磷酸基团在环丙沙星光敏损伤DNA 中的作用
2013-09-21刘艳成李海霞崔荣荣徐宇列王文锋
刘艳成 李海霞 崔荣荣 徐宇列 王文锋,*
(1中国科学院上海应用物理研究所,上海201800;2中国科学院大学,北京100049)
1 Introduction
Fluoroquinolone antibiotic(FQ)is one of the most successful drugs,which were widely used as antibiotics because of their optimal properties.Their pharmacological action consists in specific inhibition of subunit-A of the bacterial topoisomerase DNA gyrase,which controls the shape of DNA.1FQs are considered to be well tolerated drugs.Hence,the use of several compounds of the series in therapy is increasing.In the last few years,these molecules have been received growing attention from interdisciplinary areas of the scientific community due to both practical purposes and fundamental aspects.However,a serious drawback for their use is the phototoxic and photocarcinogenic activity.In order to elucidate the mechanism which leads to photosensitivity associated with this family of compounds,the photophysical and photochemical properties of FQs have been extensively studied in recent years.2-6These studies have revealed some damaging paths involved in transient parts of degradation,singlet oxygen and triplet state of FQs.
As stated above,FQs can inhibit the bacterial gyrase.Although the exact mechanism of this action is still unclear,it has been evidenced that FQs interact directly with DNA in synergy with the gyrase enzyme.7-9Therefore,DNA is thought to be one of the main cellular targets responsible for the aforementioned drug-photoinduced disorders.Evidence in literature has suggested that some FQs can be binded to DNA.However,the binding mode in details is still unclear.Other evidences have been found that a ground state association between the phosphate dianion and the positively charged 4'-NH2+moiety of some FQs.10,11However,no related reports on ciprofloxacin were found in papers published.
Ciprofloxacin(CPX)is a member of FQ family.The photounstable character of CPX has been reported in papers published,10-12which evidenced that it was a pH dependent compound and the main form was zwitterions in neutral pH solutions.13,14Reaction mechanism between ciprofloxacin hydrochloride and bovine serum albumin has been studied by Shang et al.15The co-adsorption of DNA bases with per-chlorate has been studied by Cui et al.16The binding mode and photochemical reactivity of complexes between lomefloxacin,enoxacin(two members of FQs),and calf thymus DNA has been studied by Sortino group.13The pH and phosphate dianion effects on the photochemical feature of CPX and other FQ members have been studied extensively by UV-visible spectrum method.2,14,17-19However,to the best of our knowledge,no evidence of phosphate acid base effect in the process of CPX photosensitive damage to DNAhas been found in literature published.
DNA damage photo-induced by photosensitizer is commonly considered by two types:(1)electron transfer from DNA to excited state of photosensitizer and(2)energy transfer from excited state of photosensitizer to DNA.20,21DNA damage photo-induced by CPX has been studied from the aspect of biology.7,22However,few studies on the relationship between structures and effects in the process of damage were reported.Especially,the effect of functional group in structures on the damaging process was not found in reports.Hence,it is interesting for us to study this point.
As we all know that there are so many phosphate acid bases in DNA double strand.These bases may play a very important role in the process of CPX photosensitive damage to DNA.To gain insight into the role of phosphate base of DNA in the FQs photo-induced DNA damage process,a stable study and a laser flash photolysis study were carried out.
2 Materials and methods
2.1 Materials
Ciprofloxacin(CPX),guanosine(Gua),2'-deoxyguanosine(dG),and 2'-deoxyguanosine-5'-monophosphate(dGMP)were purchased from Sigma Chemical Co.and they were used without purified.NaOH,HClO4,and phosphate(analytical grade reagent)were commercially available and used as received.Water was purified through a Millipore Milli-Q system.The pH values of the solutions were measured with a glass electrode.Structures of CPX,Gua,dG,and dGMP are shown in Scheme 1.
2.2 Absorption/emission measurements
UV-Vis absorbance was measured on a Hitachi U-3900 spectrophotometer(Hitachi Co.,Japan).Fluorescence signals were measured on a Hitachi F-4500 luminescence spectrometer(Hitachi Co.,Japan)equipped with a xenon-lamp and computer working with the Hitachi software.
2.3 Laser flash photolysis
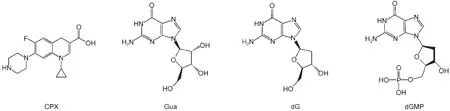
Scheme 1 Structures of CPX,Gua,dG,and dGMP
Laser flash photolysis experiments were carried out using a Nd:YAG laser(EKSPLA Co.,Lithuania).The system essential-ly consists of a Q-switched 1 J of energy at 1064 nm in pulse of 12 ns duration as the excitation source.The fundamental beam can be frequency doubled,tripled,and quadrupled to deliver light of 532,355,and 266 nm,respectively.The harmonics are separated using a beam splitter.The sample under investigation is contained in a quartz cell and replaced after each laser pulse.The analyzing beam generated by a xenon lamp,which can be pulsed to increase the analyzing light up to 200 times in the UV region,passes through the sample at right angles to the laser beam.A shutter which opens a few microseconds before the laser pulse and closes again when the event under examination is completed,and appropriate filters are placed between the analyzing lamp and sample in order to minimize the photolysis of the sample by the monitoring light.The monitoring light is dispersed in wavelength by a monochromator and then onto a photo-detector.The transmittance of light at this wavelength is detected by the photo-detector before,during and after the laser pulse.The detector is also connected to an automatic back-off box which enables changes in transmittance to be observed by feeding back a signal equal and opposite to the detector anode current prior to the laser pulse,thus maintaining the anode current close to zero.
The output of the photomultiplier(Hamamatsu R925,Japan)is displayed on a HP 54510B digitizing oscilloscope.Data processing was performed on a PC-586 personal computer using software developed in house.Back-off and energy meter readings are digitized using an Analogue to Digital Converter(ADC)attached to a DI-AN data acquisition system.Data acquisition and processing are obtained by repeating the above procedure at successive wavelengths.
3 Results and discussion
3.1 Effects on absorption properties
In neutral aqueous medium,CPX was characterized by an intense absorption band with a maximum in the region of 260-280 nm and a weak band in the region of 310-340 nm.The absorption spectra of CPX recorded in the presence of increasing amounts of phosphate buffer(PB)at pH 7.1 are shown in Fig.1.As shown in Fig.1,significant hyper-chromic effect was observed at about 270 nm with increasing the concentration of phosphate buffer.As we all know phosphate buffer has no absorption in the region of 240-360 nm.Hence,this finding provided an indication that a ground-state complex was formed.
3.2 Effects on fluorescence properties
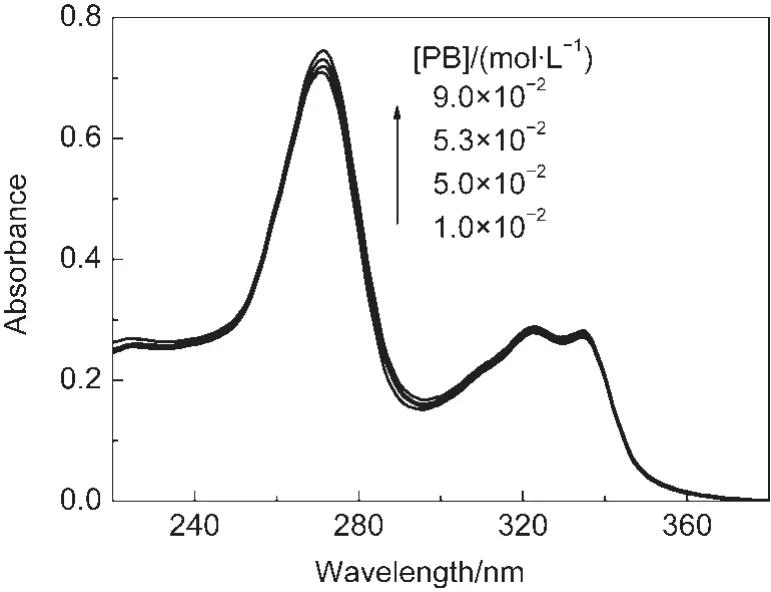
Fig.1 Absorption spectra of 2×10-5mol·L-1ciprofloxacin(CPX)in water at pH 7.1 containing different concentrations of phosphate buffer(PB)
Fluorescence emission spectra of 2×10-5mol·L-1CPX in water at neutral pH are shown in Fig.2.When excited at 355 nm,the fluorescence spectra were characterized by a broad band in the region of 350-600 nm with a peak at about 450 nm.As shown in Fig.2,the fluorescence intensity of CPX at neutral pH depended on the concentration of dissolved phosphate salt.Specific effects were observed with phosphate ions added(see Fig.2).The emission intensity decreased by increasing the concentration of the phosphate buffer.According to a linear relationship(Fig.2,inset),the slope value Kawas obtained.By applying the Stern-Volmer equation(I0/I=1+Ka[Q]with Ka=kqτ0,Q:quencher,kq:quenching rate,τ0:fluorescence lifetime),these values indicated that the short-lived excited singlet of FQs(τ0≤10-9s-1)was quenched with bimolecular rate constants kq≥1010mol-1·dm3·s-1,which was larger than the diffusion limit(in water,6.5×109mol-1·dm3·s-1).Hence,it was reasonable to think that the quenching mechanism was mainly of“static”origin.This mechanism has been proposed for other members of FQs in literature published.19,23
The presence of multiple proton binding sites in FQs makes the pattern of acid-base equilibrium rather complex.By several techniques,mainly potentiometry,UV and NMR spectroscopies and,more recent studies showed that the carboxylate group and the 4'-amino of the piperazine ring were the most significant proton binding sites from biological view.3,24,25As described above,CPX was a zwitterionic form in neutral pH solutions.Based on the results and discussion mentioned above,Scheme 2 was proposed.In the ground state association,the phosphate dianion binded the positively charged 4'-NH2+moiety with two hydrogen bonds.

Fig.2 Fluorescence emission spectra of 2×10-5mol·L-1CPX in water at pH 7.1 containing different concentrations of phosphate buffer
To investigate the phosphate base of dGMP effect on the fluorescence spectra of CPX,an experiment was carried out with dGMP,dG,and Gua as quenchers.In this experiment,the quenching effect was observed when dGMP was used as a quencher(see Fig.3).However,the similarity was not observed when dG and Gua were used as quenchers.Hence,it was reasonable to think that the phosphate base of dGMP played an important role in the quenching effect.
3.3 Laser flash photolysis

Scheme 2 Ground state association between phosphate dianion and CPX zwitterions in neutral aqueous solution
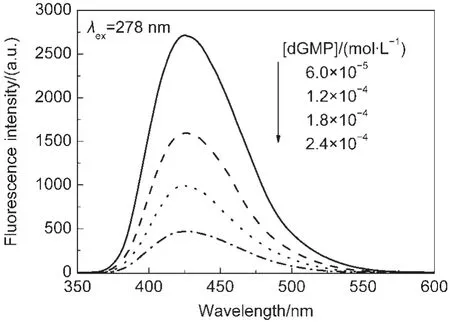
Fig.3 Fluorescence emission spectra of 2×10-5mol·L-1CPX in water at pH 7.1 containing different concentrations of dGMP
Laser flash photolysis experiments were carried out to investigate the phosphate dianion effect on the transient spectrum of the triplet state of CPX(3CPX*).The transient absorption at 550-750 nm has been evidenced to be the transient absorption of3CPX*,as it was found to be sensitive to O2and can transfer energy to naproxen forming the triplet state of naproxen(3NP*).26As shown in Fig.4,after adding phosphate buffer,a significant red shift of the transient spectrum of3CPX*was observed in the study(see Fig.4).Similarity has been found in other members of FQs.Hence,it was reasonable to think that an interaction between phosphate dianion and CPX occurred.
As we all know there is a phosphate base in the structure of dGMP(see Scheme 1).This phosphate base may play an important role in the process of CPX photo-induced DNA dam-age.To investigate this effect,following experiments were carried out by laser flash photolysis method.
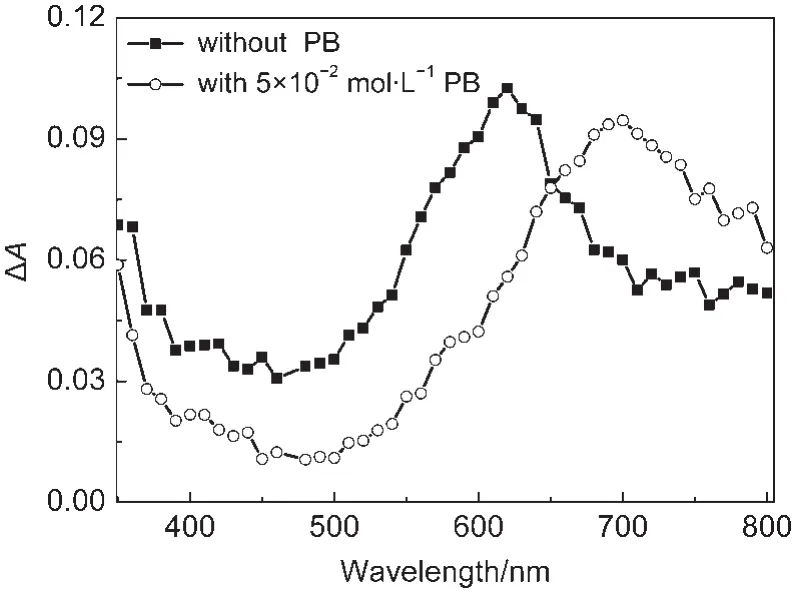
Fig.4 Transient absorption spectra recorded at 1µs after laser flash photolysis of 1×10-4mol·L-1CPX neutral aqueous solution
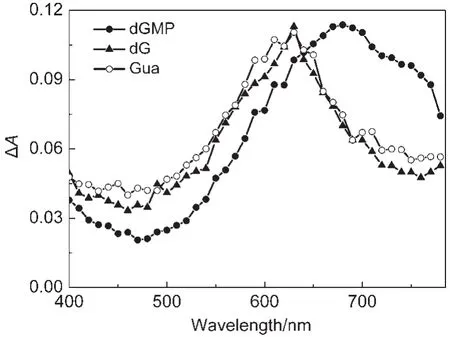
Fig.5 Transient absorption spectra recorded at 1 μs after laser flash photolysis of 1×10-4mol·L-1CPX neutral aqueous solutions containing 2.5×10-4mol·L-1dGMP,dG,and Gua
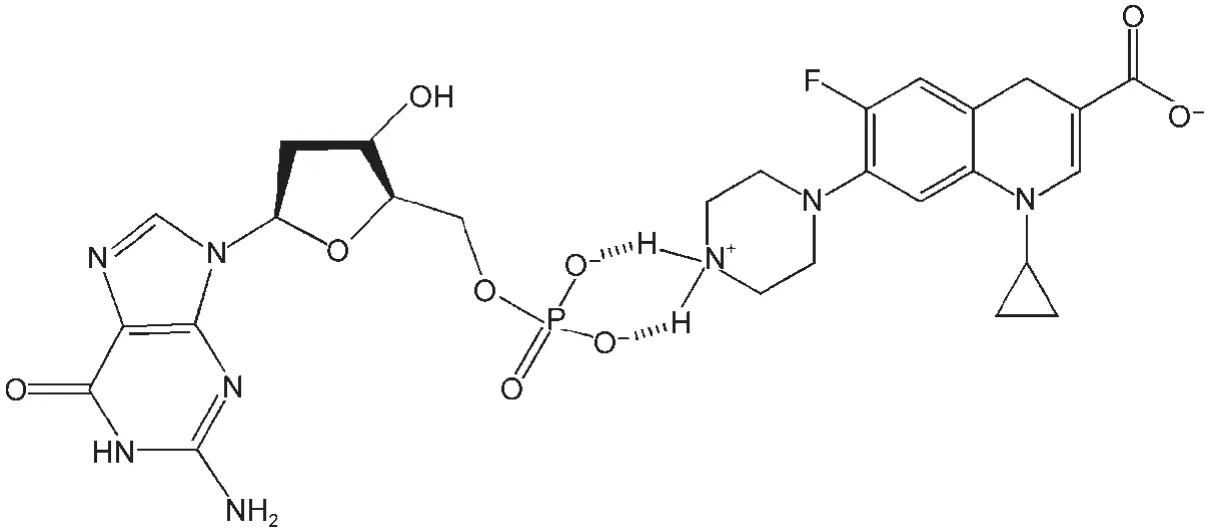
Scheme 3 Ground state association between dGMPand CPX zwitterions in neutral aqueous solution
After CPX neutral aqueous solution containing dGMP was irradiated by laser pulse,the transient absorption in the region of 650-750 nm was higher than that of the CPX neutral aqueous solutions containing dG or Gua(see Fig.5).In three solutions,the pH value was kept at 7.1 by adding drops of NaOH and HCl aqueous solution.Hence,the effect of phosphate buffer can be ruled out.Based on the results found in experiments and comparing the structures of dGMP,dG,and Gua,it was reasonable to think that the phosphate base in dGMP was the reason causing the transient absorption higher than that in the presence of dG and Gua in the region of 650-750 nm.Similar to the association between phosphate buffer and CPX,a ground state association between dGMP and CPX by the phosphate dianion of dGMP in neutral aqueous solution occurred.Because of this association,the electron distribution in the structure of CPX was changed.Hence,the transient spectrum of3CPX*was redshift and the width of spectrum became wider.Based on the results obtained,Scheme 3 was proposed.
4 Conclusions
To investigate the phosphate base effect on the photochemistry of CPX,UV-Vis,fluorescence,and Laser flash photolysis experiments were carried out in this study.Based on the results obtained in this study,the scheme of associations between dGMP and CPX zwitterions was proposed.The results obtained in our study indicated a new scheme for dGMP damage photo-induced by CPX.It may help in understanding the phototoxicity of FQs to DNA.This binding mode proposed in our study provides an insight in designing new drugs for potential photo-chemotherapeutics.To the best of our knowledge,it was the first finding about the phosphate base effect on DNA damage photo-induced by CPX.
(1) Domagala,J.M.;Hanna,L.D.;Heifetz,C.L.;Hutt,M.P.;Mich,T.F.;Sanchez,J.P.;Solomon,M.J.Med.Chem.1986,29(3),394.doi:10.1021/jm00153a015
(2)Van Doorslaer,X.;Demeestere,K.;Heynderickx,P.M.;Van Langenhove,H.;Dewulf,J.Appl.Catal.B-Environ.2011,101(3-4),540.doi:10.1016/j.apcatb.2010.10.027
(3) Cuquerella,M.C.;Bosca,F.;Miranda,M.A.;Belvedere,A.;Catalfo,A.;de Guidi,G.Chem.Res.Toxicol.2003,16(4),562.doi:10.1021/tx034006o
(4) Lorenzo,F.;Navaratnam,S.;Edge,R.;Allen,N.S.Photochem.Photobiol.2008,84(5),1118.doi:10.1111/php.2008.84.issue-5
(5) Lorenzo,F.;Navaratnam,S.;Edge,R.;Allen,N.S.Photochem.Photobiol.2009,85(4),886.doi:10.1111/php.2009.85.issue-4
(6) Martinez,L.J.;Sik,R.H.;Chignell,C.F.Photochem.Photobiol.1998,67(4),399.
(7) Gurbay,A.;Gonthier,B.;Signorini-Allibe,N.;Barret,L.;Favier,A.;Hincal,F.Neurotoxicology 2006,27(1),6.doi:10.1016/j.neuro.2005.05.007
(8) Belvedere,A.;Bosca,F.;Catalfo,A.;Cuquerella,M.;Guidi,G.;Ma,M.Chem.Res.Toxicol.2002,15(9),1142.doi:10.1021/tx025530i
(9) Hartmann,A.;Golet,E.M.;Gartiser,S.;Alder,A.C.;Koller,T.;Widmer,R.M.Arch.Environ.Con.Tox.1999,36(2),115.doi:10.1007/s002449900449
(10) Fasani,E.;Negra,F.F.B.;Mella,M.;Monti,S.;Albini,A.J.Org.Chem.1999,64(15),5388.doi:10.1021/jo982456t
(11)Vasconcelos,T.G.;Henriques,D.M.;Konig,A.;Martins,A.F.Chemosphere 2009,76(4),487.doi:10.1016/j.chemosphere.2009.03.022
(12)Paul,T.;Dodd,M.C.;Strathmann,T.J.Water Res.2010,44(10),3121.doi:10.1016/j.watres.2010.03.002
(13) Sortino,S.;Condorelli,G.New J.Chem.2001,26(1),250.
(14) Pascual-Reguera,M.I.;Parras,G.P.;Diaz,A.M.Microchem.J.2004,77(1),79.doi:10.1016/j.microc.2004.01.003
(15) Shang,Z.C.;Yi,P.G.;Yu,Q.S.;Lin,R.S.Acta Phys.-Chim.Sin.2001,17(1),48.[商志才,易平贵,俞庆森,林瑞森.物理化学学报,2001,17(1),48.]doi:10.3866/PKU.WHXB20010110
(16) Cui,L.;Ren,B.;Tian,Z.Q.Acta Phys.-Chim.Sin.2010,26(2),397.[崔 丽,任 斌,田中群.物理化学学报,2010,26(2),397.]doi:10.3866/PKU.WHXB20100136
(17) Mella,M.;Fasani,E.;Albini,A.Helv.Chim.Acta 2001,84(9),2508.doi:10.1002/1522-2675(20010919)84:9<2508::AID-HLCA2508>3.0.CO;2-Y
(18) Joshi,H.C.;Bridhkoti,J.P.;Mishra,H.;Pant,S.Spectrochim.Acta A 2011,79(3),412.doi:10.1016/j.saa.2011.02.044
(19)Albini,A.;Monti,S.Chem.Soc.Rev.2003,32(4),238.doi:10.1039/b209220b
(20) Spratt,T.E.;Schultz,S.S.;Levy,D.E.;Chen,D.;Schluter,G.;Williams,G.M.Chem.Res.Toxicol.1999,12(9),809.
(21)Wasielewski,M.R.Chem.Rev.1992,92(3),435.doi:10.1021/cr00011a005
(22) Ferguson,J.;Johnson,B.E.Brit.J.Dermatol.1990,123(1),9.doi:10.1111/bjd.1990.123.issue-1
(23) Monti,S.;Sortino,S.;Fasani,E.;Albini,A.Chem.-Eur.J.2001,7(10),2185.
(24)Chen,J.W.;Ge,L.K.;Wei,X.X.;Zhang,S.Y.;Qiao,X.L.;Cai,X.Y.;Xie,Q.Environ.Sci.Technol.2010,44(7),2400.doi:10.1021/es902852v
(25)Takacsnovak,K.;Noszal,B.;Hermecz,I.;Kereszturi,G.;Podanyi,B.;Szasz,G.J.Pharm.Sci.-Us.1990,79(11),1023.
(26)Liu,Y.C.;Zhang,P.;Li,H.X.;Wang,W.F.Photochem.Photobiol.2012,88(3),639.doi:10.1111/php.2012.88.issue-3
