TheExtractionofLacticAcidfromFermentative MarigoldWastewater
2013-08-16JIANGChuanshiZHANGHuiCUIZhenleiYUHeweiHANJiqing
JIANG Chuan-shi ZHANG Hui CUI Zhen-lei YU He-wei HAN Ji-qing
(青岛大学 化学化工与环境学院,山东 青岛266071)
0 Introduction
Marigold is an herb of ancient medicinal repute. It grows as a wild and common garden plant throughout Europe, North America and Asia.[1]Lutein has been reported in epidemiological studies that lutein reduces the risk of some chronic diseases such as cancer, heart disease and agerelated eye diseases.[2]A lot of wastewater is generated during the extraction of lutein from marigold process. lactic acid ,which contained in the wastewater, is widely used in food additives, feed sour agents and nutritional supplements.[3]
1 Materials and methods
1.1 Materials
Fermentative marigold wastewater come (Heilongjiang Leader Biotechnology Co.,Ltd)
Lactic acid(Content 80%-95%),Active carbon,Polyaluminium chloride,Ca(OH)2(content≥93%),H3PO4(AR), N-butyl alcohol(AR), Isopropyl ether(AR), chloroform(AR)
1.2 Methods
1.2.1 Organic solvent extraction method
Wastewater, of which the pH is adjusted by acid, is extracted three times with organic solvent and then concentrated by evaporation to obtain lactic acid.
1.2.2 Ca(OH)2neutralization method
Active carbon was added to the wastewater(pH6 or pH7, adjusted by Ca (OH)2). After that adding organic solvent to the solution and then concentrated by evaporation. The calcium lactate was got finally.
1.2.3 Flocculation method
Polyaluminium chloride was added to the wastewater. After long-time stirring filter,carbon was added and then concentrated by evaporation.The lactic acid was got finally.
2 Results and discussion
2.1 Calculation of lactic acid
Under certain condition, the content of lactic acid and it’s refractive index is proportional in solution, so the content of lactic acid in solution can be measured by abbe refractometer. The of lactic acid in solution can be calculated by the follow equation:
η=(n-1.3330)×1000
η——Content of lactic acid(%)
n——Refractive index
2.2 Organic solvent extraction method
Extracting 200mL of wastewater by using 200mL of isopropyl ether for three times. The organic phase was concentrated by evaporation and 2.5mL of lactic acid was got finally. The content of lactic acid in the production is 45%.
Extracting 200mL of wastewater by using 200mL of chloroform for three times. The organic phase was concentrated by evaporation and 5mL of lactic acid was got finally. The content of lactic acid in the production is 13.4%.
Extracting 200mL of wastewater by using 200mL of n-butyl alcohol for three times. The organic phase was concentrated by evaporation and 3mL of lactic acid was got finally. The content of lactic acid in the production is 76.1%.
Three kinds of organic solvent are used in the experiment, which are isopropyl ether, chloroform and n-butyl alcohol. Concluded from data, nbutyl alcohol is the best. But a lot of n-butyl alcohol is used, it is bad for environment.
2.3 Ca(OH)2 neutralization method
Ca(OH)2was added dropwise into 100mL of wastewater to adjust the solution to pH6 or pH7 at 35℃. 2g of active carbon was added to bleach.The filtration was concentrated by evaporation, and 0.5g of calcium lactate was got finally.
2.4 Flocculation method
0.25g of polyaluminium chloride was added to 100mL of wastewater,then stired the solution for 1h at 35℃.After that centrifuged the liquid for 10 minute. 0.5g of active carbon was added to bleach for 1.5h at 40℃,The filtration was concentrated by evaporation, and 13ml of lactic acid was got finally.
The content of lactic acid in experimental procedure was recorded in table 1.
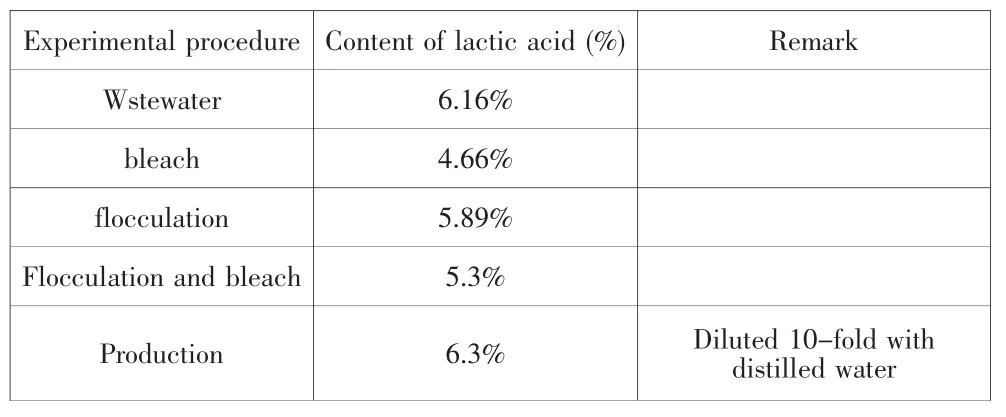
Table 1 The content of lactic acid in experimental procedure
Derived from the table, bleach and flocculation have low influence on the content of lactic acid, and the content of lactic acid in the production is high.
3 Conclusions
Extraction by using organic solvent will get high-purity production but low recovery. At the same time, the organic solvents will pollute the environment. The method of Ca(OH)2neutralization is environmental, but the recovery of calcium lactate is low.
The method of flocculation can get high-purity production and the recovery is high. It is also environmental. Compared to other sewage treatment, this method can obtain lactic acid from wastewater. This method will be widely used in the sewage treatment from fermentative marigold flower in the future.
[1]Gonzalez de Mejia,E.,Loarca-Pina,G.,&Ramos-Gomez,M.(1997). Antimutagenicity of xanthophylls present in aztec marigold (Tagetes erecta)against 1-nitropyrene[J].Mutation Research,389,219-226.
[2]Slattery, M. L., Benson, J., Curtin, K., Ma, K. N., Schaeffer, D., &Potter, J. D.(2000). Carotenoids and colon cancer[J].American Journal of Clinical Nutrition, 71,575-582.
[3]J.B. Chaudhuri, D.L. Phyle, Emulsion liquid membrane extraction of organicacids. I. A theoretical model for lactic acid extraction with emulsion swelling, Chem. Eng[J]. Sci. 47 (1992) 41-48.
