Surface Acidity of Aluminum Phosphate and Its Catalytic Performance in Benzene Alkylation with Long Chain Olefin
2013-06-07YUANHaikuan袁海宽LIUXuru刘旭汝RENJie任杰andSHENLian慎炼
YUAN Haikuan (袁海宽), LIU Xuru (刘旭汝), REN Jie (任杰)* and SHEN Lian (慎炼)
College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China
Surface Acidity of Aluminum Phosphate and Its Catalytic Performance in Benzene Alkylation with Long Chain Olefin
YUAN Haikuan (袁海宽), LIU Xuru (刘旭汝), REN Jie (任杰)* and SHEN Lian (慎炼)
College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China
The acidic properties of aluminum phosphate (AlPO4-5) solid acid catalyst were characterized by temperature programmed desorption (TPD) of ammonia (NH3), n-propylamine (n-C3H7NH2), iso-propylamine [(CH3)2CHNH2] and n-dipropylamine [(C3H7)2NH] separately, and its catalytic performance in benzene alkylation with long chain olefin was studied in a fixed-bed reactor. The characterized acid amount of catalyst increased with the basicity of adsorbates. With increase of the activation temperature of catalyst, the acid amount characterized by NH3-TPD decreased, however, it increased when characterized by TPD using three other adsorbates. The desorption kinetics of TPD process and the deactivation kinetics of catalyst were investigated. The acidity and catalytic performance of catalyst was also correlated. The results showed that the acid amount and strength are well correlated with the activity and stability using NH3as adsorbate, respectively, which indicated NH3was a better basic adsorbate. It was also found that the catalyst with higher acid amount and lower acid strength on the surface exhibited the better catalytic performance and stability.
surface acidity, temperature programmed desorption, basic adsorbate, benzene alkylation, catalytic activity
1 INTRODUCTION
Solid acid catalysts play an important role in many industrial catalytic reactions, such as alkylation, condensation, and esterification. Some of solid acid catalysts, such as zeolites [1, 2], metal oxides [3, 4], phosphates [5] and ammonium metatungstate [6], have been extensively studied and applied in benzene alkylation reaction.
The evaluation of surface acidity is of great importance when the catalytic action of solid acid catalysts is to be understood. Many attempts have been made to measure acidic properties by means of various methods, including titration, temperature programmed desorption (TPD), infra-red (IR) spectroscopy, microcalorimetry, etc. Among those methods, the TPD technique is widely used in the surface science and catalysis study because of its intrinsic simplicity and low measurement cost [7-15]. Adsorbate used in TPD experiments is very important, and mainly chosen from ammonia (NH3), pyridine, amine, hydrogen (H2) [16], oxynitride (NOx) [17], aromatic molecules [18] as probe molecules. The choices of probe molecules for characterizing acid-base surface sites of solid catalyst have been extensively discussed, and each probe molecule has its own advantages and limitations [19-21]. However, the current research lacks of comprehensive studies on the relationships between adsorbates and characterization results.
The detailed analysis of TPD-profiles may be complicated because of its dependence on the experimental conditions. These problems can be overcome using well-selected experimental conditions and applying the mathematical description for the desorption peaks [22-24]. Barrie [25] analyzed the TPD data obeying first-order desorption kinetics, and gained an initial estimate of the adsorption site distribution through the improved condensation approximation method. Panczyk et al. [26] studied the surface acidity of Ni/MgO-Al2O3catalysts through analyzing quantitatively NH3-TPD curves without using any kinetic approaches, in which the adsorption energy distributions of NH3were determined. Liu et al. [27] studied the relationship between the structure and acidity of zeolites at the molecular level using NH3-TPD method and quantum chemistry calculations. Mathematical analysis of TPD spectra highlighted the presence of acid sites on the catalysts. In addition, the relationships between the surface acidity and activity of solid catalysts were also studied widely using TPD experiments [28-30]. Nonetheless, no comprehensive approach about the choice of adsorbate and the desorption kinetics in TPD runs is developed for studying the acidity of solid catalyst.
In this paper, we report a study on systematic acidity characterization of AlPO4-5 solid catalyst by the TPD method using ammonia (NH3), n-propylamine (n-C3H7NH2), iso-propylamine [(CH3)2CHNH2], n-dipropylamine [(C3H7)2NH] as adsorbates, and its catalytic performance in benzene alkylation reaction with long chain olefin. Comparing the adsorption processes of different adsorbates on catalyst in TPD runs, the relationships of adsorbates and characterization results are discussed, and the acidity and catalytic performance of solid acid catalyst are also correlated.
2 EX PERIMENTAL
2.1 Materials and methods
Aluminum phosphate (AlPO4-5) solid acid catalyst with specific surface area of 243.5 m2·g−1(self-made in laboratory) [31], was extruded in molding, then crushed, and the fraction of 380 μm to 830 mm (20-40 mesh) was chosen as the catalyst used in benzene alkylation reaction. The mass ratio of AlPO4-5 to Al2O3in solid acid catalyst was 5∶1, and its pore volume was 0.38 cm3·g−1. Benzene and the hydrocarbon mixture of long chain alkane and alkene (C10-C13) were used as raw materials of alkylation reaction, and the components of hydrocarbon mixture was shown in Table 1, and the molar ratio of benzene to hydrocarbon mixture was 3.3∶1. High-purity liquid NH3(>99.98%) was obtained from Hangzhou Jingong Gas Co. (China). Nitrogen (N2) (>99.99%), n-C3H7NH2, (CH3)2CHNH2and (C3H7)2NH of analytical grade were purchased from Shanghai Yelian United Co. (China). All other solvents and regents were obtained from Shanghai Chemical Reagent Co. (China).
Bromine index of raw materials and products were measured by RPA-100Br Instrument (Jianghuan Analytical Instrument Co., Jiangyan, China). The TPD runs were preformed with use of WRT-2P Microcalorimetric Analyzer (Shanghai Precision & Scientific Instruments Co., China). The products were analyzed by Agilent 1790 gas chromatograph [Agilent Technologies (Shanghai) Co., China] equipped with flame ionization detector (FID) and OV-101 capillary column of 50 m×0.53 mm×1.5 μm.

Table 1 Components of hydrocarbon mixture
2.2 Acidity of catalyst characterized by TPD
In order to eliminate the effects of trace physisorbed water in catalyst on the desorption amount of adsorbate, the TPD operations were performed twice at the same conditions with and without adsorbate. 3.5 mg solid acid catalyst was filled in a fixed-bed reactor for TPD processes. Firstly, the catalyst was treated in the N2carrier flow rate of 30 ml·min−1for 2 h at a certain temperature, then it was swept by high-purity N2for 30 min. The TPD experiments heating the sample from 100 °C up to 700 °C at a rate of 10 °C·min−1were performed after the baseline being stabilized, and then the blank experiments were recorded. Afterwards, the catalyst was adsorbed by NH3or amines for 60 min, and the TPD experiments were run at the same conditions with the blank ones. The NH3-TPD data and the corresponding coverage rates of adsorbate on the catalyst surface were obtained from the difference between the desorption volumes of two TPD runs.
2.3 Alkylation reaction
The stainless steel tube of 10 mm i.d. and 900 mm long was used as the fixed-bed reactor. 4.0 g catalyst was loaded in the middle of reactor, and the both ends were filled by quartz chips. Prior to the alkylation reaction, the catalysts were activated in N2flow for 2.0 h at 100, 200 and 300 °C, respectively. Then, the raw materials were fed into the reactor through a ram pump, and the alkylation reaction of benzene with long chain olefin (all reactants in form of vapor) was carried out continuously at 5.0 MPa and 250 °C.
The bromine index of raw material (mixed hydrocarbon) is 3480 mg Br per 100 g raw materials, and the olefin conversion is defined as follows:

where IBr-rawand IBr-productsare the bromine index of the raw materials and products, respectively.
3 RESUL TS AND DISCUSSION
3.1 Acid amount characterized by TPD
Figure 1 shows the TPD curves of solid catalyst using NH3, n-C3H7NH2, (CH3)2CHNH2and (CH3)2NH as adsorbates. For the four basic adsorbates, only one peak appears approximately at 150 °C on the TPD curves, which indicates that catalysts have the weak acid sites with narrow distribution of acid strength. The acid amounts of catalysts measured by TPD of different adsorbates are shown in Table 2. Since activation was conducted at most at 300 °C, the catalyst structure was hardly altered.
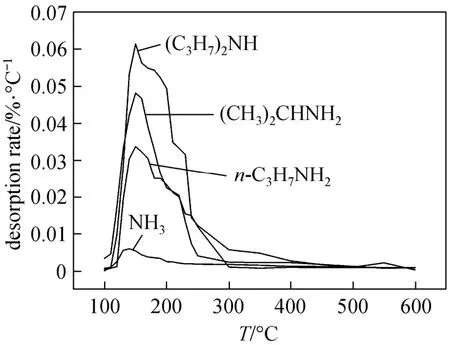
Figure 1 TPD curves of NH3, n-C3H7NH2, (CH3)2CHNH2and (C3H7)2NH
pKb, the basic dissociation constant, represents the basicity of substance. In general, the smaller pKbvalue of an adsorbate means the stronger basicity. The pKbvalues of NH3, n-C3H7NH2, (CH3)2CHNH2and(C3H7)2NH are 4.76, 3.33, 3.28 and 3.02, respectively [30]. Therefore, the order of basicity for the four adsorbates is: (C3H7)2NH>(CH3)2CHNH2>n-C3H7NH2>NH3. Because the adsorption of basic adsorbate always gives priority to the stronger acid sites of catalyst, the measured acid amount from TPD experiments increases with the basicity of adsorbates being stronger, as shown in Table 2. With the basicity of adsorbates being stronger, the weaker acid sites of catalyst are also adsorbed gradually by the adsorbates, resulting in the increase of adsorption volume in TPD experiments. In addition, due to the larger molecule of (C3H7)2NH adsorbate, several acid sites of catalyst may be covered by one adsorbate molecule, which leads to the characterized acid amount of catalyst reducing, as shown in Table 2.
As shown in Table 2, with increasing the activation temperature of catalyst, the mmol acid amount of catalyst characterized by TPD of NH3decreases, that is, the surface acid density of catalyst decreases. However, for three other basic adsorbates, the mmol acid amounts increase, meanwhile, the solid acid catalysts blacken in TPD experiments. This is because the adsorbates are not completely desorbed from the acid sites of catalyst in TPD processes, and a part of them settles on the surface of catalyst as carbides, which would affect the characterization accuracy. However, the catalyst does not being coked when using NH3as adsorbate in TPD process, therefore, NH3should be chosen as a good adsorbate in TPD process for acidity characterization of catalyst.

Table 2 The acid amounts of catalysts activated at different temperatures measured by TPD
3.2 Acid strength characterized by TPD
TPD peaks often appear at temperature where the desorption rate of adsorbate is maximal, therefore, the desorption peak temperatures (DPT) can be used to measure the acid strength of the catalyst. On the other hand, the desorption activation energy (DAE) of adsorbate calculated from TPD profiles could also be used to determine the acid strength [28, 32]. The higher DPT or DAE indicates the higher acid strength of catalyst. The data processing of the DPT method is simpler than that of the DAE method, but not accurate if the TPD peaks are of poor symmetry. Therefore, the DAE method is often chosen when characterizing the acid strength of catalyst because the shape of desorption peaks is not so important in this method.
Assuming that the adsorbate molecules adsorbed on the catalyst surface do not interact each other, and the desorption process is irreversible, that is, the re-adsorption process does not occur, the desorption rate of adsorbate in TPD process is expressed as follows [33]:

where θ is the fraction of catalyst surface covered by adsorbate, t1is the desorption time (min), k0is the desorption rate constant, R is the gas constant (J·mol−1·K−1), d is the kinetic order of desorption, and Edis the desorption activation energy (J·mol−1).
The coverage of adsorbate on the catalyst surface, θ, is defined as
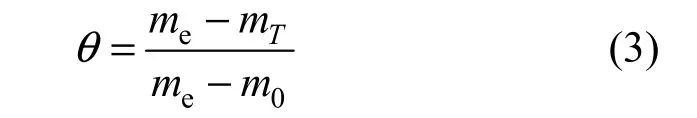
where m0, me, mTare the desorption percentage of an adsorbate at the beginning of desorption, at the end of desorption, and at the desorption temperature T, respectively. At the beginning of desorption, θ=1, and at the end of desorption, θ=0.
In TPD experiments, the fixed bed is heated at a certain rate of β (K·min−1), and the temperature of catalyst bed (T) increases linearly with t1: T=T0+βt1. Substituting it into Eq. (2), the desorption kinetic model for TPD process is obtained:

Correlation coefficient (R) for θ of experimental and calculated data as objective function, the parameters of Edand k0in Eq. (4) were estimated through the numerical method of the iterative computation combined with the fourth-order Runge-Kutta differential equations using TPD data of catalysts activated at different temperatures. The values of Ed, k0, d, R and the desorption peak temperature of different adsorbates are listed in Table 3. The value of d was assigned respectively to 1, 2, or 3, and the results of data fitting suggested that d=2 fitted the experiments the best. Therefore, the desorption processes of different adsorbates for catalysts activated at different temperatures are 2-order kinetics. The values of R are close to 1, indicating that the calculated values of θ coincides with the experimental ones.
As known from Table 3, the desorption activation energy gradually increases with the basicity of adsorbate.Edshows good correspondence with the respective basicity, and the magnitude of calculated Edfor the four adsorbates is: Ed(NH3)<Ed(n-C3H7NH2)<Ed[(CH3)2CHNH2]<Ed[(C3H7)2NH]. That is because the interactions between the basic molecules and acid sites of catalyst become stronger with the increase of basicity, consequently, resulting in larger Ed. In addition, as the activation temperature of catalyst increases, Edof all four adsorbates increases, that is, the acid strength on the catalyst surface becomes stronger. For NH3, n-C3H7NH2, (CH3)2CHNH2adsorbates, their respective desorption peak temperatures in TPD curves increase with the stronger basicity of adsorbate, therefore, the acid strength of catalyst characterized by the DPT method become stronger. Although (C3H7)2NH has the strongest basicity, the desorption peak temperature is not the highest, because of the (C3H7)2NH-TPD curve with the poor symmetry. Therefore, the characterization on the acid strength of catalyst surface through the DAE method is more accurate.

Table 3 Desorption peak temperatures and desorption activation energy in TPD of different adsorbates for catalysts activated at different temperatures

Table 4 The olefin conversion (X) of alkylation reaction using catalysts activated at different temperatures
3.3 Kinetics of alkylation reaction
Using AlPO4-5 solid acid catalysts activated at 100 °C, 200 °C, 300 °C, respectively, the alkylation reaction of benzene with long chain olefin was carried out continuously at 5.0 MPa, 250 °C. The experimental results are listed in Table 4.
As shown in Table 4, the olefin conversion decreases with alkylation reaction processing continuously, which indicates catalyst deactivates partly. With increasing the activation temperature of catalyst, the olefin conversion also decreases, which is attributed tothe decrease of activity of catalyst activated at the higher temperature. In order to evaluate quantitatively the activity and stability of catalysts activated at different temperatures, the kinetics of alkylation reaction and the deactivation kinetics of catalyst are investigated. For the benzene alkylation with long chain olefin, the effect of benzene concentration on the alkylation reaction is negligible, because it is largely excessive. Furthermore, it is considered that the effect of olefin concentration on the reaction rate is of one-order kinetics [34]. Therefore, the kinetic model of alkylation affected by the activity of catalyst is expressed as follows:

where τWis the pseudo-reaction time, and τW=S−1, S is the mass hourly space velocity (h−1), k is the reaction rate constant of alkylation, and a is the activity coefficient of catalyst.
The catalyst coked simultaneously accompanying with the alkylation reaction, therefore, the activity of catalyst decreased gradually. Assuming that the kinetics of coking deactivation of catalyst is of one-order for alkylation reaction [34, 35], the deactivation kinetic model of catalyst is obtained when [a=exp(−kdt)] is substituted into Eq. (5):

where kdis the deactivation rate constant of catalyst, and t the continuous reaction time.
Substituting the experimental data in Table 4 into Eq. (7), the values of k and kdare estimated through the Gauss-Newton iteration, which are listed in Table 5. As shown in Table 5, with increasing the activation temperature of catalyst, the k decreases, while the kdincreases gradually, which indicates that both the activity and stability of catalyst decrease. Therefore, the catalyst would have good catalytic properties for alkylation reaction when it is activated at lower temperatures. In addition, substituting the model parameters int Eq. (7), the olefin conversions (Xcal/%) are calculated and listed in Table 4, which are close to the experimental data. Moreover, their mean relative errors (erm) are very little.

Table 5 Reaction rate constant (k) and deactivation rate constant (kd) of alkylation reaction using catalysts activated at different temperatures
3.4 C orrelations betw een acidity an d catalyt ic performance of catalyst
The above results show that the mmol acid amount characterized by NH3-TPD decreases with increasing the activation temperature of catalysts, and correspondingly, the activity of catalyst gradually decreases. It also can be said that the acid amount of catalyst characterized by NH3-TPD has good correspondence with its catalytic activity. However, when using n-C3H7NH2, (CH3)2CHNH2, and (C3H7)2NH as adsorbates, the acid amount characterized by TPD runs has the reverse tendency of change in response to catalyst activities. Furthermore, the acid strength of catalyst is related closely with its stability. As the activation temperature of catalyst increases, all the Edfor TPD processes of 4 adsorbates increases, therefore, the acid strength on the catalyst surface determined by the DAE method increases. However, the increase of kdindicates the easier coking deactivation of catalyst with the acidity being stronger, and the stability of catalyst thus being worse. Compared with n-C3H7NH2, (CH3)2CHNH2, (C3H7)2NH, the acid amount and strength characterized by NH3-TPD have good corresponding correlations with the activity and stability of catalyst, respectively. Therefore, NH3is a kind of good basic adsorbate in TPD process for acidity characterization of solid catalyst. In benzene alkylation with long chain olefin, the solid acid catalyst with the higher surface acid density and the lower surface acid strength would have the better catalytic performance.
4 CONCLU SIONS
Using NH3, n-C3H7NH2, (CH3)2CHNH2and (C3H7)2NH as basic adsorbates, the acidic properties of solid acid catalyst were characterized by TPD methods, and its catalytic performance was studied in benzene alkylation with long chain olefin. The acid amount of catalyst increased with the basicity of adsorbate. With higher activation temperature of catalyst, the acid amount characterized by NH3-TPD decreased, which was different from those for n-C3H7NH2, (CH3)2CHNH2and (C3H7)2NH as adsorbates. However, the acid strength of catalyst became stronger with increasing the activation temperature. In order to evaluate quantitatively the acidity of catalyst, the kinetics of TPD process and the desorption deactivation kinetics of catalyst used in alkylation reaction were determined. In addition, the acidity of catalyst was found positively correlated to its catalytic performance. It was concluded that NH3was a good basic adsorbate in TPD process, and the catalyst with the higher surface acid density and the lower acid strength processed the better catalytic activity and stability in alkylation.
REFERENCES
1 Anand, R., Maheshwari, R., Core, K.U., Chumbhale, V.R., “Selectivealkylation of catechol with t-butyl alcohol over HY and modified HY zeolites”, Catal. Commun., 3, 321-326 (2002).
2 Bordoloi, A., Devassy, B.M., Niphadkar, P.S., Joshi, P.N., Halligudi, S.B., “Shape selective synthesis of long-chain linear alkyl benzene (LAB) with AlMCM-41/Beta zeolite composite catalyst”, J. Mol. Catal. A Chem., 253, 239-244 (2006).
3 Clark, J.H., Monks, G.L., Nightingale, D.J., Price, P.M., White, J.F.,“A new solid acid-based route to linear alkylbenzenes”, J. Catal., 193, 348-350 (2000).
4 Fu, Y., Baba, T., Ono, Y., “Vapor-phase reactions of catechol with dimethyl carbonate. Part I. o-methylation of catechol over alumina”, Appl. Catal. A Gen., 166, 419-424 (1998).
5 Zhu, X., Li, X., Jia, M., Liu, G., Zhang, W., Jiang, D., “Vapour-phase selective o-methylation of catechol with methanol over Ti-containing aluminium phosphate catalysts”, Appl. Catal. A Gen., 282, 155-161 (2005).
6 Zhu, X., Li, X., Zou, X., Wang, Y., Jia, M., Zhang, W., “Supported ammonium metatungstate as highly efficient catalysts for the vapourphase o-methylation of catechol with methanol”, Catal. Commun., 7, 579-582 (2006).
7 Auroux, A., Monaci, R., Rombi, E., Solinas, V., Sorrentino, A., Santacesaria, E., “Acid sites investigation of simple and mixed oxides by TPD and microcalorimetric techniques”, Thermochim. Acta, 379, 227-231 (2001).
8 Benaliouche, F., Boucheffa, Y., Ayrault, P., Mignard, S., Magnoux, P.,“NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites”, Micropor. Mesopor. Mat., 111, 80-88 (2008).
9 Castellón, E.R., López, A.J., Torres, P.M., Jones, D.J., Roziere, J., Trombetta, M., Busca, G., Lenarda, M., Storaro, L., “Textural and structural properties and surface acidity characterization of mesoporous silica-zirconia molecular sieves”, J. Solid State Chem., 175, 159-169 (2003).
10 Corma, A., “From microporous to mesoporous molecular sieve materials and their use in catalysis”, Chem. Rev., 97, 2373-2420 (1997).
11 Kalita, P., Gupta, N.M., Kumar, R., “Synergistic role of acid sites in the Ce-enhanced activity of mesoporous Ce-Al-MCM-41 catalysts in alkylation reactions: FTIR and TPD-ammonia studies”, J. Catal., 245, 338-347 (2007).
12 Katada, N., Igi, H., Kim, J.H., Niwa, M., “Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium”, J. Phys. Chem. B, 101, 5969-5977 (1997).
13 Martins, G.V.A., Berlier, G., Bisio, C., Coluccia, S., Pastore, H.O., Marchese, L., “Quantification of Brønsted acid sites in microporous catalysts by a combined FTIR and NH3-TPD study”, J. Phys. Chem. C, 112, 7193-7200 (2008).
14 Posey, K.L., Viegas, M.G., Boucher, A.J., Wang, C., Stambaugh, K.R., Smith, M.M., Carpenter, B.G., Bridges, B.L., Baker, S.E., Perry, D.A., “Surface-enhanced vibrational and TPD study of nitroaniline isomers”, J. Phys. Chem. C, 111, 12352-12360 (2007).
15 Trombetta, M., Busca, G., Lenarda, M., Storaro, L., Ganzerla, R., Piovesan, L., López, A.J., Rodríguez, M.A., Castellón, E.R., “Solid acid catalysts from clays: Evaluation of surface acidity of mono- and bi-pillared smectites by FT-IR spectroscopy measurements, NH3-TPD and catalytic tests”, Appl. Catal. A Gen., 193, 55-69 (2000).
16 Kanervo, J.M., Reinikainen, K.M., Krause, A.O.I., “Kinetic analysis of temperature-programmed desorption”, Appl. Catal. A Gen., 258, 135-144 (2004).
17 Guo, Y., Sakurai, M., Kameyama, H., “Temperature programmed desorption/surface-reaction study of an anodic alumina supported Ag catalyst for selective catalytic reduction of nitric oxide with propene”, Appl. Catal. B Environ., 79, 382-393 (2008).
18 Sivasankar, N., Vasudevan, S., “Temperature-programmed desorption and infrared spectroscopic studies of benzene adsorption in zeolite ZSM-5”, J. Phys. Chem. B, 108, 11585-11590 (2004).
19 Arena, F., Dario, R., Parmaliana, A., “A characterization study of the surface acidity of solid catalysts by temperature programmed methods”, Appl. Catal. A Gen., 170, 127-137 (1998).
20 Busca, G., “The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization”, Phys. Chem. Chem. Phys., 1, 723-736 (1999).
21 Davydov, A., Molecular Spectroscopy of Oxide Catalyst Surfaces, Wiley, London (2003).
22 Brazdil, J.F., Ebner, A.M., Cavalcanti, F.A.P., “Rutile vanadium antimonates: A new class of catalysts for selective reduction of NO with ammonia”, Appl. Catal. A Gen., 165, 51-55 (1997).
23 Costa, C., Dzikh, I.P., Lopes, J.M., Lemos, F., Ribeiro, F.R., “Activity–acidity relationship in zeolite ZSM-5. Application of brönsted-type equations”, J. Mol. Catal. A Chem., 154, 193-201 (2000).
24 Kanervo, J.M., Keskitalo, T.J., Slioor, R.I., Krause, A.O.I., “Temperatureprogrammed desorption as a tool to extract quantitative kinetic or energetic information for porous catalysts”, J. Catal., 238, 382-393 (2006).
25 Barrie, P.J., “Analysis of temperature programmed desorption (TPD) data for the characterisation of catalysts containing a distribution of adsorption sites”, Phys. Chem. Chem. Phys., 10, 1688-1696 (2008).
26 Panczyk, T., Gac,W., Panczyk, M., Borowiecki, T., Rudzinski, W.,“On the equilibrium nature of thermodesorption processes. TPD-NH3studied of surface acidity of Ni/MgO-Al2O3catalysts”, Langmuir, 22, 6613-6621 (2006).
27 Liu, L.C., Zhao, L.F., Sun, H., “Simulation of NH3temperature-programmed desorption curves using an ab initio force field”, J. Phys. Chem. C, 113, 16051-16057 (2009).
28 Borges, P., Pinto, R.R., Lemos, M., Lemos, F., Védrine, J.C., Derouane, E.G., Ribeiro, F.R., “Activity-acidity relationship for alkane cracking over zeolites: n-hexane cracking over HZSM-5”, J. Mol. Catal. A Chem., 229, 127-135 (2005).
29 Costa, C., Lopes, J.M., Lemos, F., Riberiro, F.R., “Activity-acidity relationship in zeolite Y: Part 2. Determination of the acid strength distribution by temperature programmed desorption of ammonia”, J. Mol. Catal. A Chem., 144, 221-231 (1999).
30 Heilbron, L., Dictionary of Organic Compounds, Science Press, Beijing (1966). (in Chinese)
31 Bai, Z.L., Chen, Q., Chen, S.Z., “Synthesis and catalytic oxidation performance of FeAPO-5 zeolite”, Journal of East China University of Science and Technology, 27, 597-600 (2001). (in Chinese)
32 Mollavali, M., Yaripour, F., Jam, S.M., Atashi, H., “Relationship between surface acidity and activity of solid-acid catalysts in vapour phase dehydration of methanol”, Fuel Process. Technol., 90, 1093-1098 (2009).
33 Yang, R.T., Long, R., Joel, P., “Adsorbent for dioxins: A new technique for sorbent screening for low-volatile organics”, Ind. Eng. Chem. Res., 38, 2726-2731 (1999).
34 Ren, J., Jin, Y.J., Zhao, Y.G., Zhou, J.L., “Study on deactivation kinetics of solid acid catalyst for alkylation reaction of benzene with long chain olefins”, Acta Petrolei Sinica (Petrol. Process Sec.), 17, 32-38 (2001). (in Chinese)
35 Hamzehlouyan, T., Kazemeini, M., Khorasheh, F., “Modeling of catalyst deactivation in zeolite-catalyzed alkylation of isobutene with 2-butene”, Chem. Eng. Sci., 65, 645-650 (2010).
2011-03-30, accepted 2012-12-01.
* To whom correspondence should be addressed. E-mail: Renjie1961@163.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
