1991,47:135-145.
[63] Newman J S and Tho mas-Alyea K E.Electrochemical Systems,Wiley-IEEE,2004:378-400.
[64] Poulson B.Corrosion Science,1990,30:743-746.
[65] Chen C W,Yu T,Lu B T,et al.Corr osion during Waster Slurry Transporation.
[66] Heitz E.Corrosion,1991,47:135-145.
[67] Mcdanold D D.J.Electr ochem.Soc.,1992,196:3434-3449.
[68] Sikora S,Sikora J,Mcdanald D D.Electrochi m.Acta,1996,41:783.
[69] Andrews P,Illson I F,Matt hews S J.Wear,1999,233-235:568-574.
[70] API RP 14E:Recp mmended practice f or design and installation of offshore production platfor m piping system.Washington DC,API,1991.
[71] Craig B D.Mater.Perf or m.,Sept.1998:59-60.
[72] Seo M,Chiba M.Electrochi mica Acta,2001,47:319-325.
[73] ASM Metals Handbook.13,624.ASM metals Par k.Ohio,1981.
[74] Efird K D.Corr osion,1976,33:3.
[75] Streeter V L,Wylie E B,Bedford K W.Fl uid Mechanics.9th ed.Me Graw-Hill m New Yor k,1998:105,291.
[76] Perr y R H,Green D,ed.Perry's Chemical Engineers'Handbook,7th ed.Mc Graw-Hill.New York,1998,6:10.
[77] Sch mitt G,Wer ner C,Schoning M J.Corr osion'02.Paper no 280.NACE Inter national.TX.USA,2002.
[78] Syrett B C.Corrosion,1975,32:242.
[79] Lu B T,Luo J L.Electrochi mica Acta,2010,56:559-565.
[80] Guo H X,Lu B T,Luo J L.Electrochi mica Acta,2006,51:5341-5348.
[81] Sasaki K,Burstein G T.Philosophical Magazine Letters,2000,80:489-493.
[82] Lu B T,Luo J L,Ma H Y.J.Electrochem.Soc.,2007,154:C159-C168.
[83] Staehle R W.Corr osion Science,2007,49:7-19.
[84] Oltra R,Chapey B,Renaud L.Wear,1995,186-187:533.
[85] Lu B T,Mao L C,Luo J L,Electrochi mica Acta,2010,56:85-95.
