Synthesis and characterization of a metal-organic framework of grid networks based on nitrilotriacetic acid and neodymium
2013-10-11ZHANGHuaiminZHAOQinfangYOUQianqianWULanzhi
ZHANG Huai-min,ZHAO Qin-fang,YOU Qian-qian,WU Lan-zhi ,
LIU Liu1,YANG Li-rong1*
(1.College of Chemistry and Chemical Engineering,Henan University,Kaifeng475004,Henan,China;2.First Middle School of Qingshui Country,Qingshui 741400,Gansu,China)
Recently,the development of metal-organic frameworks(MOFs)based on lanthanide-containing coordination polymers has been attracting enormous interest,due to the combination of inorganic and organic ligands and metal ions[1-2].The construction of a large number of intriguing aesthetic,the unusual topolo-gies of novel polymers,and the rational design strategies to assemble porous materials with high surface areas as well as predictable structures to target some specific functionalities[3]may potentially lead to industrial applications in gas storage and separation,supramolecular storage of molecules,catalysis,guestexchange,molecular recognition,sensors,molecular magnetism,chemical separations,optoelectronics,ion exchange,microelectronics,nonlinear optics and luminescent probes[4-7].In some cases,the frameworks generate spacious voids,cavities,and channels which are usually occupied by solvent molecules[8-9].Nitrilotriacetic acid(H3NTA)is one of the most important derivatives of glycine and is widely used in fabricating MOFs owing to its numerous advantages.Firstly,as a highly symmetric ligand H3NTA may be used to construct ordered frameworks.Secondly,as a multidentate carboxylate H3NTA is essential in chelating lanthanide ions to form chain-like units with O-C-O linker.Thirdly,H3NTA ligand and its deprotonated anions,i.e.,H2NTA-,HNTA2-and NTA3-,can be adopted to construct fascinating multidimensional compounds via various acidity-dependent coordination modes[10-11].Finally,H3NTA ligand with skew coordination orientation of carboxyl groups is favorable for constructing a helical structure[12].
Following our ongoing efforts toward the isolation of lanthanide MOFs[13-14],here we report the synthesis and structural characterization of 3Dlanthanide MOF of rhombic grid network[Nd(NTA)(H2O)]∞(CCDC:8775631),which is obtained from the self-assembly of bridging ligands nitrilotriacetic acid and its deprotonated anions with neodymium centers.
1 Experimental
1.1 Reagents and general techniques
All chemicals are analytical grade and used without further purification.Elemental analysis was performed with a Perkin-Elmer 240Celemental analyzer.Infrared spectra were recorded in the 4 000-400 cm-1region using KBr pellets with an AVATAR 360FT-IR spectrometer.The crystal structure was determined with a Bruker Smart CCD X-ray single-crystal diffractometer.Thermogravimetric(TG)analysis and differential thermogravimetric(DTG)analysis were conducted with a Perkin-Elmer TGA7thermogravimeter.
1.2 Synthesis of complex[Nd(NTA)(H2O)]∞
[Nd(NTA)(H2O)]∞was synthesized from the reaction mixture of nitrilotriacetic acid and neodymium nitrate at a molar ratio of 1∶1in 10mL distilled water while the pH of the solution was adjusted to 5.5 with 1mol·L-1NaOH.Resultant mixture was homogenized by stirring at ambient temperature for 20min and then transferred into 25mL Teflon-lined stainless steel autoclave to allow reaction at 160℃for 4dunder autogenous pressure.Upon completion of the reaction,the autoclave was cooled to room temperature at a rate of 5℃/h,followed by filtration,washing with distilled water and drying to afford colorless transparent block crystals suitable for X-ray diffraction(XRD)analysis.Elemental analysis calcd(%)for C6H8NO7Nd:C,20.57;H,2.30;N,4.00.Found(%):C,20.02;H,2.56;N,4.32.
1.3 X-ray crystallographic determination
Single-crystal XRD measurements of[Nd(NTA)(H2O)]∞were carried out at 296(2)K (excitation source:graphite monochromated Mo Kα-radiation(λ=0.071 073nm);ω-scan mode).All independent reflections were collected in a range of 2.06°-25.00°and determined in subsequent refinement.SADABS multi-scan empirical absorption corrections were applied to the data processing[15].The crystal structures were solved by direct methods and Fourier synthesis.Positional and thermal parameters were refined by the full-matrix least-squares method onF2using the SHELXTL software package[16].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.Analytical expressions of neutral-atom scattering factors were employed,and anomalous dispersion corrections were incorporated.The crystallographic data,selected bond lengths and angles for the title complex are listed in Table 1,Table 2and Table 3,respectively.

Table 1 Summary of crystallographic data for[Nd(NTA)(H2O)]∞
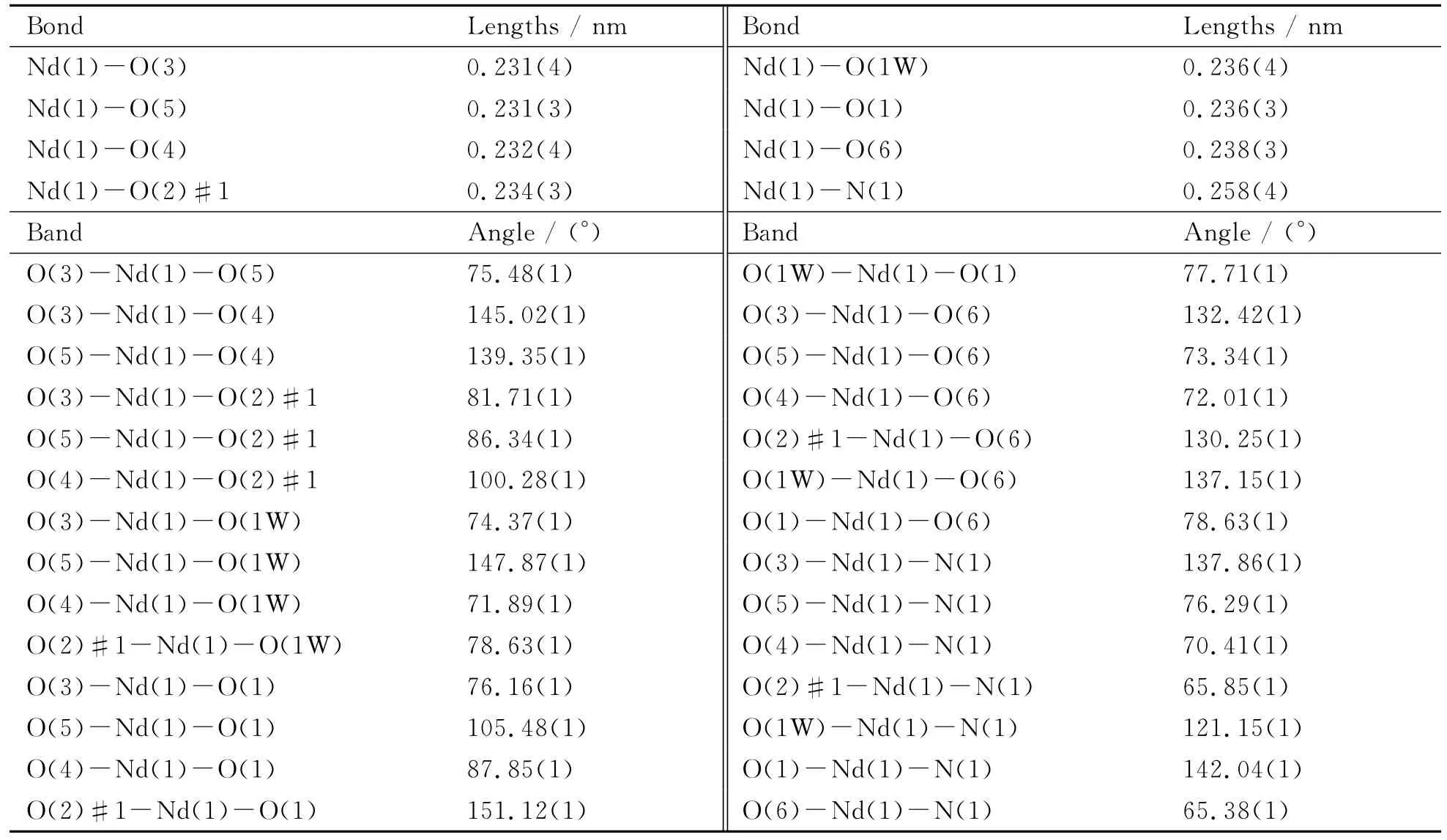
Table 2 Selected bond lengths and angles for[Nd(NTA)(H2O)]∞

Table 3 Data of hydrogen bonds in[Nd(NTA)(H2O)]∞
2 Results and discussion
2.1 IR spectrum of the complex
[Nd(NTA)(H2O)]∞is insoluble in CH3COCH3,CH3CH2OH,CH3CN and tetrahydrofuran,but slightly soluble in CH3OH or dimethylformamide.The strong and broad absorption bands in the ranges of 3 408-3 403cm-1and 918-915cm-1are assigned to the characteristic peaks of water molecules in the complex[17].The strong vibrations at about 1 645cm-1and 1 335cm-1are ascribed to the coordinated carboxylates.TheδO-C-Ovibration in plane occurs in the range of 680-770cm-1.The absence of the characteristic bands ranging from 1 690cm-1to 1 730cm-1indicates that the H3NTA ligands are completely deprotonated in the form of NTA3-anions upon reaction with the metal ions[18].
2.2 Structural description of the complex
Single-crystal XRD analysis reveals that crystal[Nd(NTA)(H2O)]∞belongs to monoclinic space groupP21/n.In the asymmetric unit of the crystal,there exists one crystallographically independent Nd(Ⅲ),one NTA and one water.Six carboxylic oxygen atoms(O(1),O(2),O(3),O(4),O(5)and O(6))and one nitrogen(N1)coming from four NTA ligands as well as one oxygen atom(O1W)coming from one water molecule comprise the coordinated sphere of Nd(Ⅲ).Besides,the eight-coordinated Nd(Ⅲ)adopts a distorted triangular dodecahedral coordination geometry(see Fig.1a).The Nd-ONTAdistances are in the range of 0.231(4)-0.238(3)nm,the Nd-OWdistance is 0.236(4)nm,and the Nd-N distance is 0.257 6(4)nm.In general,the bond length data in the present work are consistent with those in previous work covering lanthanide coordination polymers[14].Moreover,through the bridging of O-C-O linkers,the asymmetrical units form one dimensional(1D)right-handed helical chains along theadirection((Nd-OCO-Nd-OCO)n)(see Fig.1b).Adjacent 1Dhelical chains are further connected into 2Dlayers with an interlayer distance of 0.415nm by carboxylic groups,and the 2Dlayers are finally assembled into 3D frameworkviaO-C-O linkers and hydrogen bonds(see Fig.1dand Fig.2d).

Fig.1 (a)Polyhedral view of the Nd(Ⅲ)ion in[Nd(NTA)(H2O)]∞;(b)the 1Dchain in[Nd(NTA)(H2O)]∞;(c)the 2Dlayer in[Nd(NTA)(H2O)]∞ ;(d)the formation of 3Dframework of[Nd(NTA)(H2O)]∞

Fig.2 Schematic representations of 3Dsupramolecular topological metal-organic framework of square grid networks structure observed in[Nd(NTA)(H2O)]∞
The most striking feature of as-synthesized MOF is that Nd8C14O28rhombic tetrahedron acts as its motif building block.Better insight into the nature of this intricate skeleton frame of rhombic tetrahedron networks can be achieved by topological approach,that is,by reducing the multidimensional structures to simple node and connection nets,as depicted in Fig.2band Fig.2c.The coordination environment of the complex is shown in Fig.2a.It is seen that the central Nd(Ⅲ)atom is bonded to four NTA ligands simul-taneously,and each NTA molecule is connected with four Nd(Ⅲ)atoms to form rhombic tetrahedral Nd8C14O28unit with the dimensions of 0.673nm×0.655nm×0.661nm and corresponding angles of 119.697°and 60.303°.Besides,the rhombic tetrahedral Nd8C14O28unit generates a large nanoscale cage which can be considered to consist of eight Nd(Ⅲ)vertices and fourteen bended O-C-O edges(see Fig.2b).Moreover,there are four large quadrilateral windows on the surface of each cage with the dimensions of 0.673 1nm×0.661 3nm.As a result,a novel 3Dframework is prolonged alonga,bandcaxes through the above cage-to-cage connections(Fig.2d).
[1]DENG H,GRUNDER S,CORDOVA K E,et al.Large-pore apertures in a series of metal-organic frameworks[J].Science,2012,336:1018-1023.
[2]FARRUSSENG D.Metal-organic frameworks:applications from catalysis to gas storage[M].Wiley-VCH,Germany,2011.
[3]SUH M P,CHEON Y E,LEE E Y.Syntheses and functions of porous metallosupramolecular networks[J].Coord Chem Rev,2008,252:1007-1026.
[4]SUH K S K,YUTKIN M P,DYBTSEV D N,et al.Enantioselective sorption of alcohols in a homochiral metal-organic framework[J].Chem Commun,2012,48:513-515.
[5]FRISˇCˇIC′T.Supramolecular concepts and new techniques in mechanochemistry:cocrystals,cages,rotaxanes,open metal organic frameworks[J].Chem Soc Rev,2012,41:3493-3510.
[6]WANG Q,WU X,ZHANG W,et al.Solids with rhombic channels:syntheses and crystal structures of[Cu3(NTA)2(4,4′-bpy)2(H2O)2]·9H2O and[{Cu(4,4′-bpy)(H2O)4}{Cu2(NTA)2(4,4′-bpy)}]·7H2O (NTA = nitrilotriacetate,4,4′-bpy=4,4′-bipyridine)[J].Inorg Chem,1999,38:2223-2226.
[7]ZHANG Q,LU C,YANG W,et al.Hydrothermal syntheses and crystal structures of two lanthanum compounds La(OH)SO4and LaO(NO3)[J].Inorg Chem Commun,2004,7:889-892.
[8]DUVAL S,FLOQUET S,SIMONNET-JÉGAT C,et al.Capture of the[Mo3S4]4+cluster within a{Mo18}macrocycle yielding a supramolecular assembly stabilized by a dynamic H-bond network[J].J Am Chem Soc,2010,132:2069-2077.
[9]ILYUKHIN A,LOGVINOVA V,DAVIDOVICH R.Structures of lead(II)nitrilotriacetates and ethylenediaminetetraacetates[J].Russ J Inorg Chem,1999,44:1571-1577.
[10]LI D M,CUI L F,XING Y H,et al.Synthesis and structural characterization of new tungsten(VI)complexes with polycarboxylate ligands[J].J Mol Struct,2007,832:138-145.
[11]JIANG Y B,KOU H Z,WANG R J,et al.Synthesis,crystal structure,and magnetic properties of oxime-bridged polynuclear Ni(II)and Cu(II)complexes[J].Inorg Chem,2005,44:709-715.
[12]THUÉRY P.Uranyl-lanthanide heterometallic complexes with cucurbit[6]uril and perrhenate ligands[J].Inorg Chem,2009,48:825-827.
[13]YANG L R,SONG S,ZHANG W,et al.Synthesis,structure and luminescent properties of neodymium(III)coordination polymers with 2,3-pyrazinedicarboxylic acid[J].Synth Met,2011,161:647-654.
[14]YANG L R,SONG S,ZHANG H M,et al.Synthesis,structure of 3Dlanthanide(La(III),Pr(III))nanoporous coordination polymers containing 1Dchannels as selective luminescent probes of Pb2+,Ca2+and Cd2+ions[J].Synth Met,2011,161:2230-2240.
[15]SHELDRICK G M.SADABS:Empirical absorption correction software[CP].University of Göttingen,Institut fur Anorganische Chemieder Universitat,Tammanstrasse 4,D-3400Göttingen.Germany,1999.
[16]SHELDRICK G M.SHELXTL:version 5[CP].Reference manual,Siemens Analytical X-ray Systems.USA,1996.
[17]NAKAMOTO K.Infrared and raman spectra of inorganic and coordination compounds[M].John Wiley and Sons,New York,1997.
[18]TANCREZ N,FEUVRIE C,LEDOUX I,et al.Lanthanide complexes for second order nonlinear optics:Evidence for the direct contribution of electrons to the quadratic hyperpolarizability 1[J].J Am Chem Soc,2005,127:13474-13475.
