EEDs对鱼类性激素合成途径干扰作用研究进展
2013-08-20王慧田华汝少国
王慧,田华,汝少国
中国海洋大学海洋生命学院,青岛266003
性激素在鱼类生殖系统发育中起着决定性的作用。性激素主要由性腺合成并分泌,可以通过血液循环运输到其他的靶组织,与细胞核内的雌激素受体、雄激素受体等核受体结合而发挥作用。许多环境内分泌干扰物如双酚A(bisphenol A,BPA)、4–壬基酚、己烯雌酚(diethylstilbestrol,DES)、o,p'–滴滴涕等具有与生物体内雌激素相类似的结构,可作为配体与雌激素受体结合[1-2],通过性激素受体介导途径发挥类雌激素效应,这种作用方式被认为是环境内分泌干扰物(environmental endocrine disruptors,EEDs)发挥内分泌干扰作用的经典作用途径,这类化合物也被称为环境雌激素。此外,环境中还存在很多化学物质,虽然其结构与内源性激素并不相似,不能直接与激素受体相结合,但在体内实验中却也表现出内分泌干扰效应,这可能是其影响了生物体内性激素的合成、转运、代谢、清除等过程,从而影响了性激素的含量,这些作用被统称为非性激素受体介导途径[3]。近年来,研究发现,某些典型的环境雌激素如 17α-炔雌醇(17α-ethinylestradiol,EE2)、BPA也可以通过非性激素受体介导途径发挥内分泌干扰作用[4-5]。性激素的合成涉及到一系列的酶促反应,EEDs对性激素合成途径的干扰可能是影响了性激素合成底物的含量,也可能是影响了类固醇生成酶mRNA的表达和/或活性。已经证实,芳香化酶抑制剂法倔唑、杀菌剂咪鲜胺、除草剂阿特拉津等可通过非受体介导途径,影响性腺性激素合成途径中相关酶的基因表达和/或活性[6],导致性激素水平紊乱,进而干扰生物体的生殖和发育过程。本文综述了鱼类的性激素合成途径,EEDs对性激素合成底物和类固醇生成酶的影响,EEDs影响性激素合成的信号转导机制,以及EEDs对性激素水平的影响和生殖危害,以期为EEDs发挥内分泌干扰效应的作用机制研究提供借鉴。
1 鱼类性激素合成途径
在垂体促性腺激素(gonadotropins,GtHs)包括卵泡刺激素(follicle-stimulating hormone,FSH)和促黄体激素(luteinizing hormone,LH)的调控下,性腺特化的体细胞分泌产生性激素,性激素对性腺发育、卵黄形成、卵细胞成熟、精子生成和精子排放等生理过程具有重要的调控作用。一般硬骨鱼体内发挥作用的主要性激素为17β-雌二醇(17β-estradiol,E2)、睾酮(testosterone,T)和11-酮基睾酮(11-ketotestosterone,11-KT)[7]。LH 调控雄激素的合成和分泌,FSH控制雄激素向雌激素的转化。性激素合成的反应底物为胆固醇,在类固醇激素合成急性调节蛋白(steroidogenic acute regulatory protein,StAR)[8]的作用下,胆固醇由线粒体外膜转运至内膜,然后在胆固醇侧链裂解酶(cholesterol side chain cleavage enzyme,CYP11A1/P450scc)的作用下,转化为孕烯醇酮。在滑面内质网中,3β-羟类固醇脱氢酶(3βhydroxysteroid dehydrogenase,3β-HSD)催化孕烯醇酮转化为孕酮,17α羟化酶(又名17,20-裂解酶,cytochrome P450 17 alpha-hydroxylase,17,20-lyase,CYP17/P450c17/P45017α)催化孕烯醇酮和孕酮转化为雄激素。雄烯二酮转化为E2的合成途径有2条:一是芳香化酶(cytochrome P450 aromatase,CYP19/P450arom)催化雄烯二酮转化为雌酮,然后雌酮经17β-羟类固醇脱氢酶 I(17β-hydroxysteroid dehydrogenase type I,17β-HSD1)催化转化为 E2;二是17β-羟类固醇脱氢酶 III(17β-hydroxysteroid dehydrogenase type III,17β-HSD3)催化雄烯二酮转化为T,T再经芳香化酶催化转变为E2[9]。其中,芳香化酶是E2合成过程的限速酶,其所催化的雄激素向雌激素的转化过程对鱼类性腺发育是必不可少的。11β-羟化酶(11β-hydroxylase,P45011β)催化 T 向11β-羟基睾酮的转化[10],再经 11β-羟类固醇脱氢酶II(11β-hydroxysteroid dehydrogenase type II,11β-HSD2)作用生成11-KT,这2种酶对鱼类精巢中11-KT的合成具有重要作用。各种酶类催化的反应过程如图1所示。
FSH和LH通过激活cAMP/PKA信号转导途径,调节性激素的合成。类固醇生成酶基因启动子上的顺式作用元件是cAMP/PKA刺激类固醇生成酶基因转录的作用位点[11-12]。GtHs与其膜受体结合后,激活G蛋白,活化腺苷酸环化酶合成cAMP,细胞内cAMP浓度增加可激活蛋白激酶A(protein kinase A,PKA),PKA进而通过调节某些转录因子的磷酸化水平实现对类固醇生成酶转录水平的调控。目前已知与类固醇生成酶表达相关的转录因子有甾体生成因子(steroidogenic factor 1,SF-1)和cAMP应答元件结合蛋白(cAMP response element binding protein,CREB)等。研究证实CREB通过133位丝氨酸的磷酸化/去磷酸化作用调节P450arom基因启动子上的cAMP应答元件(cAMP response element,CRE),从而起始P450arom基因的转录[13-14]。SF-1是孤儿核受体超家族的一员,是许多cAMP依赖的靶基因包括P450arom、CYP11A1、StAR等的转录因子。已有研究证实SF-1是cAMP/PKA途径的应答基因,SF-1存在潜在的PKA磷酸化作用位点,体外实验直接证实了SF-1磷酸化依赖于PKA的激活[15-16]。Michael等[17]研究表明,cAMP 对人卵巢芳香化酶转录的刺激作用至少部分是由于SF-1水平升高及其与芳香化酶基因结合活性增强所致。
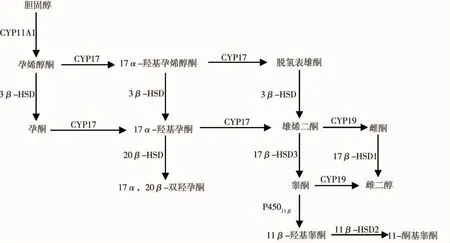
图1 鱼类性激素合成途径Fig.1 Sex hormone biosynthesis pathway in fish
2 EEDs对性激素合成途径的影响及其信号转导机制
2.1 EEDs对性激素合成底物的影响
理论上,性激素合成途径中的任何因子均可作为外源化合物作用的潜在靶位点。哺乳动物中,性激素合成的底物——胆固醇的来源包括3个方面:①从头合成的胆固醇;② 细胞内以胆固醇酯形式储存的胆固醇;③ 脂蛋白从血浆中摄取的胆固醇[18]。在硬骨鱼类中,类固醇合成的底物主要为从血浆中摄取的外源胆固醇[19]。在脂蛋白的作用下,胆固醇经血液循环从合成或吸收部位运送到作用部位。在哺乳动物中,低密度脂蛋白(low density lipoprotein,LDL)起主要作用,Becker等[20]研究表明,谷甾醇是一种治疗重度小儿家族性高胆固醇血症的药物,同时具有内分泌干扰活性,服用3个月后患者血清中LDL的浓度降低了20%。而在鱼类中,大多数的胆固醇转运过程由高密度脂蛋白(high density lipoproteins,HDL)负责完成[21]。Sharpe 等[19]研究表明,雄性金鱼(Carassius auratus)暴露于10μg·g-1E2中5个月,血浆中HDL和T显著降低。因此,EEDs也可以通过降低/提高鱼类血浆中HDL的含量,影响运输到性腺细胞的胆固醇含量,最终干扰性激素的合成。
胆固醇被转运至线粒体内膜才能启动性激素合成途径。研究发现,胆固醇从线粒体外膜转运至内膜的跨膜转运是性激素合成的限速步骤[22]。StAR是胆固醇的一种转运蛋白,主要参与胆固醇的代谢。StAR蛋白受损可导致性激素合成水平的急剧下降或性激素合成途径的中断,此外EEDs也可能通过改变StARmRNA表达水平,导致性激素合成底物的减少。例如,雄性金鱼(Carassius auratus)暴露于200 μg·g-1的 β-谷甾醇(纯度为 72.6%)5 个月,精巢中StAR的转录水平显著降低,使进入线粒体内膜的胆固醇含量降低,血浆中的T浓度显著减少[19]。
2.2 EEDs对类固醇生成酶的影响
2.2.1 体内实验检测EEDs对类固醇生成酶的影响
EEDs可通过干扰性激素合成途径中类固醇生成酶的mRNA表达水平或活性,干扰性激素的合成,影响鱼类正常的生殖功能。不同的EEDs作用于不同的酶类,例如法倔唑是一种典型的性腺P450arom抑制剂[23],曲洛司坦可选择性降低 3β-HSD的活性[24],氨鲁米特可特异性抑制 CYP11A1的活性[25]。而某些外源化合物可同时作用于多种类固醇生成酶,例如杀菌剂咪鲜胺可抑制性腺P450arom 和 CYP17 的 活 性[26],查 尔 酮 是 性 腺P450arom 和17β-HSD 的抑制剂[27],而酮康唑是细胞色素P450酶(cytochrome P450 enzymes,CYPs)的非特异性抑制剂,可降低脊椎动物性激素合成途径中不同CYPs的活性,其中CYP11A1和CYP17为主要的作用靶点[28]。
类固醇生成酶基因表达的上调/下调会导致酶活性的增强/减弱,从而升高/降低鱼类体内的性激素水平。Ma等[29]研究发现,2,4-二氯酚可减少雌性斑马鱼(Danio rerio)性腺P450arom mRNA表达量,进而降低E2浓度。但近年来,有研究表明,补偿效应可能会使类固醇生成酶mRNA水平与酶活性的变化趋势相反[30]。某些EEDs可降低性激素的合成,鱼类对这类EEDs的应答之一是通过提高类固醇生成酶mRNA的表达水平,增强自身性激素的合成[28]。Skolness 等[26]对雌性黑头呆鱼(Pimephales promelas)研究表明,咪鲜胺减少了卵巢中E2的合成,但CYP19 mRNA和CYP17 mRNA的转录水平上调。Ankley等[28]研究表明,酮康唑降低了雌雄黑头呆鱼(Pimephales promelas)性腺中T的合成及雌鱼中E2的合成,但CYP11A1 mRNA和CYP17 mRNA的表达增强。
2.2.2 体外实验证实EEDs对类固醇生成酶的直接影响
H295R细胞系能够表达多种类固醇生成酶[31],Sanderson等[32]采用H295R细胞系研究发现,咪唑类杀菌剂可以抑制芳香化酶活性,乙烯菌核利和阿特拉津可通过增强细胞内的cAMP水平,诱导芳香化酶的转录和催化活性。Ma等[33]研究发现,五氯酚和 2,4,6-三氯苯酚抑制了 H295R细胞系CYP11A1、CYP17、CYP19、3β-HSD 以及 17β-HSD mRNA表达水平,从而显著降低了T和E2的含量。Villeneuve等[34]分别采用 H295R细胞系和黑头呆鱼(Pimephales promelas)卵巢组织离体培养方法,检测了法倔唑、氯苯嘧啶醇、酮康唑、咪鲜胺、乙烯菌核利和扑灭通对性激素合成的影响,发现这6种化学物质均能够显著影响E2和/或T的合成。
此外,可在共培养的条件下,以癌细胞系中特异性雌激素调控基因的转录水平来反映原代培养细胞中芳香化酶的表达情况。例如,人乳腺成纤维细胞和人乳腺癌细胞系(human breast adenocarcinoma cell line,MCF-7)共培养条件下,外源化学物质通过刺激乳腺成纤维细胞中芳香化酶活性导致雌激素合成增强,雌激素水平升高能够刺激MCF-7细胞雌激素调控基因pS2的表达,因此可用pS2基因的表达水平变化表征外源化合物的雌激素活性大小。Heneweer等[35]采用此体系检测了 E2、DES、BPA 和地塞米松(dexamethasone,DEX)等化学物质的雌激素活性,其中E2、DES和BPA使pS2的转录水平呈现3~7倍增长。共培养体系中雌激素化合物如BPA显示出比仅在MCF-7细胞培养体系中更强的雌激素活性,即在较低的浓度下即可诱导pS2的表达[36]。
2.3 EEDs影响类固醇生成的信号转导机制
外源化合物之所以能在转录水平上影响鱼类类固醇生成酶基因的表达,与cAMP、PKA、SF-1等所介导的胞内信号转导途径有关。cAMP和PKA活性增强是类固醇生成酶基因表达的第一步[37],在人、大鼠、小鼠等哺乳动物中已经证实EEDs可通过影响cAMP和/或PKA浓度,造成孕酮、睾酮、雌激素水平异常。48 h 的 10 nmol·L-12,3,7,8-四氯二苯并二恶英(2,3,7,8-tetrachlorodibenzo-p-dioxin,TCDD)暴露使人卵巢黄素化颗粒细胞(human luteinizing granulosa cells,LGCs)中PKA和孕酮的含量显著降低[38];Qu 等[13]推测氰戊菊酯可能是通过减少cAMP的形成而抑制小鼠间质细胞瘤细胞(mouse Leydig tumor cells,MLTC-1)中人绒毛膜促性腺激素(human chorionic gonadotropin,hCG)诱导的孕酮分泌。Ronco等[39]认为,林丹对大鼠精巢中睾酮生成的抑制作用(至少部分)是由间质细胞cAMP水平降低引起的。磷酸二酯酶4(phosphodiesterase4,PDE4)可特异性水解 cAMP,Kucka等[40]证实,PDE4抑制剂阿特拉津可以造成细胞内和细胞外cAMP的积累,进而激活PKA。
cAMP激活PKA,引发一系列的信号级联放大,最终增强转录因子如SF-1的活性和类固醇生成酶基因的表达。EEDs可能通过改变某些转录因子的表达,影响类固醇生成酶的mRNA表达水平或酶活性,进而干扰性激素的合成途径。斑马鱼(Danio rerio)脑芳香化酶基因cyp19a2包含一个雌激素应答元件(estrogen responsive element,ERE),对雌激素的应答有重要作用,而性腺芳香化酶基因cyp19a1包含SF-1转录调控元件,阿特拉津暴露导致斑马鱼cyp19a1表达上调,而cyp19a2表达无显著变化[41],这表明阿特拉津是通过非性激素受体介导途径发挥内分泌干扰效应的。Govoroun等[42]检测到虹鳟鱼(Oncorhynchus mykiss)经 E2暴露后精巢中 3β-HSD、P450c17、P45011βmRNA表达水平均降低,推测可能受同一个上游转录因子如SF-1的调控。这种“转录因子SF-1水平异常”与“性激素浓度紊乱”的相关关系已经在啮齿类、哺乳类中得到证实。如妊娠期暴露邻苯二甲酸二辛酯(diethylhexyl phthalate,DEHP)导致新生小鼠精巢中SF-1 mRNA表达水平降低,进而造成StAR和P450scc 基因表达下调[43];Du 等[44]研究表明,全氟辛酸(perfluorooctanoic acid,PFOA)能够显著降低人肾上腺皮质瘤H295R细胞中SF-1mRNA和蛋白水平的表达,进而进一步抑制类固醇生成酶CYP11A1和CYP17 mRNA的表达。
3 EEDs对性激素水平的影响及其生殖危害
EEDs可通过对鱼类性激素合成途径的干扰作用改变性激素正常的合成和/或分泌水平,进而干扰鱼类正常的生殖功能[45]。如 Govoroun 等[42]证实了外源雌激素暴露情况下,虹鳟鱼(Oncorhynchusmykiss)精巢分化期和分化后期P450c17、3β-HSD和P45011βmRNA的表达水平受抑制与雄鱼雌性化有关;Yokota等[46]研究表明≥238 μg·L-14-叔戊基苯酚(4-tertpentylphenol,4-PP)暴露使基因型雄性青鳉(Oryzias latipes)精巢中P45011β的mRNA水平受到显著抑制,并导致性逆转现象;对于与E2结构差异较大的有机磷农药久效磷,体外实验证实其不能模拟E2与雌激素受体结合[47],却能够通过促进性腺芳香化酶的表达,造成雄性金鱼(Carassius auratus)体内E2水平升高和T水平降低[48],并诱导雄性孔雀鱼(Poecilia reticulata)及斑马鱼(Danio rerio)雌性化[49-50]。
EEDs在环境中不易降解,一旦进入水体等环境介质,将会对鱼类造成生殖危害。早在20世纪90年代初,Purdom等[51]在英国污水处理厂出水口下游的泻湖中发现了具有雌雄两性特征的斜齿鳊鱼(Rutilus rutilus)。近年来研究表明,污水处理厂排水中含有E2、17α-炔雌醇、烷基酚和双酚A,导致类固醇生成酶基因表达异常、体内性激素水平改变,从而诱导鱼类产生卵精巢兼性结构[52-54]。自然界中EEDs对鱼类的繁殖影响已非常普遍,对鱼类的生存和繁衍构成了严重的威胁,佛罗里达州的阿波普卡湖污染严重,抗雄激素污染物使雄性食蚊鱼(Gambusia holbrooki)精子数目减少,生殖足变短[55]。密西西比河有机氯杀虫剂污染严重,其中29%的雄性密西西比铲鲟(Scaphirhynchus platyorynchus)出现雌雄同体现象[56]。
4 研究展望
4.1 重视生殖轴线多个位点的正负反馈调节机制
性激素的调控是一个复杂的过程,涉及到生殖轴线上多个位点的正负反馈调节机制。因此,体内实验中所表现出的类固醇生成酶mRNA表达和/或活性的变化,既可能是外源化合物对性腺组织的直接作用,也可能是上游生殖内分泌系统扰乱的间接体现。例如,酮康唑可以直接作用于性激素合成途径中的CYP11A1和CYP17,是类固醇生成酶的抑制剂[28],Villeneuve 等[57]研究表明,酮康唑(400 μg·L-1)暴露可导致雄性黑头呆鱼(Pimephales promelas)FSH-βmRNA表达水平升高,LH-βmRNA表达水平降低,进一步增强其对性激素合成的抑制效应。Tian 等[48,58]研究表明,久效磷农药暴露造成雄性和雌性金鱼(Carassius auratus)体内FSH水平升高,同时LH水平降低,从而进一步强化性腺芳香化酶基因表达升高所造成的雄雌激素比例失衡。性激素的合成与分泌受到GtHs的调节,同时性激素水平的变化对上游激素的合成与分泌调控也具有反馈作用,从而维持生殖轴线中各种激素的正常水平[59]。因此,某些 EEDs是否通过影响 GtHs间接干扰性激素合成,需要结合GtHs与性激素的合成与分泌水平以及GtHsmRNA表达水平等进行综合探讨。
4.2 明确性激素合成途径中多种转录因子间的相互作用
信号转导通路涉及多个转录因子,这些转录因子并不是唯一和独立的,可能是彼此联系、相互调节、协同或拮抗的。已有研究证实了SF-1和CREB的相互作用[60],其相互作用包括直接和共激活作用[61],也有研究认为,cAMP途径可能是通过磷酸化作用增加SF-1的转录活性,但Zheng等[62]的研究表明,SF-1的磷酸化作用不是其与CREB共同作用的关键。Lan等[63]建立了3个常见转录因子同源结构域相互作用蛋白激酶3(homeodomain-interacting protein kinase 3,HIPK3)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)和 c-Jun与 SF-1作用间的联系。研究表明,在cAMP刺激下,HIPK3使转录因子c-Jun和JNK磷酸化,此过程对SF-1的活性和CYP11A1的表达具有重要作用。采用哺乳动物细胞系实验证实,阿特拉津不直接与SF-1结合发挥内分泌干扰作用,而是通过激活SF-1的磷酸化作用、增强SF-1配体的产生、促进cAMP的产生等3种途径间接影响SF-1的表达,导致性腺芳香化酶过量表达[41]。可见,EEDs对类固醇生成酶基因表达水平的影响可能与上游的多种转录因子、多个调节途径有关,其精细的分子调控机制有待于进一步研究。
4.3 探讨性激素合成途径的物种差异
黑头呆鱼(Pimephales promelas)、斑马鱼(Danio rerio)和日本青鳉(Oryzias latipes)被认为是内分泌干扰物筛选和测试的模式生物[64]。不同硬骨鱼类的性激素合成步骤大致相同,但在类固醇生成酶及其所调控性激素的类别、时空分布及活性方面略有差异。例如,在虹鳟鱼(Oncorhynchusmykiss)中,仅存在一个 3β-HSD 基因[65];而 Kazeto 等[66]从日本鳗鲡(Anguilla japonica)卵巢中克隆得到了2种3β-HSD亚型,即3β-HSDI和3β-HSDII,这2种亚型的核苷酸和氨基酸序列高度同源,3β-HSDI活性高于3β-HSDII。在精子发生的不同阶段,某些类固醇生成酶的表达情况存在差异。例如,精子发生早期,P45011β的表达水平较低,精子形成期急剧上升并最终达到最高水平[67]。而同一种类固醇生成酶在不同物种间所发挥的催化作用也不尽相同。例如,在日本鳗鲡(Anguilla japonica)、斑马鱼(Danio rerio)和尼罗罗非鱼(Oreochromis niloticus)中,17β-HSD1的功能主要是高效地催化从雌酮到E2的反应,但尼罗罗非鱼17β-HSD1是一种多功能的酶,还能催化雄烯二酮和睾酮之间的反应,只是其催化效率较低[68]。此外,发挥主要生殖调节作用的性激素种类也具有一定的物种特异性,在大多数鱼类中11-KT是最主要的雄激素,但对雄性食蚊鱼(Gambusia holbrooki)和孔雀鱼(Poecilia reticulates)等花鳉亚科鱼性征发育起主要调控作用的是睾酮[69]。这些差异可能会导致EEDs对不同物种、同一物种不同发育时期性激素合成干扰效应的不同。因此,在设计EEDs非受体介导机制实验、解释结果并进行物种间外推时,应充分考虑到上述因素。
4.4 证实各个内分泌轴线间的交互作用
鱼类生殖不仅受生殖轴线的调控,而且受生殖轴线、甲状腺轴和肾间腺轴的协同调控。正是由于各个轴线间存在着复杂的交互作用,使得环境中的某些化学物质可同时引起靶向多个内分泌系统的复合毒性效应。一方面,EEDs对类固醇生成途径的影响可引起甲状腺轴和/或肾间腺轴的相关变化。例如:芳香化酶抑制剂法倔唑处理不仅可使黑头呆鱼(Pimephates promelas)和日本青鳉(Oryzias latipes)血浆中E2浓度降低,产卵量下降,还可调节甲状腺激素相关基因的表达[70-71]。另一方面,在体内实验中EEDs引起的类固醇生成酶的变化也可能是甲状腺轴和/或肾间腺轴扰乱的间接效应。Nelson等[72]研究表明,体内三碘甲腺原氨酸(3,3’,5-triiodo-L-thyronine,T3)处理能够下调金鱼(Carassius auratus)精巢和卵巢中P450arom mRNA表达水平;丙基硫氧嘧啶(propylthiouracil,PTU)为临床上最常用的一种治疗甲状腺功能亢进症的药物,Liu等[73]对成熟的雌性斑马鱼(Danio rerio)的研究表明,PTU暴露引起的甲状腺素(L-thyroxine,T4)和T3浓度降低可进一步导致FSH和LH分泌升高,进而促进类固醇合成;Kirby等[74]认为,肾上腺轴和生殖轴线在多个层面存在相互作用,研究表明,应激所诱导的大鼠肾上腺糖皮质激素含量增加可以导致血浆LH浓度降低。由此可见,EEDs可能通过复杂的交互作用机制影响鱼类内分泌系统,这也为EEDs非性激素受体介导机制研究提出了新的难题,需要结合更多的体内和体外实验进行综合探讨。
[1] Lee H R,Jeung E B,Cho M H,etal.Molecularmechanism(s)of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors[J].Journal of Cellular and Molecular Medicine,2013,17(1):1-11
[2] Jung E M,An B S,Yang H,et al.Biomarker genes for detecting estrogenic activity of endocrine disruptors via estrogen receptors[J].International Journal of Environmental Research and Public Health,2012,9(3):698-711
[3] 史熊杰,刘春生,余珂,等.环境内分泌干扰物毒理学研究[J].化学进展,2009,21(2-3):340-349 Shi X J,Liu CS,Yu K,et al.Toxicological research on environmental endocrine disruptors[J].Progress in Chemistry,2009,21(2-3):340-349(in Chinese)
[4] Liu SZ,Qin F,Wang H P,et al.Effects of17α-ethinylestradiol and bisphenol A on steroidogenic messenger ribonucleic acid levels in the rare minnow gonads[J].Aquatic Toxicology,2012,122-123:19-27
[5] Rhee JS,Kim BM,Lee C J,et al.Bisphenol A modulates expression of sex differentiation genes in the selffertilizing fish,Kryptolebias marmoratus[J].Aquatic Toxicology,2011,104(3-4):218-229
[6] Sanderson JT.The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals[J].Toxicological Sciences,2006,94(1):3-21
[7] Thibaut R,Porte C.Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish[J].Journal of Steroid Biochemistry and Molecular Bi-ology,2004,92(5):485-494
[8] MillerW L.Steoidogenic acute regulatory protein(StAR),a novel mitochondrial cholesterol transporter[J].Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids,2007,1771(6):663-676
[9] Miller W L.Minireview:Regulation of steroidogenesis by electron transfer[J].Endocrinology,2005,146(6):2544-2550
[10] Zhang W L,Zhou L Y,Senthilkumaran B,et al.Molecular cloning of two isoforms of 11β-hydroxylase and their expressions in the Nile tilapia,Oreochromis niloticus [J].General and Comparative Endocrinology,2010,165(1):34-41
[11] Stocco D M.StAR protein and the regulation of steroid hormone biosynthesis[J].Annual Review of Physiology,2001,63:193-213
[12] Ascoli M,Fanelli F,Segaloff D L.The lutropin/choriogonadotropin receptor,a 2002 perspective [J].Endocrine Reviews,2002,23(2):141-174
[13] Qu JH,Hong X,Chen J F,et al.Fenvalerate inhibits progesterone production through cAMP-dependent signal pathway[J].Toxicology Letters,2008,176(1):31-39
[14] Gonzalez G A,Montminy M R.Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133[J].Cell,1989,59(4):675-680
[15] Carlone D L,Richards JS.Evidence that functional interactions of CREB and SF-1 mediate hormone regulated expression of the aromatase gene in granulose cells and constitutive expression in R2C cells[J].The Journal of Steroid Biochemistry and Molecular Biology,1997,61(3-6):223-231
[16] Jacob A L,Lund J.Mutations in the activation function-2 core domain of steroidogenic factor-1 dominantly suppresses PKA-dependent transactivation of the bovine CYP17 gene[J].The Journal of Biological Chemistry,1998,273(22):13391-13394
[17] Michael M D,Kilgore M W,Morohashi K I,et al.Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter(PII)of the human aromatase P450(CYP19)gene in the ovary[J].The Journal of Biological Chemistry,1995,270(22):13561-13566
[18] Sharpe R L,DroletM,MacLatchy D L.Investigation of de novo cholesterol synthetic capacity in the gonads of goldfish(Carassius auratus)exposed to the phytosterol beta-sitosterol[J].Reproductive Biology and Endocrinology,2006,4(1):60-70
[19] Sharpe R L,Woodhouse A,Moon TW,et al.β-Sitosterol and 17β-estradiol alter gonadal steroidogenic acute regulatory protein(StAR)expression in goldfish,Carassius auratus[J].General and Comparative Endocrinology,2007,151(1):34-41
[20] Becker M,Staab D,Bergmann K V.Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol[J].The Journal of Pediatrics,1993,122(2):292-296
[21] Babin P J,Vernier JM.Plasma lipoproteins in fish[J].Journal of Lipid Research,1989,30:467-489
[22] Jefcoate CR,Mcnamara BC,Artemenko I,etal.Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis[J].The Journal of Steroid Biochemistry and Molecular Biology,1992,43(8):751-767
[23] Villeneuve D L,Mueller N D,Martinovi D,et al.Direct effects,compensation,and recovery in female fatheadminnows exposed to amodelaromatase inhibitor[J].Environmental Health Perspectives,2009,117(4):624-631
[24] Ankley G T,Cavallin JE,Durhan E J,etal.Temporal evaluation of effects of amodel 3β-hydroxysteroid dehydrogenase inhibitor on endocrine function in the fathead minnow[J].Environmental Toxicology and Chemistry,2011,30(9):2094-2102
[25] Moore RW,Jefcoate CR,Peterson R E.2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450scc[J].Toxicology and Applied Pharmacology,1991,109(1):85-97
[26] Skolness SY,Durhan E J,Garcia R N,et al.Effects of a short-term exposure to the fungicide prochloraz on endocrine function and gene expression in female fathead minnows(Pimephales promelas)[J].Aquatic Toxicology,2011,103(3-4):170-178
[27] Bail JL,PougetC,Fagnere C,etal.Chalconesare potent inhibitors ofaromatase and 17β-hydroxysteroid dehydrogenase activities[J].Life Sciences,2001,68:751-761
[28] Ankley G T,Cavallin JE,Durhan E J,et al.A timecourse analysis of effects of the steroidogenesis inhibitor ketoconazole on components of the hypothalamic-pituitary-gonadal axis of fathead minnows[J].Aquatic Toxicology,2012,114(115):88-95
[29] Ma Y B,Han J,Guo Y Y,et al.Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol[J].A-quatic Toxicology,2012,106-107:173-181
[30] Ankley G T,Jensen K M,Kahl M D,et al.Ketoconazole in the fathead minnow(Pimephales promelas):Reproductive toxicity and biological compensation[J].Environmen-tal Toxicology and Chemistry,2007,26(6):1214-1223
[31] Sanderson JT,Seinen W,Giesy JP,et al.2-Chloro-striazine herbicides induce aromatase(CYP19)activity in H295R human adrenocortical carcinoma cells:A novelmechanism for estrogenicity?[J].Toxicological Sciences,2000,54(1):121-127
[32] Sanderson JT,Boerma J,Lansbergen GW,et al.Induction and inhibition of aromatase(CYP19)activity by various classes of pesticides in H295R human adrenocortical carcinoma cells[J].Toxicology and Applied Pharmacology,2002,182(1):44-54
[33] Ma Y B,Liu CS,Lam PK S,etal.Modulation of steroidogenic gene expression and hormone synthesis in H295R cells exposed to PCP and TCP[J].Toxicology,2011,282(3):146-153
[34] Villeneuve D L,Ankley G T,Makynen E A,et al.Comparison of fatheadminnow ovary explantand H295R cell-based steroidogenesis assays for identifying endocrine-active chemicals[J].Ecotoxicology and Environmental Safety,2007,68(1):20-32
[35] Heneweer M,Muusse M,Dingemans M,et al.Co-culture of primary human mammary fibroblasts and MCF-7 cells as an in vitro breast cancermodel[J].Toxicological Sciences,2005,83(2):257-263
[36] Heneweer M,Berg M,Geest M C,et al.Inhibition of aromatase activity bymethyl sulfonyl PCBmetabolites in primary culture of humanmammary fibroblasts[J].Toxicology and Applied Pharmacology,2005,202(1):50-58
[37] Simpson E R,Waterman M R.Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH[J].Annual Review of Physiology,1988,50:427-440
[38] Enan E,Lasley B,Stewart D,et al.2,3,7,8-Tetrachlorodibenzo-p-dioxin(TCDD)modulates function of human luteinizing granulosa cells via cAMP signaling and early reduction of glucose transporting activity[J].Reproductive Toxicology,1996,10(3):191-198
[39] Ronco A M,Valdós K,Marcus D,etal.Themechanism for lindane-induced inhibition of steroidogenesis in cultured rat Leydig cells[J].Toxicology,2001,159(1-2):99-106
[40] Kucka M,Majkic K P,Fa S,et al.Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4[J].Toxicology and Applied Pharmacology,2012,265(1):19-26
[41] Suzawa M,Ingraham H A.The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells[J].PLoSONE,2008,3(5):e2117
[42] Govoroun M,Mcmeel OM,Mecherouki H,et al.17β-Estradiol treatment decreases steroidogenic enzymemessenger ribonucleic acid levels in the rainbow trout testis[J].Endocrinology,2001,142(5):1841-1848
[43] Borch J,Metzdorff SB,Vinggaard A M,et al.Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis [J].Toxicology,2006,223(1-2):144-155
[44] Du G Z,Huang H Y,Hu JL,et al.Endocrine-related effects of perfluorooctanoic acid(PFOA)in zebrafish,H295R steroidogenesis and receptor reporter gene assays[J].Chemosphere,2013,http://dx.doi.org/10.1016/j.chemosphere.2013.01.012
[45] Arukwe A.Cellular and molecular responses to endocrinemodulators and the impacton fish reproduction[J].Marine Pollution Bulletin,2001,42(8):643-655
[46] Yokota H,Abe T,Nakai M,et al.Effects of 4-tertpentylphenol on the gene expression of P450 11βhydroxylase in the gonad of medaka(Oryzias latipes)[J].Aquatic Toxicology,2005,71(2):121-132
[47] Chen H,Xiao J,Hu G,et al.Estrogenicity of organophosphorus and pyrethroid pesticides[J].Journal of Toxicology and Environmental Health,Part A:Current Issues,2002,65(19):1419-1435
[48] Tian H,Ru S,Bing X,et al.Effects ofmonocrotophos on the reproductive axis in themale goldfish(Carassius auratus):Potential mechanisms underlying vitellogenin induction[J].Aquatic Toxicology,2010,98(1):67-73
[49] Tian H,Li Y,Wang W,et al.Exposure tomonocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success ofmale guppies(Poecilia reticulata)[J].Toxicology and Applied Pharmacology,2012,263(2):163-170
[50] Zhang X,Gao L,Yang K,et al.Monocrotophos pesticide modulates the expression of sexual differentiation genes and causes phenotypic feminization in zebrafish(Danio rerio)[J].Comparative Biochemistry and Physiology Part C:Toxicology& Pharmacology,2013,157(1):33-40
[51] Purdom CE,Hardiman PA,Bye V J,etal.Estrogenic effects of effluents from sewage treatment works[J].Chemistry and Ecology,1994,8(4):275-285
[52] Vajda A M,Barber L B,Gray JL,et al.Reproductive disruptions in fish downstream from an estrogenic wastewater effluent[J].Environmental Science &Technology,2008,42(9):3407-3414
[53] Vigan L,Benfenati E,Botteroc S,et al.Endocrinemodulation,inhibition of ovarian developmentand hepatic alterations in rainbow troutexposed to polluted riverwater[J].Environmental Pollution,2010,158(12):3675-3683
[54] Vajda A M,Barber L B,Gray J L,et al.Demasculinization ofmale fish by wastewater treatment plant effluent[J].Aquatic Toxicology,2011,103(3-4):213-221
[55] Toft G,Edwards TM,Baatrup E,et al.Disturbed sexual characteristics inmalemosquitofish(Gambusia holbrooki)from a lake contaminated with endocrine disruptors[J].Environmental Health Perspectives,2003,111(5):695-701
[56] Harshbarger JC,Coey M J,Young M Y.Intersexes in Mississippi River shovelnose sturgeon sampled below Saint Louis,Missouri,USA[J].Marine Environmental Research,2000,50(1-5):247-250
[57] Villeneuve D L,Miracle A L,Jensen K M,et al.Development of quantitative real-time PCR assays for fathead minnow(Pimephales promelas)gonadotropinβ subunitmRNAs to support endocrine disruptor research[J].Comparative Biochemistry and Physiology,Part C,2007,145(2):171-183
[58] Tian H,Ru S,Wang W,et al.Effects of monocrotophos on the reproductive axis in the female goldfish(Carassius auratus)[J].Comparative Biochemistry and Physiology,Part C,2010,152(1):107-113
[59] Nett T M,Turzillo A M,Baratta M,et al.Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone[J].Domestic Animal Endocrinology,2002,23(1-2):33-42
[60] Manna P R,Eubank D W,Lalli E,et al.Transcriptional regulation of themouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1[J].Journal of Molecular Endocrinology,2003,30:381-397
[61] Jacob A L,Lund J,Martinez P,et al.Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5[J].The Journal of Biological Chemistry,2001,276(40):37659-37664
[62] ZhengW C,Jefcoate CR.Steroidogenic factor-1 interacts with cAMP response element-binding protein to mediate cAMP stimulation of CYP1B1 via a far upstream enhancer[J].Molecular Pharmacology,2005,67(2):499-512
[63] Lan H C,Li H J,Lin G,et al.Cyclic AMP stimulates SF-1-dependent CYP11A1 expression through homeodomain-interacting protein kinase 3-mediated Jun N-terminal kinase and c-Jun phosphorylation[J].Molecular and Cellular Biology,2007,27(6):2027-2036
[64] Ankley G T,Johnson R D.Small fishmodels for identifying and assessing the effects of endocrine-disrupting chemicals[J].ILAR Journal,2004,45(4):469-483
[65] Sakai N,Tanaka M,Takahashi M,et al.Ovarian 3βhydroxysteroid dehydrogenase/△5–△4 isomerase of rainbow trout:Its cDNA cloning and properties of the enzyme expressed in amammalian cell[J].FEBS Letters,1994,350(2-3):309-313
[66] Kazeto Y,Ijiri S,Matsubara H,et al.Molecular cloning and characterization of 3β-hydroxysteroid dehydrogenase/△5–△4 isomerase cDNAs from Japanese eel ovary[J].Journal of Steroid Biochemistry and Molecular Biology,2003,85(1):49-56
[67] Maugars G,Schmitz M.Gene expression profiling during spermatogenesis in early maturing male Atlantic salmon parr testes[J].General and Comparative Endocrinology,2008,159(2-3):178-187
[68] Zhou L Y,Wang D S,Senthilkumaran B,et al.Cloning,expression and characterization of three types of 17β-hydroxysteroid dehydrogenases from the Nile tilapia,Oreochromis niloticus[J].Journal of Molecular Endocrinology,2005,35:103-116
[69] Borg B.Androgens in teleost fishes[J].Comparative Biochemistry and Physiology Part C:Pharmacology,Toxicology and Endocrinology,1994,109(3):219-245
[70] Ankley G T,Kahl M D,Jensen KM,etal.Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fatheadminnow(Pimephates promelas)[J].Toxicological Sciences,2002,67(1):121-130
[71] Zhang X,Hecker M,Park JW,et al.Real-time PCR array to study effects of chemicals on the Hypothalamic-Pituitary-Gonadal axis of the Japanese medaka[J].A-quatic Toxicology,2008,88(3):173-182
[72] Nelson E R,Allan E R,Pang F Y,et al.Thyroid hormone and reproduction:Regulation of estrogen receptors in goldfish gonads[J].Molecular Reproduction and Development,2010,77(9):784-794
[73] Liu C S,Zhang X W,Deng J,et al.Effects of prochloraz or propylthiouracil on the cross-talk between the HPG,HPA,and HPT axes in zebrafish[J].Environmental Science& Technology,2011,45(2):769-775
[74] Kirby E D,Geraghty A C,Ubuka T,et al.Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats[J].Proceedings of the National Academy of Sciences of the United States of America,2009,106(27):11324-11329 ◆
