RNA 干涉介导大鼠成肌细胞系L6中MuRF-1与FOXO3a基因表达变化的研究
2013-05-05达志峰贾英伟丁洁朱志祥冯勇韦建梁炳生
达志峰 贾英伟 丁洁 朱志祥 冯勇 韦建 梁炳生
•论著•
RNA 干涉介导大鼠成肌细胞系L6中MuRF-1与FOXO3a基因表达变化的研究
达志峰 贾英伟 丁洁 朱志祥 冯勇 韦建 梁炳生
目的探讨RNAi技术体外抑制MuRF-1或FOXO3a基因表达的效果,为RNAi介导的失神经骨骼肌萎缩基因治疗奠定基础。方法体外培养大鼠成肌细胞系L6,将MuRF-1和(或)FOXO3a siRNA重组质粒在Lipofectamine 2000介导下转染,优化与检测系统的转染效率;将2 μg MuRF-1或FOXO3a基因siRNA重组质粒转染L6,转染后48 h与72 h,采用实时定量PCR检测siRNA重组质粒对MuRF-1和FOXO3a的mRNA的抑制效果,使用Western印迹检测siRNA重组质粒对MuRF-1和FOXO3a蛋白水平的抑制效果。用单因素方差分析方法和LSD法进行组间比较。结果(1)质粒转染后24 h,荧光显微镜下可见细胞中有大量明亮的绿色荧光表达,显示系统有较高的转染效率。(2)实时定量PCR分析结果显示:MuRF-1与FOXO3a各自的siRNA重组质粒转染后48 h,二者干扰序列在mRNA水平分别明显抑制了MuRF-1和FOXO3a的表达,抑制率达67﹪和54﹪,与对照组相比差异均有统计学意义(P < 0.05),联合MuRF-1与FOXO3a两基因siRNA重组质粒转染后48 h,干扰序列在mRNA水平明显抑制了MuRF-1及FOXO3a的表达,抑制率达61﹪及58﹪,与对照组相比差异有统计学意义(P < 0.05);转染后72 h,MuRF-1与FOXO3a各自的siRNA重组质粒干扰序列对MuRF-1和FOXO3a的mRNA的抑制率分别达79﹪和81﹪,联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a的mRNA的抑制率达77﹪及72﹪,与对照组相比差异均有统计学意义(P < 0.05),与48 h相比,抑制效应更为明显。(3)Western印迹灰度分析结果显示:转染后48 h,干扰序列MuRF-1明显抑制了MuRF-1蛋白的表达,抑制率达61﹪,与对照组相比差异有统计学意义(P <0.05),干扰序列FOXO3a明显抑制了FOXO3a蛋白的表达,抑制率达46﹪,与对照组相比差异有统计学意义(P < 0.05),联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a蛋白表达的抑制率达64﹪及42﹪,与对照组相比差异有统计学意义(P < 0.05);转染后72 h,干扰序列MuRF-1对MuRF-1蛋白表达的抑制率达70﹪,与对照组相比差异有统计学意义(P < 0.05),干扰序列FOXO3a对FOXO3a蛋白表达的抑制率达72﹪,与对照组相比差异有统计学意义(P < 0.05),联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a蛋白表达的抑制率达73﹪及74﹪,与对照组相比差异有统计学意义(P < 0.05),与48 h相比,抑制效应更为明显,与对mRNA水平的影响一致。(4)MuRF-1的siRNA重组质粒与联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列MuRF-1对MuRF-1的mRNA和蛋白抑制效果相比差异无统计学意义(P >0.05),FOXO3a的siRNA重组质粒与联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列FOXO3a对FOXO3a的mRNA和蛋白抑制效果相比差异无统计学意义(P > 0.05)。结论(1)RNA干扰技术在体外能够明显抑制泛素连接酶MuRF-1和叉头蛋白转录因子FOXO3a基因的表达。(2)体外研究中MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的mRNA和蛋白抑制效果无差别。(3)活体实验中MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的mRNA和蛋白抑制效果尚不明确,这为RNAi介导的失神经骨骼肌萎缩基因治疗提供了一种新的思路。
肌萎缩; MuRF-1; FOXO3a; L6; 去神经支配; RNA干扰; 基因表达
随着科学技术的迅猛发展,工农业生产日趋机械化、现代化,另外国内私家车数量大量增加,由此引发众多的各种生产和交通事故,从而加大了周围神经损伤的发生率和致残率,尤其是臂丛神经损伤,可导致严重的肢体功能障碍,给患者造成巨大的痛苦[1]。延缓神经损伤后骨骼肌的萎缩,为肌肉重获神经支配争取时间,是手内肌功能恢复的关键。最新研究发现泛素连接酶MuRF-1与“feak-headbox”(FOXO)转录因子是调控骨骼肌萎缩最关键的两个分子,叉头蛋白转录因子3a(fork-head protein,FoxO3a)是肌肉蛋白降解的分子开关之一[2-3]。另一方面,病理状态下蛋白质降解的主要机制是通过泛素蛋白酶体水解通路的激活[4]。Bodine等[5]利用基因敲除小鼠研究发现,MuRF-1能有效缓解肌肉萎缩过程,证实泛素连接酶是骨骼肌肉萎缩过程中的关键酶。本研究通过RNA干涉技术,在大鼠成肌细胞系L6中特异性地阻断MuRF-1和FOXO3a,探讨其中MuRF-1和FOXO3a基因与蛋白的表达变化,探索失神经骨骼肌萎缩的发生机制,为RNAi介导的失神经骨骼肌萎缩基因治疗打下基础。
材料与方法
一、材料
大鼠骨骼肌成肌细胞系L6购自美国ATCC公司,pcDNA6.2-GW/EmGFP-miR质粒购自上海锐赛生物技术有限公司,胎牛血清、DMEM(高糖)细胞培养液、胰蛋白酶为美国Gibco公司产品,Genelute Plassmid Miniprep Kit为美国Sigma公司产品,Lipofectamine 2000转染试剂盒为美国Invitrogen公司产品,兔抗大鼠FOXO3a(一抗)为美国Cell Signal公司产品,兔抗大鼠MuRF-1(一抗)为美国Santa Cruz公司产品,山羊抗兔IgG为北京中杉金桥生物技术有限公司产品,总RNA抽提试剂(Trizol)购自上海生工工程有限公司,cDNA合成随机引物及PCR引物委托上海生工工程有限公司进行合成。
二、实验方法
1.获 取MuRF-1和FOXO3a两 基 因 的siRNA重组质粒:参照文献[5]获取MuRF-1和FOXO3a基因miRNA干扰序列,委托上海锐赛生物技术有限公司构建并鉴定siRNA重组质粒。
2.分组说明:阴性对照组(序列siRNA CON组)、实验组A(含MuRF-1基因siRNA重组质粒)、实验组B(含FOXO3a基因siRNA重组质粒)、实验组C(含MuRF-1与FOXO3a基因siRNA重组质粒);转染:取对数生长期的大鼠成肌细胞系L6,用胰酶消化后,将细胞接种于6孔细胞培养板中,加入适量含10﹪胎牛血清的DMEM(高糖)完全培养液,置于37℃、5﹪CO2培养。待细胞融合度达60﹪~70﹪时,取2 μg siRNA重组质粒和5 μl Lipofectamine 2000,分别加入Opti-MEM培养液中,终体积为500 μl,室温孵育20 min;吸取细胞培养板中的培养液,将DNA-脂质体复合物转移入6孔细胞培养板中,轻柔混匀,加培养液至终体积2 ml,置37℃、5﹪CO2孵育48 ~ 72 h,并以未加任何试剂的大鼠成肌细胞系L6作为对照。其间24 h给细胞更换DMEM完全培养液。
3.分别于转染后48 h和72 h弃细胞培养液,按说明书提取细胞总RNA,并反转录成cDNA。应用荧光定量PCR试剂检测转染后细胞的MuRF-1和FOXO3a基因表达,并用PCR软件分析达到荧光阈值时的循环次数(cycle threshold,Ct值),用2-ΔΔCt来计算各组标本FOXO3a和MuRF-1基因的表达强度(表1)。
4.转染48 h和72 h后的细胞,弃上清,用适量细胞蛋白裂解液提取蛋白,经离心获得蛋白上清液并行蛋白定量,经SDS-PAGE蛋白电泳后,通过电转将蛋白转移至PVDF膜上。封闭后加入一抗(兔抗大鼠FOXO3a多克隆抗体1:300,兔抗大鼠MuRF-1多克隆抗体1:100)和二抗(山羊抗兔IgG1:4000),最后ECL化学发光法显色,并用BIO-RAD图像处理软件进行灰度测定,以各因子灰度与内参照灰度之比代表组织内FOXO3a和MuRF-1蛋白的含量,以各灰度比与对照组灰度比的比值作为各蛋白因子的表达强度。
三、统计学分析方法
用SPSS 19.0统计软件处理各组数据,所有PCR及Western印迹检测数据用± s表示。采用单因素方差分析方法和LSD法进行各组比较和组间比较,以P < 0.05为差异有统计学意义。
结 果
一、获取MuRF-1和FOXO3a两基因的siRNA重组质粒
根据大鼠MuRF-1(Gene ID:140939)和FOXO3a(Gene ID:294515)的基因序列分别自行设计并合成miRNA oligo,并行测序分析证实其序列完全准确(表2)。
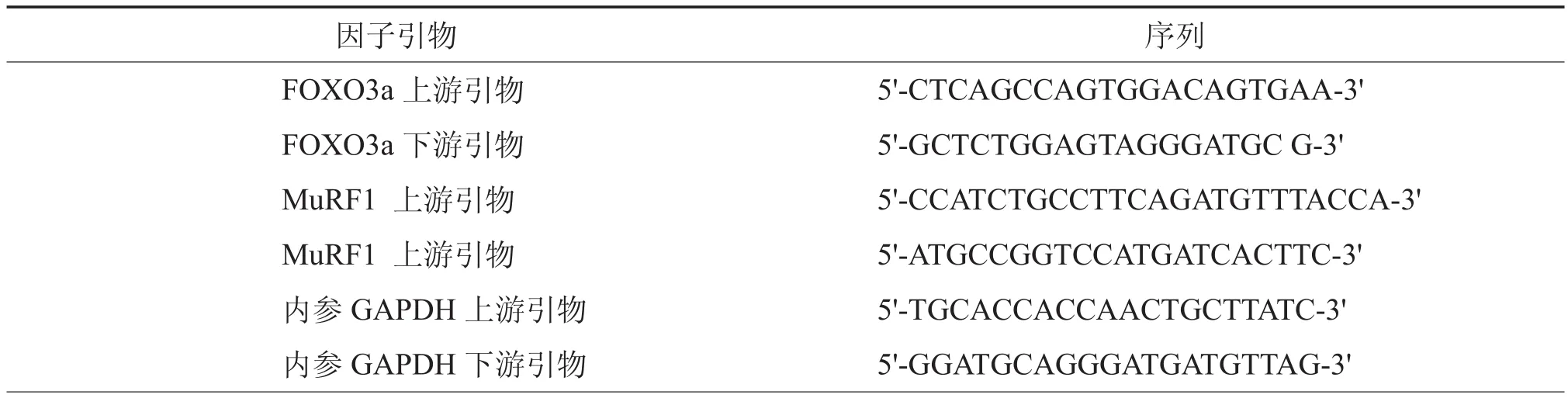
表1 实时定量PCR引物
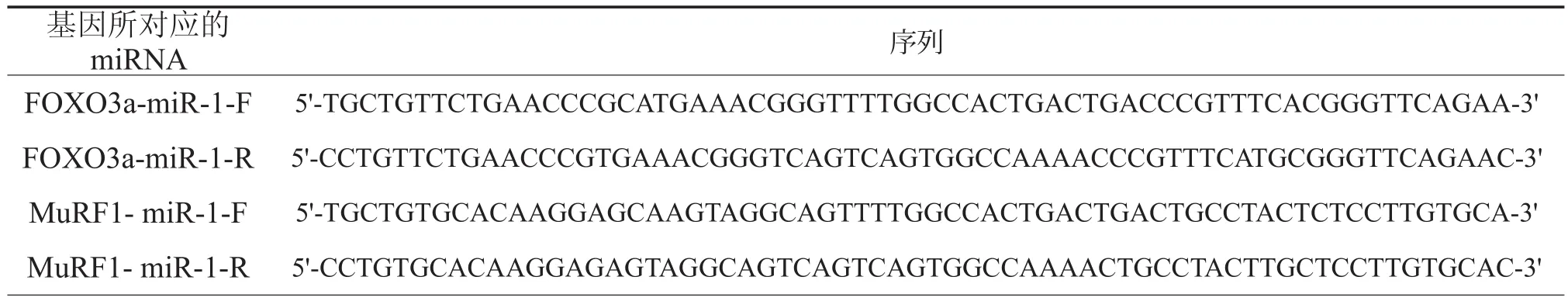
表2 目的基因miRNA oligo序列
二、MuRF-1和FOXO3a基因siRNA重组质粒瞬时转染后mRNA表达变化结果(图1 ~ 2)
转染后48 h,MuRF-1与FOXO3a各自的siRNA重组质粒干扰序列在mRNA水平分别明显抑制了MuRF-1和FOXO3a的表达,抑制率达67﹪和54﹪,与对照组相比差异均有统计学意义(t对照-MuRF-1= 6.401,P < 0.001;t对照-FOXO3a = 5.563,P = 0.001),联合MuRF-1与FOXO3a两基因siRNA重组质粒转染后48 h,干扰序列在mRNA水平明显抑制了MuRF-1及FOXO3a的表达,抑制率达61﹪及58﹪,与对照组相比差异有统计学意义[t对照-联合(MuRF-1)= 5.828,P = 0.001;t对照-联合(FOXO3a)= 5.975,P = 0.001]。
转染后72 h,MuRF-1与FOXO3a各自的siRNA重组质粒干扰序列对MuRF-1和FOXO3a的mRNA的抑制率分别达79﹪和81﹪,联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a的mRNA的抑制率达77﹪及72﹪,与对照组相比差异均有统计学意义[t对照-MuRF-1 = 10.425,P < 0.001;t对照-FOXO3a = 9.872,P < 0.001;t对照-联合(MuRF-1)= 10.034,P < 0.001;t对照-联合(FOXO3a)= 8.878,P < 0.001],与48 h相比,抑制效应更为明显。MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的mRNA抑制效果相比较差异无统计学意义[48 h:tMuRF-1-联 合(MuRF-1)=0.573,P = 0.587,tFOXO3a-联合(FOXO3a)= 0.412,P = 0.695;72 h:tMuRF-1-联合(MuRF-1)= 0.391,P = 0.709,tFOXO3a-联合(FOXO3a)=1.110,P = 0.310]。三、MuRF-1和FOXO3a基因siRNA重组质粒瞬时转染后蛋白表达变化结果(图3 ~ 5)
转染后48 h,干扰序列MuRF-1明显抑制了MuRF-1蛋白的表达,抑制率达61﹪,与对照组相比差异有统计学意义(t对照-MuRF-1 = 4.679,P = 0.003),干扰序列FOXO3a明显抑制了FOXO3a蛋白的表达,抑制率达46﹪,与对照组相比差异有统计学意义(t对照-FOXO3a = 3.117,P = 0.021),联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a蛋白表达的抑制率达64﹪及42﹪,与对照组相比差异有统计学意义[t对照-联合(MuRF-1)= 4.909,P = 0.003;t对照-联合(FOXO3a)= 2.846,P = 0.029]。
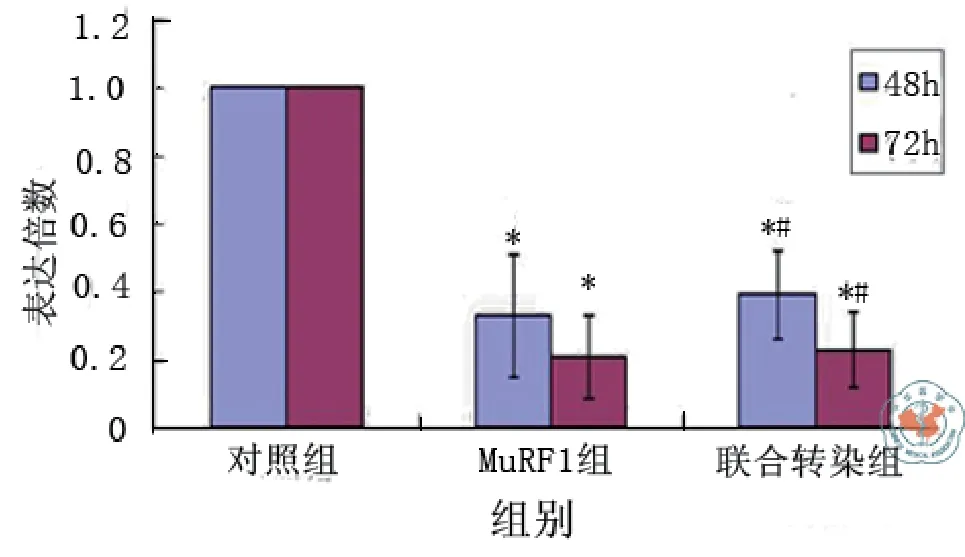
图1 MuRF1基因siRNA重组质粒转染后48 h与72 h后RT-PCR结果
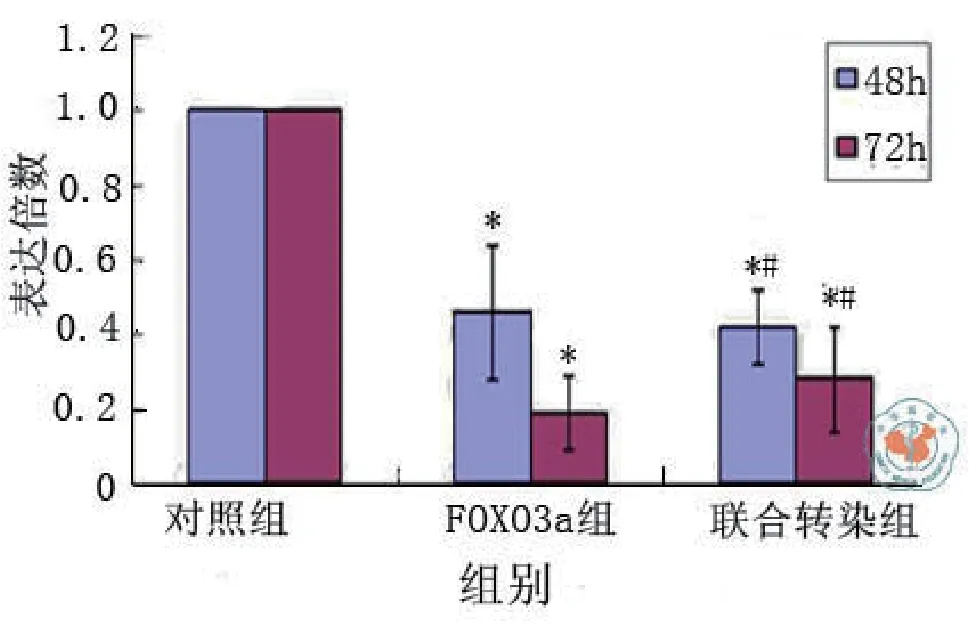
图2 FOXO3a基因siRNA重组质粒转染后48 h与72 h后RT-PCR结果
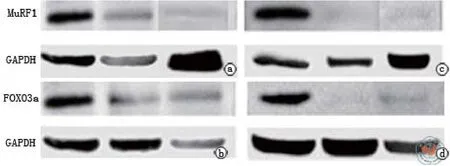
图3 转染后Western印迹检测条带
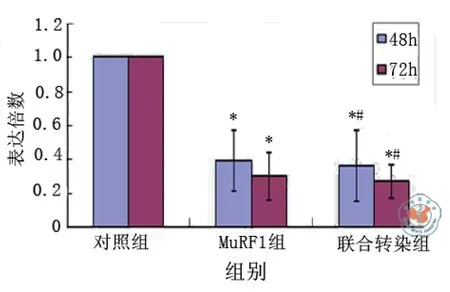
图4 MuRF1基因siRNA重组质粒转染后48 h与72 h后Western印迹结果
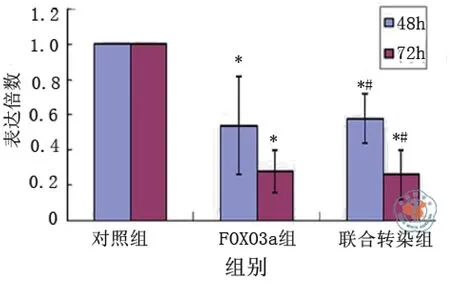
图5 FOXO3a基因siRNA重组质粒转染后48 h与72 h后Western印迹结果
转染后72 h,干扰序列MuRF-1对MuRF-1蛋白表达的抑制率达70﹪,与对照组相比差异有统计学意义(t对照-MuRF-1 = 8.631,P < 0.001),干扰序列FOXO3a对FOXO3a蛋白的表达的抑制率达72﹪,与对照组相比差异有统计学意义[t对照-FOXO3a = 8.283,P < 0.001),联合MuRF-1与FOXO3a两基因siRNA重组质粒干扰序列对MuRF-1和FOXO3a蛋白表达的抑制率达73﹪及74﹪,与对照组相比差异有统计学意义(t对照-联合(MuRF-1)= 9.008,P < 0.001;t对照-联合(FOXO3a)=8.513,P < 0.001],与48 h相比,抑制效应更为明显,与对mRNA水平的影响一致。
MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的蛋白抑制效果相比较差异无统计学意义[48 h:tMuRF-1-联合(MuRF-1)= 0.230,P = 0.826,tFOXO3a -联合(FOXO3a)= 0.271,P = 0.795;72 h:tMuRF-1-联合(MuRF-1)= 0.370,P = 0.724,tFOXO3a-联合(FOXO3a)=0.436,P = 0.698]。
讨 论
臂丛神经损伤后再生距离长,再生速度慢,再生轴突到达手内肌所需时间长,而手内肌肌肉短小,功能精细,一旦失去神经支配,半年内即发生不可逆的组织变性,即使神经获得再支配,也很难恢复有效的功能[6]。因此,臂丛神经损伤所致的手内在肌萎缩的防治是周围神经领域亟待解决的一大难题。骨骼肌在失神经支配后早期出现快速萎缩的事实表明:骨骼肌中蛋白代谢的动态平衡被打破了,蛋白降解速率超过了蛋白合成速率[7],由此可见,采取有效措施调节失神经骨骼肌的蛋白代谢,促进蛋白合成,抑制蛋白水解甚至逆转失神经骨骼肌代谢的负平衡,将是延缓失神经骨骼肌萎缩、为失神经骨骼肌重新获得神经支配争取时间,并且是促进骨骼肌功能恢复的有效途径[8]。Tintignac等[9]发现FOXO3a是与肌萎缩密切相关的基因,它存在于几乎全部的肌萎缩类型中。FOXO3a第253位的丝氨酸去磷酸化后,可促进骨骼肌蛋白的降解,从而促进肌萎缩[10-11]。MuRF-1属于泛素连接酶,激活MuRF-1是导致肌萎缩的主要机制,研究发现,小鼠缺乏MuRF-1可抵抗肌萎缩,故MuRF-1可作为在临床中对抗肌萎缩的靶点[12]。
RNA干涉(RNA interference,RNAi)是一种阻抑基因表达的新方法,是一种简单、有效的代替基因敲除的遗传工具,已成为基因功能研究极其重要的工具[13]。
因此本研究采用RNAi技术在体外下调泛素连接酶MuRF-1和FOXO3a基因的表达,借此为失神经骨骼肌萎缩寻找新的治疗方法。本研究结果显示:采用RNAi技术特异性的敲除掉与失神经骨骼肌萎缩有密切关系的两个基因FOXO3a与MuRF-1,在48 h后检测发现联合转染组、单独转染组与对照组相比较肌萎缩相关因子的mRNA及蛋白表达量均被显著抑制,在72 h后这种抑制效果更加明显,这表明RNAi技术在体外能够明显抑制泛素连接酶MuRF-1和FOXO3a基因的表达。但是本研究还发现MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的mRNA和蛋白抑制效果无明显差别。分析原因可能与FOXO3a可通过不同的方式激活MuRF-1[14]有关,当MuRF-1基因被敲除后FOXO3a也就失去这种激活作用,所以联合转染后并没有出现协同或者拮抗作用。活体实验中MuRF-1与FOXO3a两基因siRNA重组质粒联合转染与各基因siRNA重组质粒单独转染其干扰序列对MuRF-1或FOXO3a的mRNA和蛋白抑制效果是否与体外研究结果一致尚不明确,本研究为RNAi介导的失神经骨骼肌萎缩基因治疗提供了一种新的思路,即联合干预两类在失神经骨骼肌萎缩过程中起重要作用的因子MuRF-1和FOXO3a进行基因治疗,可能会有更好的疗效,这有待于进一步研究。
1 Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy[J]. Biochim Biophys Acta, 2008, 1782(12):730-743.
2 Zheng B, Ohkawa S, Li HY, et al. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/ MAFbx expression during glucocorticoid-induced skeletal muscle atrophy[J]. Fsseb Journal, 2010, 24 (8):2660-2669.
3 Stitt T, Drugjan D. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors[J]. Mol Cell, 2004, 14(3):395-403.
4 Tisdale MJ. Is there a common mechanism linking muscle wasting in various disease types?[J]. Curr Opin Support Palliat Care, 2007, 1(4):287-292.
5 Bodine SC, Latres E, Baumhueter S, et al. Identi fi cation of Ubiquitin Ligases required for skeletal muscle atrophy[J]. Science, 2001, 294(5547):1704-1708.
6 Katoch SS, Garg A, Sharma S. Histological evidences of reparative and regenerative effects of beta-adrenoceptor agonists, clenbuterol and isoproterenol, in denervated rat skeletal muscle[J]. Indian J Exp Biol, 2006, 44(6):448-458.
7 Garg A, Sharma S. Isoproterenol ameliorates work stressinduced rat skeletal muscle degeneration[J]. Indian J Biochem Biophys, 2006, 43(2):82-87.
8 Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways[J]. Int J Biochem Cell Biol, 2005, 37(10):1974-1985.
9 Tintignac LA, Lagirand J, Batonnet S, et al. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase[J]. J Biol Chem, 2005, 280(4):2847-2856.
10 Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy[J]. Cell, 2004, 117(3):399-412.
11 Nakae J, Oki M, Cao Y. The Foxo transcription factors and metabolic regulation[J]. FEBS lett, 2008, 582(1):54-67.
12 Janácek J, Cebasek V, Kubínová L, et al. 3D visualization and measurement of capillaries supplying metabolically diffe rent fi ber types in the rat extensor digitorum longus muscle during denervation and reinnervation[J]. J Histochem Cytochem, 2009, 57(5):437-447.
13 Haman GJ. RNA interference[J].Nature, 2002, 418(6894): 244-251.
14 Deouxm M, Vanbeneden R, Paskon, et al. Role of the insulin-like growth factor I decline in the induction of atrogin-1/mafbx during fasting and diabetes[J]. Endocrinology, 2004, 145(11):4806-4812.
Study of RNAi-mediated gene downr egulation of ubiquitin ligase MuRF-1 and forkhead activin signal transducer FOXO3a in vitro
DA Zhi-feng*, JIA Ying-wei, DING Jie, ZHU Zhixiang, FENG Yong, WEI Jian, LIANG Bing-sheng,*Department of Orthopaedics (Micro and Hand Surgery), The 2nd Hospital of Shanxi Medical University, Taiyuan 030001, China
LIANG Bing-sheng, Email:liangbs707@yahoo.com
Objective To investigate the effect of RNAi technology in inhibiting gene expression of MuRF-1 or FOXO3a in vitro, establishing a foundation for RNAi mediated gene therapy for denervated skeletal muscle atrophy.MethodsRat muscle cell line L6 was transfected with MuRF-1 or FOXO3a siRNA plasmid using Lipofectamine 2000. After transfection was optimized, 2 μg of MuRF-1 or FOXO3a siRNA recombinant plasmid was transfted. At 48 h and 72 h after transfection, the real-time quantitative PCR was used to detect the inhibition effect of MuRF-1 and FOXO3a. Protein level was evaluated using Western blotting.Results(1) At 24 h after transfection, strong bright green fluorescence was observed under fluorescence microscopy, indicating high transfection efficiency. (2) Real time quantitative PCR analysis showed mRNA expression was significantly inhibited for both MuRF-1 and FOXO3a after 48 h, with an inhibition rate of 67﹪ and 54﹪ respectively. When MuRF-1 and FOXO3a siRNA recombinant plasmids were co-transfectied, MuRF-1 and FOXO3a expression was inhibite by 61﹪ and 58﹪ respectively compared with the control (P < 0.05 for both). At 72 h after transfection, the inhibitory rates for MuRF-1 and FOXO3a siRNA recombinant plasmids at mRNA level were 79﹪ and 81﹪ respectively. When MuRF-1 and FOXO3a siRNA recombinant plasmids were co-transfected, MuRF-1 and FOXO3a mRNA was inhibited by 77﹪ and 72﹪ respectively, significantly higher than the control group (P < 0.05) and higher than that at 48 h. (3) Western blotting showed MuRF-1 and FOXO3a were inhibited by 61﹪ and 46﹪ respectively.When MuRF-1 and FOXO3a siRNA recombinant plasmids were co-transfected, MuRF-1 and FOXO3a protein expression was inhibited by 64﹪ and 42﹪ respectively, both higher than the control (P < 0.05). At 72 h, MuRF-1 and FOXO3a protein expression was inhibited by 70﹪ and 72﹪respectively, significantly greater than the control. Whenunited MuRF-1 and FOXO3a siRNA recombinant plasmids were co-transfected, MuRF-1 and FOXO3a inhibition rates were 73﹪and 74﹪ respectively, significantly greater than the control (P < 0.05). Again, the inhibition was greater at 72 h than at 48 h.ConclusionsRNA interference can obviously inhibit the expression of ubiquitin ligase MuRF-1 and fork protein transcription factors FOXO3a in vitro. Co-transfection of MuRF-1 and FOXO3a siRNA recombinant plasmids can inhibit MuRF-1 and FOXO3a mRNA and protein expression. The inhibition effects were similat to those when the plasmid was tranfected alone.
Muscle atrophy; MuRF-1; FOXO3a; L6; Denervation; RNA Interference; Gene expression
2012-04-19)
(本文编辑:李少婷)
10.3877/cma.j.issn.2095-1221.2013.02.007
国家自然科学青年基金(81000805)
030001 太原,山西医科大学第二医院骨科(达志峰、贾英伟、丁洁、朱志祥、韦建、梁炳生);上海市第六人民医院骨科(冯勇)
梁炳生,Email:liangbs707@yahoo.com
达志峰,贾英伟,丁洁,等. RNA干涉介导大鼠成肌细胞系L6中MuRF-1与FOXO3a基因表达变化的研究[J/CD].中华细胞与干细胞杂志:电子版, 2013, 3(2):87-93.
