三元杂化TiO2-SiO2-POMs 催化剂的合成及其在可见光降解罗丹明B 中的应用
2013-09-17王晓夏徐华龙RUHLMANNLaurent
王晓夏 徐华龙,* 沈 伟 RUHLMANN Laurent 秦 枫
SORGUES Sébastien3 COLBEAU-JUSTIN Christophe3
(1复旦大学化学系,上海市分子催化与功能材料重点实验室,复旦大学先进材料实验室,上海200433;2Laboratoire d'Electrochimie et de Chimie Physique du Corps Solide,Institut de Chimie,UMR au CNRS n°7177,Université de Strasbourg,France;3Laboratoire de Chimie Physique,UMR au CNRS n°8000,Université Paris-Sud 11,Orsay,France)
1 Introduction
Titania(TiO2)has been found as a promising photo-catalyst because of its relatively high activity and stability.1,2However,the band gap of rutile TiO2is 3.0 eV and that of anatase TiO2is 3.2 eV,3therefore TiO2can be hardly activated to photodegrade organics under visible light.Its application of solar energy is thus limited.4Meanwhile,the generated positive hole(h+)and negative electron(e−)pairs over TiO2are prone to recombination,thus the catalyst easily loses its photoability.Polyoxometalates(POMs)have diverse structures and abundant optical and electronic capacities.5POMs take effects in photolysis,6especially in water purification.7The band gap of POMs ranges from 3.1 to 4.6 eV.8So POMs mainly rely on UV or near-UV light for irritation.
A catalyst with decreased band gap and good h+-e−pair stability is desired.Yang et al.8have discovered that the photodegradation ability of TiO2-POMs on dyes rises compared with each monomer.They believe that the combination of POMs and TiO2prompts lateral electron transfer and then increases the photocatalytic efficiency.In view of the limitation of using visible light,doping carbon or nitrogen into TiO2eminently reinforced its photo-conversion under visible light illumination,because these doped atoms substitute for some of the lattice titanium atoms close to/on the surface.This change narrows the band gap and results in faster electron transfer,so the doped TiO2absorbs more visible light.9−11As in the same family of carbon,silicon doped into TiO2is also potential in photocatalysis.For example,De Witte et al.12synthesized mesoporous molecular sieves TiO2-SBA-15 to degrade Rhodamine-6G under UV light with high photocatalytic activity.Although SiO2has no photocatalysis effect itself,13it promotes high dispersion of TiO2to achieve higher specific surface.14In addition,some researchers have discovered that as a substrate for TiO2,hydrophobic SiO2prompts pre-concentration of the organics during the photocatalytic procedure.15In fact,some TiO2-SiO2-POM catalysts have been synthesized by impregnation method to degrade organics under UV light.16Li and Liu17have reported that Fenton-assisted TiO2-SiO2-FeW11O399−photodegraded Congo red oxidized by H2O2in Fenton reagent.To take account of beneficial improvement in materials and structure,the ternary hybrid TiO2-SiO2-POM catalyst synthesized by sol-gel method has potential phtocatalytic ability under visible light illumination.
Stepped sol-gel method was applied in the following experiment,based on the balance of photocatalytic ability,moderate surface adsorption,and availability in synthesis.We propose the hybrid TiO2-SiO2-POMs as the catalyst,where POMs accelerate the lateral transfer of electrons to stabilize h+-e−pair,and SiO2can reinforce dispersion of TiO2and absorbance of visible light.
2 Experimental
2.1 Synthesis and characterization of TiO2-SiO2-POMs catalyst
Pure TiO2gel and SiO2gel were synthesized by stepped solgel method and then the gel was mixed with POMs:6 mL titanium tetraisopropoxide(TTIP,Aldrich,97%)was dissolved in 30 mL isopropyl alcohol(Sinopharm,AR),and 8 mol·L−1HCl(Taicang Zhitang,AR)was added into the mixture to adjust pH to 1−2.The mixture was stirred at room temperature until TiO2hydrogel was formed.42.08 g tetraethyl orthosilicate(TEOS,Shanghai Lingfeng,AR)was added into the mixture of 60 g,2 mol·L−1concentrated HCl(Taicang Zhitang,AR)and 15 g water.The resulting mixture was maintained in 40°C water bath for 24 h.Thus the SiO2gel was obtained.Two gels were mixed and stirred at room temperature.H4SiW12O40(Sinopharm,AR)was chosen for the representative of incorporated POMs(Fig.S1,Supporting Information).The hydrogel was transferred into a crucible,and then heated to 200 °C with heating rate of 2 °C·min−1.At last the temperature was kept at 200 °C for 1 h.4The cooled catalysts were denoted as TiO2-SiO2and TiO2-SiO2-SiW
The wide-angle XRD patterns(2θ,20°−70°)were collected on a Bruker D8 Advance X-ray Diffractometer(D8 Advance,Bruker,Germany)using nickel-filtered Cu Kαradiation(λ>0.15406 nm)at a voltage and a current of 40 kV and 40 mA,respectively.The Raman patterns were collected on a Dilor LABRAM-1B(LABRAM-1B,Dilor,France),with an excitation wavelength of 632.8 nm.The incident microwaves of Time Resolved Microwave Conductivity(TRMC)were generated by a Gunn diode in theKαband(28−38 GHz).A Nd:YAG laser(5022DNS10,BMI,USA)was used as the pulsed light source providing an IR radiation atλ=1064 nm.Full width at half-maximum of one pulse was 7 ns,and the repetition frequency of the pulses was 10 Hz.UV light(3rd harmonic,355 nm)was obtained by tripling the IR radiation.Diffuse reflection spectrophotometry(DRS)data were collected on a Shimadzu UV-2450 UV-Visible Spectrophotometer(UV-2450,Shimadzu,Japan).X-ray photoelectron spectroscopy(XPS)data were fit out of the curves from a PerkinElmer PHI 5000C ESCA system(PHI 5000C,PerkinElmer,USA)equipped with a hemispherical electron energy analyzer,using Mg-Kα(1253.6 eV)anode operated at 14 kV and 20 mA.The carbonaceous C 1s line(284.6 eV)was used as the reference to calibrate the binding energies(BEs).The Brunauer-Emell-Teller(BET)surface areas of samples were detected in Quantachrome Quadra-Sorb SI(QuadraSorb SI,Quantachrome,USA)with a relative pressure(p/p0)ranged from 1×10−3−0.995,kept at 200 °C for 3 h as pretreatment.Transmission electron microscopy(TEM)images were recorded on a JEOL JEM 2010 microscope(JEM 2010,JEOL,Japan)operated at 200 kV.
2.2 Catalytic performance:photodegradation of Rhodamine B
The photodegradation of Rhodamine B(RhB)was chosen as the probe reaction.The light source was CEL-HXUV300 from Beijing China Education Au-light Co.,Ltd.Certain amount of catalyst and 100 mL RhB solution were put into the reactor and stirred in dark for mixing.The mixture was bubbled with air.The experiment was performed under light wavelength longer than 420 nm with the light filter UVCUT420 nm.Every 5 or 10 min,1 mL mixture was taken up and filtered.RhB in the clear solution was detected by Cintra 10e UV-Vis Spectrometer(Cintra 10e,GBC,Australia).
XRD results exhibit that the amount of anatase TiO2increases while the amount of rutile TiO2decreases after SiO2is introduced in the TiO2-POMs(Fig.1).Due to the relatively low calcination temperature,the sol-gel catalysts are mainly amorphous with a small amount of crystallized part.Before introduced with SiO2,TiO2-SiW12presents more rutile peaks than anatase,especially at R(110),R(101),and R(211).After introduced,TiO2manifests more anatase peaks,especially at A(101),referred from Fig.1(a)vs Fig.1(b).The phenomena may result from TiO2dispersion state and interaction with SiO2after introduced by SiO2.The interaction stabilizes more anatase at calcination temperature.18It facilitates more electron transfer because of more oxidized sites and higher degree of hydroxylation on the surface of anatase(i.e.,numbers of hydroxyl groups).19
Raman(Fig.S2)and XPS(Fig.S3)results demonstrate that during the synthesis of TiO2-SiO2-SiW12,new Ti―O―Si
3 Results and discussion
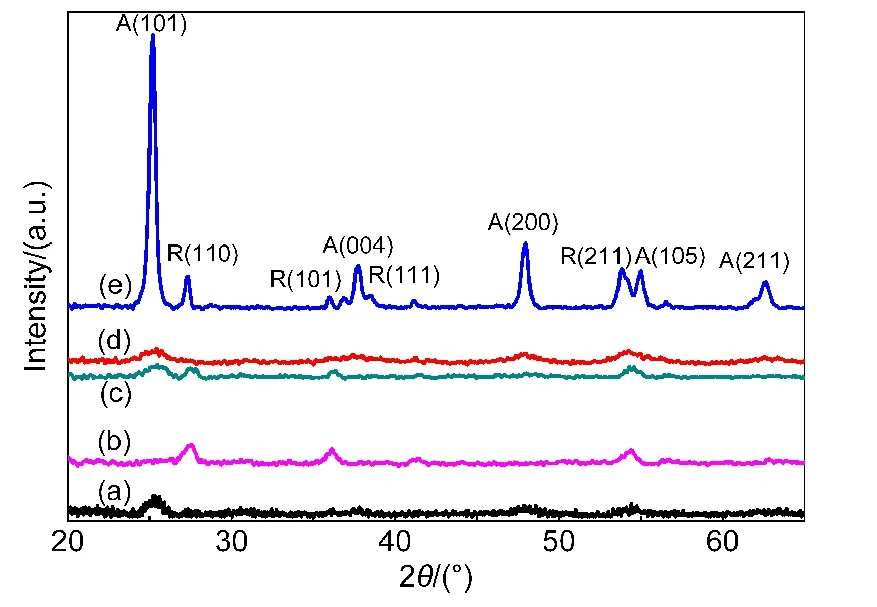
Fig.1 XRD patterns of the catalysts
bonding is formed.20Therefore TiO2,SiO2,and POMs are proved to form one composite but no mixed photocatalysts.TEM images(Fig.S4)show that the introduction of SiO2improves the dispersion of TiO2.To learn specifically on the synergistic interaction,time resolved microwave conductivity(TRMC)is applied for characterization.The influence of laser wavelength and intensity on TRMC decay shows the importance of the fast-recombination processes.Ahigh value of maximum value(Imax)in TRMC signal can be considered as reflecting good electronic properties of the sample.The measurement has been applied as a tool to investigate the excess chargecarrier lifetimes in TiO2powders,21where laser illumination on the catalysts initiates microwave power.From nanosecond to microsecond,the decrease of the number of h+-e−pairs after the pulse is caused by recombination or quenching.And this change is recorded by the variation of microwave power.The presence of a long time tail in the TRMC signals of the ternary hybrid TiO2-SiO2-SiW12s attributed to quenching of this recombination by hole-trapping at the surface.More stable the h+-e−pairs are,longer it needs to recover.The stability of h+-e−pairs depends on the convenience of electron transfer.Fig.2 shows clearly that the materials with remarkable interaction possess stable TRMC signals.It manifests that the synergistic effect facilitates charge transfer amongst components.The vertical return of excited e−back to valence band(VB)is diminished by the horizontal electron or hole transfer.The active sites are therefore stabilized for longer time.POMs are strong electron acceptors where electrons are delocalized on the structure.During visible illumination,we induce the formation of h+-e−pairs near the interface of TiO2-POMs.Then,the reduced POMs increase a lot the lifetime of h+-e−pairs and avoid recombination between h+and e−because the distance between h+and e−is longer.POMs enhance the lateral transfer of electrons in order to form h+-e−pairs(Fig.2(b)vs Fig.2(c)),and the introduction of SiO2(Fig.2(a)vs Fig.2(c))shows that in the presence of SiO2,more h+-e−pairs are observed.The hybrid TiO2-SiO2-POM catalyst is more stable than P25.
DRS shows that SiO2increases absorbance across the visible region in comparison with that of pristine TiO2(Fig.3).The function of SiO2resembles the surface plasmon resonance(SPR)features of metal nanoparticles in TiO2-POMs-metal nanocomposite.22The phenomena indicate that the hybrid TiO2-SiO2-POM catalysts exhibit high activity under visible light illumination.
Estimation of band gap is based on Fig.3 and is according to the method of Barton et al.23Together with the surface area data,the results have been summarized in Table 1.The band gaps of TiO2and TiO2-SiW12are 2.95 eV,thus the presence of SiW12does not change the band gap evidently.The introduced SiO2reduces the band gap of the catalyst,as shown in Table 1.The reduction enables more electrons to transfer from valence band(h+)to conduct band(e−).The electron transfer can be activated because the energy of reaction light source(al-most 2.95 eV)is higher than the band gap of hybrid TiO2-SiO2-POM catalyst.In another hand,the hybrid TiO-SiO-SiW2212catalyst possesses obvious absorbance across visible spectra,exhibiting as a lifting section with the wavelength longer than 420 nm in Fig.3(d).Meanwhile,SiO2significantly enlarges the surface area of catalyst,rendering RhB enriched on the active sites(Table 1).
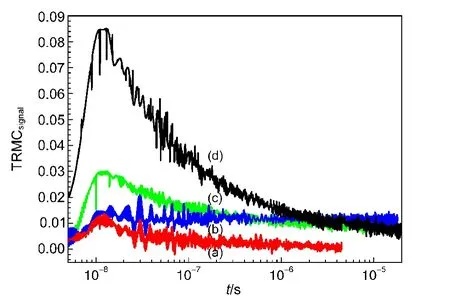
Fig.2 TRMC curves of the catalysts
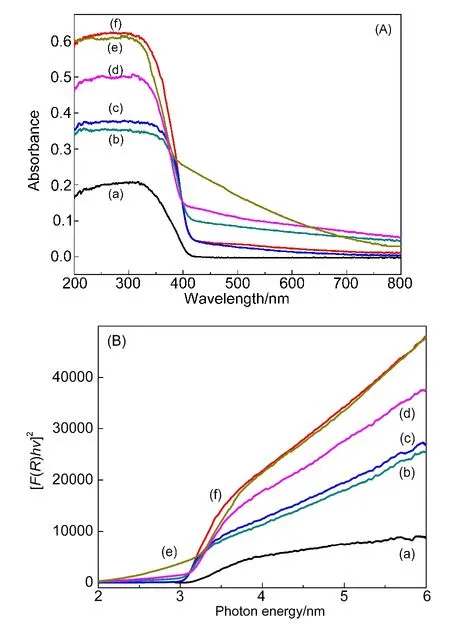
Fig.3 DRS patterns of the catalysts
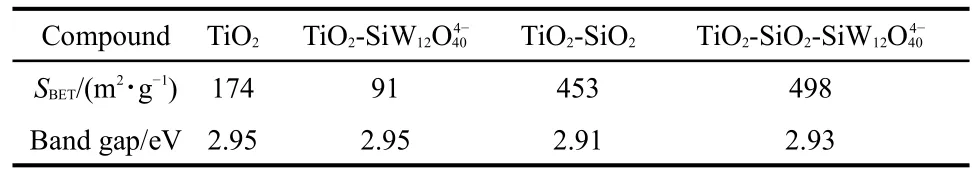
Table 1 DRS results and surface areas of catalysts
As a typical kind of dye,RhB is selected as a rapid and sensitive probe.4However,RhB decomposes very slowly under visible light illumination.RhB is mainly oxidized by photogenerated hole on the surface of the irradiated catalyst,leading to an adsorbed RhB cation radical(RhB+·).RhB+·combines with molecular O2and leads to its destruction.4The active oxygen species produced during the photocatalysis process is superoxide species(O2−·).The holes react with water molecules to produce hydroxyl radicals(OH·),and they attack dye molecules.OH·is a strong oxidant species and oxidizes RhB into radical species.The break of benzene ring and de-ethylation happen simultaneously during the photodegradation of RhB24(Fig.S5).In Fig.S6,the decrease in UV spectra indicates the conjugate structures,e.g.,benzene rings,were destructed in illumination,and the shift of maximum peak indicates the separation of ethyl groups.24According to Chen et al.25TiO2dominates the decomposition of conjugate structures and POMs lead to de-ethylation process.After step-by-step oxidation,RhB is mineralized.Curves of(f−b)in Fig.4(A)exhibit photocatalytic activity of SiO2,TiO2,P25,TiO2-SiO2,and TiO2-SiO2-SiW1with the same 1 mol·L−1total amount of catalyst reacted at room temperature.After 60 min,the photodegradation conversions of RhB by different catalysts are approximately as follows:TiO2,6%;P25,26%;TiO2-SiO2,46%;TiO2-SiO2-SiW1,76%.The background curve of un-photoactive SiO2reflects that RhB degrades very slowly under visible light illumination.The ternary hybrid TiO2-SiO2-SiW12possesses the best photoactivity.Individual impact factor like lifetime of h+-e−pairs(Fig.2)or absorbance of visible light(Fig.3)does not directly determine the photoactivity.A proper combination of two factors leads to the best performed catalyst.Afterwards,the photocatalytic reaction is furthered over TiO2-SiO2-SiW12O440−under optimal reaction conditions as shown in Fig.4(A(a)).The conversion of RhB catalyzed by 1.4 mol·L−1TiO2-SiO2-SiW12O440−and kept at 60°C water bath exceeds 99%after 60 min.Compared with the results over TiO2-PW1catalyst by Yang et al.(60 min,98%),4with ternary hybrid TiO2-SiO2-SiW1catalyst,only 80%active amount was needed to get the same activity.At these conditions,ternary hybrid TiO2-SiO2-SiWexhibited good photocatalytic stability,the conversion of RhB still keeps almost at 80%after 4 cycles,while it is only 30%over P25 catalyst(Fig.4(B)).
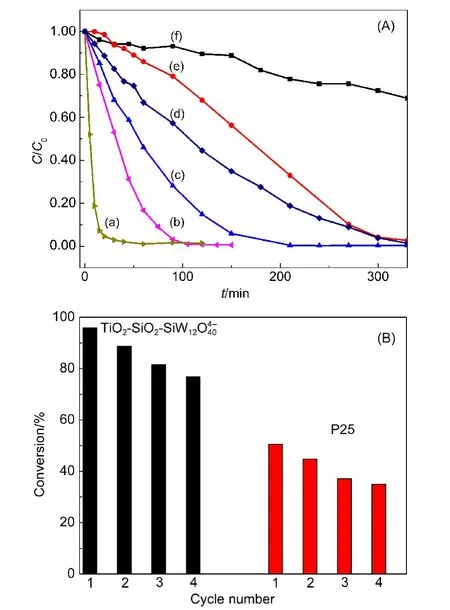
Fig.4 (A)Photocatalytic abilities of the catalysts calcined at 200 °C,stirred at 700 r·min−1in 100×10−6RhB solution,bubbled in air,reacted under light longer than 420 nm and(B)percentage of photodegradation of RhB on its exposure to 1.4 mol·L−1TiO2-SiO2-SiW12and 1 mol·L−1P25 photocatalysts
4 Conclusions
The ternary hybrid TiO2-SiO2-POM catalyst is synthesized by stepped sol-gel method,and it exhibits remarkable efficiency in photocatalysis under visible light illumination.SiO2promotes the dispersion of TiO2and reduces the threshold of catalyst.Therefore introduced SiO2eminently reinforces absorbance in the visible spectra.The h+-e−pairs are excited and formed on the surface of TiO2and POMs by lower energy,from conventional UV light to visible light.High surface area brought by SiO2provides more active sites and absorbs more reactive species,which could have been one of the reasons for its enhanced photocatalytic activity.POMs mainly form synergistic effect together with TiO2.They improve the lateral charge transfers,as h+→h+and e−→e−.This contributes to stabilizing h+-e−pairs for longer time.The hybrid ternary TiO2-SiO2-POM catalyst attributes its high activity to the decrease of the band gap and the increase of the electron transfer under visible light illumination simultaneously.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Fox,M.A.;Dulay,M.T.Chem.Rev.1993,93,341.doi:10.1021/cr00017a016
(2) Hu,C.;Wang,Y.;Tang,H.Appl.Catal.B:Environ.2001,30,277.doi:10.1016/S0926-3373(00)00237-X
(3)Schaming,D;Farha,R.;Xu,H.;Goldmann,M.;Ruhlmann,L.Langmuir 2011,27,132.doi:10.1021/la1024923
(4)Yang,Y.;Wu,Q.;Guo,Y.;Hu,C.;Wang,E.J.Mol.Catal.A:Chem.2005,225,203.doi:10.1016/j.molcata.2004.08.031
(5) Sadakane,M.;Steckhan,E.Chem.Rev.1998,98,291.
(6) Schaming,D.;Ahmed,I.;Hao,J.;Alain-Rizzo,V.;Farha,R.;Goldmann,M.;Xu,H.;Giraudeau,A.;Audebert,P.;Ruhlmann,L.Electrochim.Acta 2011,56,10454.doi:10.1016/j.electacta.2011.02.064
(7) Mylonas,A.;Hiskia,A.;Papaconstantinou,E.J.Mol.Catal.A:Chem.1996,114,191.doi:10.1016/S1381-1169(96)00317-2
(8)Yang,Y.;Guo,Y.;Hu,C.;Wang,E.Appl.Catal.A:Gen.2003,252,305.doi:10.1016/S0926-860X(03)00446-0
(9) Khan,S.U.M.;Al-Shahry,M.;Ingler,W.B.,Jr.Science 2002,297,2243.doi:10.1126/science.1075035
(10) Ren,W.;Ai,Z.;Jia,F.;Zhang,L.;Fan,X.;Zou,Z.Appl.Catal.B:Environ.2007,69,138.doi:10.1016/j.apcatb.2006.06.015
(11) Serpone,N.J.Phys.Chem.B 2006,110,24287.doi:10.1021/jp065659r
(12)De Witte,K.;Busuioc,A.M.;Meynen,V.;Mertens,M.;Bilba,N.;Van Tendeloo,G.;Cool,P.;Vansant,E.F.Microporous Mesoporous Mat.2008,110,100.doi:10.1016/j.micromeso.2007.09.035
(13) Guo,Y.;Hu,C.J.Mol.Catal.A:Chem.2007,262,136.doi:10.1016/j.molcata.2006.08.039
(14) Gao,X.;Wachs,I.E.Catal.Today 1999,51,233.doi:10.1016/S0920-5861(99)00048-6
(15)Anderson,C.;Bard,A.J.J.Phys.Chem.B 1997,101,2611.doi:10.1021/jp9626982
(16) Bai,B.;Zhao,J.;Feng,X.Mater.Lett.2003,57,3914.doi:10.1016/S0167-577X(03)00240-4
(17) Li,D.;Liu,Y.Gas-Sensitive Behavior Based on Charge Transfer Processes of a Novel Polyoxometalate(POM)/TiO2/SiO2Composite Film.InAdvanced Materials Research,International Conference on Soft Magnetic Materials,Male,Maldives,May 23−24,2011;Zhang,T.B.,Tian,J.Eds.;Trans Tech Publications:Zurich,2011;p 102.
(18) Li,D.;Guo,Y.;Hu,C.;Jiang,C.;Wang,E.J.Mol.Catal.A:Chem.2004,207,183.doi:10.1016/S1381-1169(03)00491-6
(19) Carp,O.;Huisman,C.L.;Reller,A.Prog.Solid State Chem.2004,32,33.doi:10.1016/j.progsolidstchem.2004.08.001
(20) Zhang,X.;Zhang,F.;Chan,K.Y.Appl.Catal.A:Gen.2005,284,193.doi:10.1016/j.apcata.2005.01.037
(21) Colbeau-Justin,C.;Kunst,M.;Huguenin,D.J.Mater.Sci.2003,38,2429.doi:10.1023/A:1023905102094
(22) Pearson,A.;Bhargava,S.K.;Bansal,V.Langmuir 2011,27,9245.doi:10.1021/la201655n
(23) Barton,D.G.;Shtein,M.;Wilson,R.D.;Soled,S.L.;Iglesia,E.J.Phys.Chem.B 1999,103,630.doi:10.1021/jp983555d
(24) Liu,H.J.;Peng,T.Y.;Peng,Z.H.;Dai,K.J.Wuhan Univ.(Nat.Sci.Ed.)2007,53,127.[刘华俊,彭天右,彭正合,戴 珂.武汉大学学报(理学版),2007,53,127.]
(25) Chen,C.;Zhao,W.;Lei,P.;Zhao,J.;Serpone,N.Chem.Eur.J.2004,10,1956.
