湿式空气非均相催化氧化处理有机废水中难降解有机物的研究
2013-09-06宋丽红
宋丽红
(深圳市环境科学研究院,深圳 518001)
1 前言
随着造纸、印染、化学及石油加工等工业的发展,大量的废水排入自然水体。废水含有大量的有毒、难降解有机物,造成严重的环境问题并威胁着人类的健康,在环境问题严峻、水资源短缺的今天,如何高效、低耗地进行有机废水中难降解有机物的去除,是水处理领域的一大研究热点。
在过去的几十年,水处理技术主要依赖传统物理化学及生物方法。这些传统的方法技术诸如活性炭吸附、化学氧化和生物降解都有难以避免的缺陷,如仅仅转移污染物、能耗物耗量大、对难降解物质去除效果差、需时过长等缺点,让这些传统方法在难降解有机物处理方面颇有局限[1]。近年来,高级氧化技术 (Advanced Oxidation Processes,AOPs)在有机污染物的降解处理方面得到广泛的关注,原因是高级氧化技术能产生羟基自由基(Hydroxyl Radicals,·OH),羟基自由基是水体系下氧化性仅次于氟的自由基[2],能具有高活性和无选择性,能有效降解难以生物降解的有机污染物[3,4],在替代传统技术中极具前景。在众多的高级氧化技术中,湿式空气氧化技术 (Wet air oxidation,WAO)是最为经济可行的技术之一。湿式空气氧化技术是在高温 (200℃ ~320℃)高压 (0.5~20 MPa)下,以气态氧源 (纯氧或空气)作为氧化剂[5,6],使得部分大分子有机物氧化成能生物降解的有机物或完全矿化成水与二氧化碳。湿式空气催化氧化 (Catalytic Wet Air Oxidation,CWAO)是基于湿式空气氧化,添加适当的催化剂形成高效催化体系,能大幅提高反应速率,提高难降解有机污染物的去除效率,提高体系污染物负荷,降低反应耗时,降低反应温度及压力,从而获得更好的效率并降低技术的运行成本[7~10],实现高浓度、有毒有害、难降解的工业有机废水的高效处理[11],是对湿式空气氧化的重要改进。在催化剂方面,用于湿式空气催化氧化技术的均相、非均相催化剂都得到了一定的发展[6]。均相催化剂中,溶解性铜盐显示出最高的催化活性[11~13],然而,尽管均相催化剂具有高催化活性,其使用要求对催化体系进行后续处理以去除催化剂,且无法简单实现催化剂回收再用增加了技术运行成本,因此,高效非均相催化的研发及运用成为该领域近年来的研究热点。本文以催化剂种类为主线,以酚类废水为处理目标,对近年来湿式空气非均相催化氧化研究与进展进行了评述,并探讨湿式空气非均相催化氧化的机理及催化剂失活问题。
2 湿式空气非均相催化氧化技术
在众多的有机污染物质中,酚类物质因其显著的毒性和总量大的排放特性而受到广泛的关注[5,14,15],此外,酚类还是多种芳香族化合物氧化降解过程的中间产物,是研究湿式空气非均相催化氧化技术降解有机污染物的研究的主要降解目标物质之一[16,17]。
2.1 金属氧化物催化湿式空气氧化
金属氧化物具有造价相对低廉,具有一定催化性能的优点而作为非均相催化剂广泛运用到湿式空气催化氧化体系中以解决传统湿式空气氧化的问题。运用在降解苯酚的金属氧化物催化剂有铜(Cu)、铈(Ce)、锰(Mn)、铁(Fe)、镍(Ni)、铝(Al)、铬(Cr)及钴(Co)等,这些金属氧化物常被固定在催化剂载体上以提高其湿热稳定性。金属氧化物催化湿式空气氧化苯酚的研究见表1。
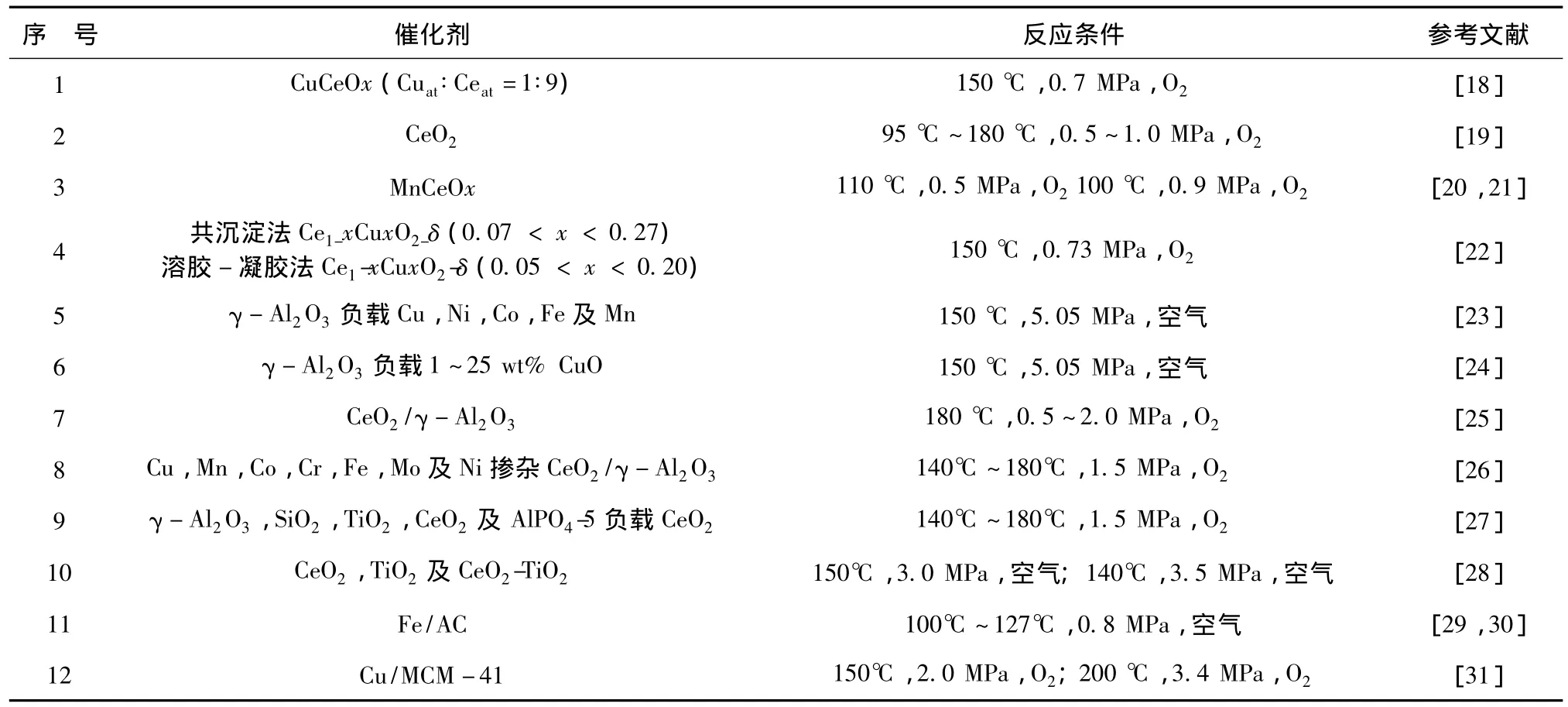
表1 金属氧化物催化湿式空气氧化苯酚的研究Tab.1 Phenol degradation in CWAO with metal oxide catalysts
在众多的金属氧化物催化剂中,含铜催化剂表现出较高催化活性,被广泛应用在湿式催化氧化研究中。在 γ-Al2O3负载铜(Cu)、镍(Ni)、钴(Co)、铁(Fe)及锰(Mn)5种常见过渡金属氧化物的催化应用研究中,CuOx/γ-Al2O3由于其极高的表面还原活性而表现出最高的催化活性[32]。稀土元素铈氧化物因具有特殊的氧化还原及形态特性,已被广泛运用在催化研究中[33~35]。有研究指出[19],CeO2应用在湿式空气催化氧化体系中能发挥较高的催化活性,最佳工艺条件下能对初始浓度为400~2500mg/L的苯酚废水实现高于90%的降解率;对CeO2进行载体固定用在降解苯酚废水的研究,也取得成功的实践,如γ-Al2O3、SiO2、TiO2、CeO2、AlPO4负载。此外,CeO2掺杂过渡金属,如锰(Mn)[20,36,37]、钛(Ti)[28]等,能大幅提高催化剂氧化活性、比表面积及稳定性。尽管金属氧化物在CWAO体系中催化活性低于贵金属,但由于造价相对低廉,仍受到广泛关注。
2.2 贵金属催化湿式空气氧化
相对于普通金属氧化物,贵金属作为催化剂应用在CWAO中,体现出更高的催化活性及结构稳定性。常见贵金属有钌 Ru、铑 Rh、铂 Pt、钯 Pd等。贵金属一般通过负载在催化剂载体上形成,一些贵金属催化剂应用在CWAO中降解苯酚的研究见表2。
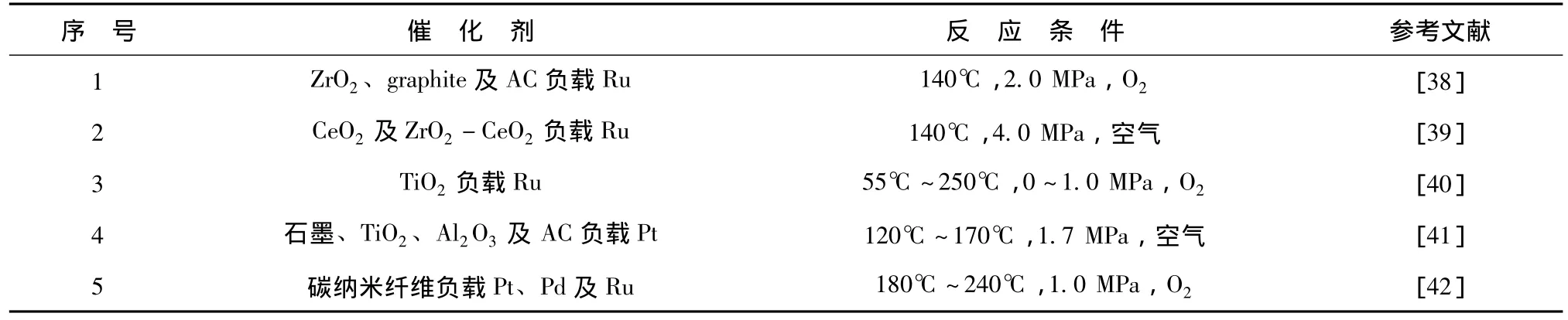
表2 贵金属催化湿式空气氧化苯酚的研究Tab.2 Phenol degradation in CWAO with noble metal catalysts
贵金属催化剂已广泛运用到CWAO降解苯酚的研究中。其中含Ru催化剂表现出最高的催化活性。不同的催化剂载体对贵金属催化剂的催化氧化效率有一定的影响[38]。
2.3 碳材料催化湿式空气氧化
金属氧化物及贵金属催化剂因其较高的催化活性而得到较快的发展和深入的研究,但由于使用过程中有不同程度的金属成分的溶出及含碳化合物的表面沉积及吸附,造成了金属氧化物及贵金属催化剂钝化,催化活性降低[43~46]。因此,具有稳定催化活性的非均相湿式空气氧化催化剂研发成为新的研究热点之一。碳材料因其良好的化学稳定性及优良的表面性质而常被应用为催化剂载体,如活性炭(Activated carbon,AC),石墨 (Graphite),碳纳米管 (Carbon nanotubes,CNTs)及活性炭纤维(Activated carbon fibers,ACF)。近年来一些研究表明,碳材料在不含活性金属或金属氧化物成分情况下[47~51],仍表现出一定的催化性能。催化活性来自碳材料表面的活性表面官能团,这些官能团可能是碳材料原有的,也可能是经特殊化学修饰后形成的[52,53]。应用在苯酚湿式空气氧化降解的碳材料催化剂见表3。
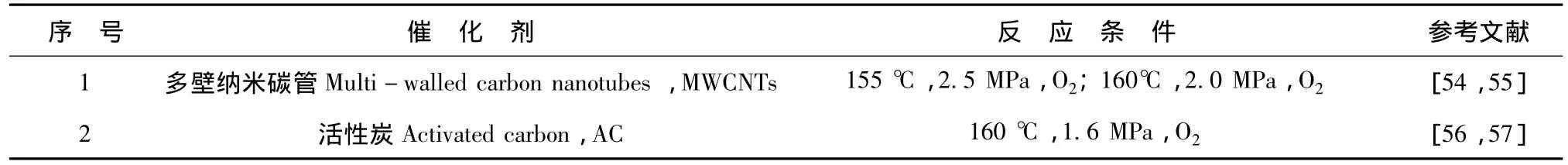
表3 碳材料催化湿式空气氧化苯酚的研究Tab.3 Phenol degradation in CWAO with carbon materials
多壁纳米碳管 (Multi-walled carbon nanotubes,MWCNTs)经HCl及HNO3-H2SO4处理后在湿式空气氧化降解苯酚的体系中显示出优良的催化活性及稳定性[55]。在反应温度为 160℃,2.0MPa,O2条件下经2小时反应苯酚转化率达到100%,76%TOC实现矿化。MWCNTs经HNO3/H2SO4,H2O2,O3及空气等氧化剂处理后实现功能化[54],经处理的MWCNTs均表现出良好的湿式空气催化氧化活性,其中,O3处理之MWCNTs表现出最优的催化活性,最佳工艺条件下苯酚去除率达100%,80%TOC实现矿化,MWCNTs表面的羧酸根和弱酸性质在催化活性方面起到重要作用。
3 湿式空气催化氧化机理
目前,绝大多数研究表明,湿式空气氧化技术的关键作用因子是强氧化性自由基,在传统湿式空气氧化体系中能观察到自由基的产生、传播及扑灭[58]。虽然湿式空气催化氧化降解有机污染物的机理尚不明确[59],一般认为其降解机理与传统湿式空气氧化体系一致[60]。对于有机物降解技术,副产物及最终产物的种类及毒性强弱是重要的评价因子,湿式空气催化氧化体系最终产物与传统湿式空气氧化体系产物相似,除二氧化碳外,多为短链有机物如甲酸、乙酸及草酸等,体系中初始有机物质的形态与结构对最终形成产物类型影响较少[61]。
非均相催化剂在湿式空气催化氧化技术中的运用则是利用其活化、加速反应物降解产生自由基,同时,催化剂通过络合等作用加速了体系中氧原子的传递[62],同样增强了体系氧化作用。
4 技术存在问题
尽管非均相催化剂的使用克服了均相催化剂的诸多问题,且在湿式空气氧化降解有机污染物的运用中起到重要的提高氧化效率的作用,但非均相催化剂的使用普遍存在失活问题,这是该技术主要存在问题[45,46]。众多研究表明,在湿式空气催化氧化技术中,非均相催化剂失活的原因主要有催化剂表面碳沉积及催化剂溶出。如何解决非均相催化剂的失活问题,成为该技术研究的重点之一。
4.1 催化剂表面碳沉积
与众多的高级氧化技术一样,催化剂表面碳沉积影响湿式空气催化氧化体系主要是通过阻隔反应物及溶解氧与催化剂表面活性位的接触。湿式空气催化氧化体系通过催化剂的使用能有效降低反应体系要求的温度与压力,但温和的反应环境增加了催化剂表面中间产物及聚合物的吸附,降低了氧化反应效率[63]。在锰(Mn),铁(Fe),钴(Co),镍(Ni)及铜(Cu)等常见的应用在湿式空气催化氧化的过度金属中,锰 (Mn)表现出最严重的碳沉积现象[23],贵金属催化剂同样存在严重的表面碳沉积现象[64,65]。
4.2 催化剂溶出
催化剂溶出是均相催化氧化技术的主要问题之一。非均相催化湿式空气氧化降解有机污染物体系中,随着反应进行,有机酸中间产物生成降低体系的pH值[66],加之反应温度较高,压力较大,在这样的体系中,酸性介质对金属催化剂及催化剂载体的溶出作用明显[67],活性组分的流失将严重影响催化剂的活性,降低体系的氧化反应速率,此外,严重的金属溶出还将影响水质。众多研究表明[68~70],拥有高催化活性的铜系非均相催化剂在湿式空气催化氧化体系中存在严重的溶出问题,在pH低于4的介质中,铜溶出极为严重[68]。
4.3 解决催化剂失活问题
解决催化剂失活或活性降低的问题有多重途径,针对催化剂表面碳沉积,热处理是主要的催化剂再生方法,通常是将碳沉积催化剂置于400℃左右的氧化性气流中[71],在热处理过程中去除表面沉积碳成分;有机溶剂冲洗也是去除表面沉积碳成分的主要方法[72]。针对金属溶出,有研究针对反应体系pH进行控制,加入缓冲溶液以控制pH使得氧化反应在中性环境中进行,能有效降低催化剂中金属成分的溶出,但严重影响了氧化反应效率[69];金属溶出的主要解决思路是对催化剂进行掺杂、改性等,如对MnO2-CeO2进行铂(Pt)或银(Ag)的掺杂,能有效降低催化剂反应过程的碳沉积[73];有研究对催化剂进行有机模板改性,利用模板的憎水性减少催化剂跟液相的接触,但不阻碍污染物在催化剂表面的吸附及分散,能有效减低溶液介质对金属成分的溶解作用[74]。
5 结论与展望
非均相催化湿式空气氧化技术能解决传统湿式空气氧化技术的缺点,大幅提高反应速率,提高难降解有机污染物的去除效率,提高体系污染物负荷,降低反应耗时,降低反应温度及压力,从而获得更好的效率并降低技术的运行成本,是极具前景的有机废水处理技术。运用在湿式空气催化氧化技术中的非均相催化剂主要有过度金属氧化物、贵金属及碳材料等。研究表明,自由基的氧化作用为该技术的作用因子,催化剂失活导致反应速率下降为该技术的主要问题,包括碳沉积及金属溶出为均相催化剂失活的主要原因。针对技术特点及研究现状,高催化活性、可重复利用非均相催化剂的研发为其主要发展方向之一。
[1]Yap P,Lim T.Effect of aqueous matrix species on synergistic removal of bisphenol-A under solar irradiation using nitrogen-doped TiO2/AC composite[J].Applied Catalysis B:Environmental,2011,101(3-4):709-717.
[2]Liu H,Wang C,Li X,et al.A novel electro-fenton process for water treatment:reaction-controlled pH adjustment and performance assessment.[J].Environ Sci Technol,2007,41(8):2937-2942.
[3]Pignatello J J.Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide[J].Environmental Science & Technology,1992,26(5):944-951.
[4]Sun Y,Pignatello J J.Photochemical reactions involved in the total mineralization of 2,4-D by iron(3+)/hydrogen peroxide/UV[J].Environmental science & technology,1993,27(2):304-310.
[5]Mishra V S,Mahajani V V,Joshi J B.Wet air oxidation[J].Industrial& engineering chemistry research,1995,34(1):2-48.
[6]Hua L,Ma H,Zhang L.Degradation process analysis of the azo dyes by catalytic wet air oxidation with catalyst CuO/γ-Al2O3[J].Chemosphere,2013,90(2):143-149.
[7]Vallet A,Ovejero G,Rodríguez A,et al.Ni/MgAlO regeneration for catalytic wet air oxidation of an azo-dye in trickle-bed reaction[J].Journal of Hazardous Materials,2013,244-245,46-53.
[8]Oliviero L,Barbier Jr.J,Duprez D.Wet Air Oxidation of nitrogen-containing organic compounds and ammonia in aqueous media[J].Applied Catalysis B:Environmental,2003,40(3):163-184.
[9]Levec J,Pintar A.Catalytic wet-air oxidation processes:A review[J].Catalysis Today,2007,124(3-4):172-184.
[10]Dai Q,Zhou M,Lei L.Wet electrolytic oxidation of cationic red X-GRL[J].Journal of Hazardous Materials,2006,137(3):1870-1874.
[11]Bhargava S K,Tardio J,Prasad J,et al.Wet oxidation and catalytic wet oxidation[J].Industrial & engineering chemistry research,2006,45(4):1221-1258.
[12]Lopes R J G,Silva A M T,Quinta-Ferreira R M.Screening of catalysts and effect of temperature for kinetic degradation studies of aromatic compounds during wet oxidation[J].Applied Catalysis B:Environmental,2007,73(1-2):193-202.
[13]Kim S,Lee D.Catalytic wet peroxide oxidation of dyehouse effluents with Cu/Al2O3and copper plate[J].Studies in Surface Science and Catalysis,2006,159:393-396.
[14]Yu H,Nie E,Xu J,et al.Degradation of Diclofenac by Advanced Oxidation and Reduction Processes:Kinetic Studies,Degradation Pathways and Toxicity Assessments[J].Water research,2013,47(5):1909-1918.
[15]Mukherjee S,Basak B,Bhunia B,et al.Potential use of polyphenol oxidases(PPO)in the bioremediation of phenolic contaminants containing industrial wastewater[J].Reviews in Environmental Science and Bio/Technology,2013,12(1):61-73.
[16]Ding Z,Aki S N V K,Abraham M A.Catalytic Supercritical Water Oxidation:Phenol Conversion and Product Selectivity[J].Environmental Science & Technology,1995,29(11):2748-2753.
[17]Santos A,Yustos P,Durban B,et al.Catalytic Wet Oxidation of Phenol:Kinetics of the Mineralization Rate[J].Industrial& Engineering Chemistry Research,2001,40(13):2773-2781.
[18]Arena F,Giovenco R,Torre T,et al.Activity and resistance to leaching of Cu-based catalysts in the wet oxidation of phenol[J].Applied Catalysis B:Environmental,2003,45(1):51-62.
[19]Lin S S,Chen C L,Chang D J,et al.Catalytic wet air oxidation of phenol by various CeO2catalysts[J].Water Research,2002,36(12):3009-3014.
[20]Chen H,Sayari A,Adnot A,et al.Composition-activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation[J].Applied Catalysis B:Environmental,2001,32(3):195-204.
[21]Arena F,Trunfio G,Negro J,et al.Synthesis of highly dispersed MnCeOxcatalysts via a novel“redox-precipitation”route[J].Materials Research Bulletin,2008,43(3):539-545.
[22]Hoc∨evar S,Krašovec U O,Orel B,et al.CWO of phenol on two differently prepared CuO-CeO2catalysts[J].Applied Catalysis B:Environmental,2000,28(2):113-125.
[23]Kim S,Ihm S.Nature of carbonaceous deposits on the alumina supported transition metal oxide catalysts in the wet air oxidation of phenol[J].Topics in catalysis,2005,33(1-4):171-179.
[24]Kim S,Kim K,Ihm S.The characteristics of wet air oxidation of phenol over CuOx/Al2O3catalysts:Effect of copper loading[J].Chemosphere,2007,68(2):287-292.
[25]Chang L,Chen I,Lin S.An assessment of the suitable operating conditions for the CeO2/γ-Al2O3catalyzed wet air oxidation of phenol[J].Chemosphere,2005,58(4):485-492.
[26]Chen I,Lin S,Wang C,et al.CWAO of phenol using CeO2/γ-Al2O3with promoter—Effectiveness of promoter addition and catalyst regeneration[J].Chemosphere,2007,66(1):172-178.
[27]Chen I,Lin S,Wang C,et al.Preparing and characterizing an optimal supported ceria catalyst for the catalytic wet air oxidation of phenol[J].Applied Catalysis B:Environmental,2004,50(1):49-58.
[28]Yang S,Zhu W,Wang J,et al.Catalytic wet air oxidation of phenol over CeO2-TiO2catalyst in the batch reactor and the packed-bed reactor[J].Journal of Hazardous Materials,2008,153(3):1248-1253.
[29]Quintanilla A,Menéndez N,Tornero J,et al.Surface modification of carbon-supported iron catalyst during the wet air oxidation of phenol:Influence on activity,selectivity and stability[J].Applied Catalysis B:Environmental,2008,81(1-2):105-114.
[30]Quintanilla A,Casas J A,Zazo J A,et al.Wet air oxidation of phenol at mild conditions with a Fe/activated carbon catalyst[J].Applied Catalysis B:Environmental,2006,62(1-2):115-120.
[31]Wu Q,Hu X,Yue P L,et al.Copper/MCM-41 as catalyst for the wet oxidation of phenol[J].Applied Catalysis B:Environmental,2001,32(3):151-156.
[32]Kim S,Ihm S.Nature of carbonaceous deposits on the alumina supported transition metal oxide catalysts in the wet air oxidation of phenol[J].Topics in Catalysis,2005,33(1-4):171-179.
[33]Fuku K,Goto M,Sakano T,et al.Efficient degradation of CO and acetaldehyde using nano-sized Pt catalysts supported on CeO2and CeO2/ZSM-5 composite[J].Catalysis Today,2013,201:57-61.
[34]De Leitenburg C,Goi D,Primavera A,et al.Wet oxidation of acetic acid catalyzed by doped ceria[J].Applied Catalysis B:Environmental,1996,11(1):L29-L35.
[35]Imamura S,Okumura Y,Nishio T,et al.Wet-Oxidation of a Model Domestic Wastewater on a Ru/Mn/Ce Composite Catalyst[J].Industrial& Engineering Chemistry Research,1998,37(3):1136-1139.
[36]Santiago A F J,Sousa J F,Guedes R C,et al.Kinetic and wet oxidation of phenol catalyzed by non-promoted and potassium-pro-moted manganese/cerium oxide[J].Journal of Hazardous Materials,2006,138(2):325-330.
[37]Arena F,Negro J,Parmaliana A,et al.Improved MnCeOxSystems for the Catalytic Wet Oxidation(CWO)of Phenol in Wastewater Streams[J].Industrial& Engineering Chemistry Research,2007,46(21):6724-6731.
[38]Castillejos-López E,Maroto-Valiente A,Nevskaia D M,et al.Comparative study of support effects in ruthenium catalysts applied for wet air oxidation of aromatic compounds[J].Catalysis Today,2009,143(3-4):355-363.
[39]Wang J,Zhu W,Yang S,et al.Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts[J].Applied Catalysis B:Environmental,2008,78(1-2):30-37.
[40]Pintar A,Batista J,Ti ler T.Catalytic wet-air oxidation of aqueous solutions of formic acid,acetic acid and phenol in a continuous-flow trickle-bed reactor over Ru/TiO2 catalysts[J].Applied Catalysis B:Environmental,2008,84(1-2):30-41.
[41]Masende Z,Kuster B,Ptasinski K J,et al.Support and dispersion effects on activity of platinum catalysts during wet oxidation of organic wastes[J].Topics in catalysis,2005,33(1-4):87-99.
[42]Taboada C D,Batista J,Pintar A,et al.Preparation,characterization and catalytic properties of carbon nanofiber-supported Pt,Pd,Ru monometallic particles in aqueous-phase reactions[J].Applied Catalysis B:Environmental,2009,89(3-4):375-382.
[43]Alejandre A,Medina F,Fortuny A,et al.Characterisation of copper catalysts and activity for the oxidation of phenol aqueous solutions[J].Applied Catalysis B:Environmental,1998,16(1):53-67.
[44]Zhang Q,Chuang K T.Treatment of Combined Bleach Plant Effluents via Wet Oxidation over a Pd-Pt-Ce/Alumina Catalyst[J].Environmental Science & Technology,1999,33(20):3641-3644.
[45]Lee D,Kim D,Kim T,et al.Deactivation of Pt catalysts during wet oxidation of phenol[J].Catalysis Today,2010,154(3-4):244-249.
[46]Kouraichi R,Delgado J J,López-Castro J D,et al.Deactivation of Pt/MnOx-CeO2catalysts for the catalytic wet oxidation of phenol:Formation of carbonaceous deposits and leaching of manganese[J].Catalysis Today,2010,154(3-4):195-201.
[47]Liu Z,Ma J,Cui Y,et al.Influence of different heat treatments on the surface properties and catalytic performance of carbon nanotube in ozonation[J].Applied Catalysis B:Environmental,2010,101(1-2):74-80.
[48]Keller N,Maksimova N I,Roddatis V V,et al.The Catalytic Use of Onion-Like Carbon Materials for Styrene Synthesis by Oxidative Dehydrogenation of Ethylbenzene[J].Angewandte Chemie International Edition,2002,41(11):1885-1888.
[49]Li L,Ye W,Zhang Q,et al.Catalytic ozonation of dimethyl phthalate over cerium supported on activated carbon[J].Journal of Hazardous Materials,2009,170(1):411-416.
[50]Serp P,Corrias M,Kalck P.Carbon nanotubes and nanofibers in catalysis[J].Applied Catalysis A:General,2003,253(2):337-358.
[51]Rocha R P,Sousa J P S,Silva A M T,et al.Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid:The role of the basic nature induced by the surface chemistry[J].Applied Catalysis B:Environmental,2011,104(3-4):330-336.
[52]Besson M,Gallezot P,Perrard A,et al.Active carbons as catalysts for liquid phase reactions[J].Catalysis Today,2005,102-103:160-165.
[53]Korovchenko P,Donze C,Gallezot P,et al.Oxidation of primary alcohols with air on carbon-supported platinum catalysts for the synthesis of aldehydes or acids[J].Catalysis Today,2007,121(1-2):13-21.
[54]Yang S,Wang X,Yang H,et al.Influence of the different oxidation treatment on the performance of multi-walled carbon nanotubes in the catalytic wet air oxidation of phenol[J].Journal of Hazardous Materials,2012,233-234:18-24.
[55]Yang S,Li X,Zhu W,et al.Catalytic activity,stability and structure of multi-walled carbon nanotubes in the wet air oxidation of phenol[J].Carbon,2008,46(3):445-452.
[56]Cordero T,Rodríguez-Mirasol J,Bedia J,et al.Activated carbon as catalyst in wet oxidation of phenol:Effect of the oxidation reaction on the catalyst properties and stability[J].Applied Catalysis B:Environmental,2008,81(1-2):122-131.
[57]Santos A,Yustos P,Cordero T,et al.Catalytic wet oxidation of phenol on active carbon:stability,phenol conversion and mineralization[J].Catalysis Today,2005,102-103:213-218.
[58]Robert R E L,Barbati S E P,Ricq N,et al.Intermediates in wet oxidation of cellulose:identification of hydroxyl radical and characterization of hydrogen peroxide[J].Water research,2002,36(19):4821-4829.
[59]Mantzavinos D,Hellenbrand R,Livingston A G,et al.Catalytic wet oxidation of p-coumaric acid:Partial oxidation intermediates,reaction pathways and catalyst leaching[J].Applied Catalysis B:Environmental,1996,7(3-4):379-396.
[60]Debellefontaine H,Foussard J N E L.Wet air oxidation for the treatment of industrial wastes.Chemical aspects,reactor design and industrial applications in Europe[J].Waste management,2000,20(1):15-25.
[61]Eftaxias A,Font J,Fortuny A,et al.Kinetic modelling of catalytic wet air oxidation of phenol by simulated annealing[J].Applied Catalysis B:Environmental,2001,33(2):175-190.
[62]Cavani F,Trifir O F.Classification of industrial catalysts and catalysis for the petrochemical industry[J].Catalysis today,1997,34(3-4):269-279.
[63]Cybulski A,Trawczy N Ski J.Catalytic wet air oxidation of phenol over platinum and ruthenium catalysts[J].Applied Catalysis B:Environmental,2004,47(1):1-13.
[64]Mikulov A J,Barbier J,Rossignol S,et al.Wet air oxidation of acetic acid over platinum catalysts supported on cerium-based materials:Influence of metal and oxide crystallite size[J].Journal of catalysis,2007,251(1):172-181.
[65]Lee D,Kim D,Kim T,et al.Deactivation of Pt catalysts during wet oxidation of phenol[J].Catalysis Today,2010,154(3):244-249.
[66]Alejandre A,Medina F,Rodriguez X,et al.Cu/Ni/Al layered double hydroxides as precursors of catalysts for the wet air oxidation of phenol aqueous solutions[J].Applied Catalysis B:Environmental,2001,30(1-2):195-207.
[67]Kouraichi R,Delgado J J,López-Castro J D,et al.Deactivation of Pt/MnOx-CeO2catalysts for the catalytic wet oxidation of phenol:Formation of carbonaceous deposits and leaching of manganese[J].Catalysis Today,2010,154(3-4):195-201.
[68]Santos A,Yustos P,Quintanilla A,et al.Study of the copper leaching in the wet oxidation of phenol with CuO-based catalysts:Causes and effects[J].Applied Catalysis B:Environmental,2005,61(3-4):323-333.
[69]Santos A,Yustos P,Durbán B,et al.Oxidation of phenol in aqueous solution with copper catalysts[J].Catalysis Today,2001,66(2-4):511-517.
[70]Kim K,Kim J,Ihm S.Wet oxidation of phenol over transition metal oxide catalysts supported on Ce0.65Zr0.35O2prepared by continuous hydrothermal synthesis in supercritical water[J].Journal of Hazardous Materials,2009,167(1-3):1158-1162.
[71]Massa P,Ivorra F,Haure P,et al.Catalytic wet air oxidation of phenol aqueous solutions by 1%Ru/CeO2-Al2O3catalysts prepared by different methods[J].Catalysis Communications,2007,8(3):424-428.
[72]Chen I,Lin S,Wang C,et al.CWAO of phenol using CeO2/γ-Al2O3with promoter—Effectiveness of promoter addition and catalyst regeneration[J].Chemosphere,2007,66(1):172-178.
[73]Hamoudi S,Sayari A,Belkacemi K,et al.Catalytic wet oxidation of phenol over PtxAg1-xMnO2/CeO2catalysts[J].Catalysis Today,2000,62(4):379-388.
[74]Massa P,Ivorra F,Haure P,et al.Optimized wet-proofed CuO/Al2O3catalysts for the oxidation of phenol solutions:Enhancing catalytic stability[J].Catalysis Communications,2009,10(13):1706-1710.
