Toxicity evaluation of botanical insecticides and their mixture against the spiraling whitefly, Aleurodicus dispersus
2012-12-05JianmingWANGBingLINGXiCAOShulinBAOMaoxinZHANG
Jian-ming WANG, Bing LING, Xi CAO, Shu-lin BAO, Mao-xin ZHANG
Laboratory of Insect Ecology, South China Agriculture University, Guangzhou, Guangdong 510642, China
Toxicityevaluationofbotanicalinsecticidesandtheirmixtureagainstthespiralingwhitefly,Aleurodicusdispersus
Jian-ming WANG, Bing LING, Xi CAO, Shu-lin BAO, Mao-xin ZHANG*
LaboratoryofInsectEcology,SouthChinaAgricultureUniversity,Guangzhou,Guangdong510642,China
【Background】AleurodicusdispersusRussell, a destructive invasive pest was first found in Hainan, China, in April 2006. Botanical insecticides have long been suggested as attractive alternatives to synthetic ones for pest management because of their high potency, efficacy and more eco-friendly nature. 【Method】The spraying and film contact methods were used to measure the toxicity of nine botanical insecticides and their mixture againstA.dispersus. 【Result】The maximum mortality of adults was recorded for pyrethrum and rotenone withLC50at 24 h being 2.56 mg·L-1and 34.15 mg·L-1, respectively. These were followed by azadirachtin (24 hLC50being 158.36 mg·L-1) and bitter melon leaf extracts (24 hLC50being 311.02 mg·L-1). Pyrethrum showed significant insecticidal action to the eggs and nymphs ofA.dispersuswith 24 hLC50being 77.39 mg·L-1and 61.42 mg·L-1, respectively. Bioassay results indicated that mixed azadirachtin or rotenone with pyrethrum (1∶1) exhibited a significant synergism againstA.dispersus, and their cotoxicity coefficient (CTC) were 193.11 and 224.35, respectively. 【Conclusion and significance】Several of the examined botanical pesticides showed potential to controlA.dispersus. The mixture of azadirachtin or rotenone with pyrethrum could not only have synergism but also delay the resistance ofA.dispersus.
pyrethrum; azadirachtin; rotenone;Aleurodicusdispersus; insecticidal activity
Introduction
The spiraling whitefly (AleurodicusdispersusRussell) is a destructive invasive pest. This is native to the Caribbean islands and Central America (Russell,1965), but has spread very fast in African and Asian countries besides several Pacific islands and poses serious problem (Martin amp; Lucas,1984; Neuenschwander,1994).A.dispersusis a highly polyphagous pest (Lambkin,1999; Srinivasa,2000). Its extensive host range covers 182 plants belonging to 146 genera and 57 families in Hainan (Yu,2011). The major host plants of economic concern arePsidiumguajavaL.,CaricapapayaL.,MusasapientumL.,AnnonasquamosaL.,EuphorialonganaLam,SolanummelongenaL.,PhaseolusvulgarisL., andManihotglazioviiMuell., in addition to several species of avenue trees such asPterocarpusindicusWilld.,TerminaliacatappaL.,CannaindicaL., andCercischinensisBunee in the urban environment (Hanetal.,2008). Both adults and nymphs ofA.dispersussuck sap from the leaf surfaces and can cause leaf wilting and dropping. Honeydew and wax excreted by the nymphs and adults on leaf surfaces can decrease the photosynthetic capacity of the host plants (Ramanietal.,2002).
A.dispersuswas first found in Hainan, China, in April 2006 (Yu,2011; Yuetal.,2007). Within a short period of time, the species has spread to the whole island, and damage to both economic crops and street trees have been documented (Hanetal.,2008; Zhengetal.,2008).
Some synthetic insecticides are proven effective in spiraling whitefly control (Kambrekaretal.,2003; Linetal.,2007; Liuetal.,2007). However, they are hazardous to human health because of the pest′s widespread distribution and its presence in densely populated areas. In addition, indiscriminate use of synthetic insecticides kills beneficial insects and leads to the development of pest resistance to synthetic insecticides. Thus, there is an urgent need for alternative approaches to pest management that can completely or partially replace current chemically-based pest management practices (Dubey amp; Sundararaj,2004). Botanical insecticides can contribute towards this goal as attractive alternatives to synthetic chemical insecticides for pest management because of their high potency, efficacy and more eco-friendly nature (Isman,2006). With this in mind, nine botanically-based insecticide products and their mixtures were evaluated for the suppression ofA.dispersus. We found several promising alternatives, and identified a combination with a synergistic effect, holding out promise for a more enviornmentally friendly control of this invasive species.
Materials and methods
Insects
The various developental stages ofA.dispersusused in assays were obtained from a natural population ofA.dispersusinTerminaliacatappaL.trees growing on the grounds of the Environment and Plant Protection Institute, Chinese Academy of Tropical Agriculture, Danzhou, Hainan Province, where they were grown free from any insecticides.A.dispersusadults, at least 24 h old, were collected randomly from populations of several hundred individuals reared onT.catappafor use in the bioassays.
Botanical insecticides
Technical grade of Matrine [98% active ingredient (AI)], Stemonine (2% AI), Sanguinarine (15% AI) and Celangulin (6% AI), were purchased from Shanxi Fangsheng Biological Development Co.Ltd., China. Osthole technical (98% AI) was purchased from Nanjing Zelang Medical Technology Co.Ltd., China. Rotenone (40% AI) and pyrethrum (50% AI) were obtained from Yunnan Nanbao Plant Chemicals Co.Ltd., China. Azadirachtin (20% AI) was purchased from Luxi Light Neem Industry Development Co.Ltd., China.
The chloroform extract of bitter melon (MomordicacharantiaL., BM) leaves were obtained and isolated following Abo amp; Matsuda (2000). Fresh leaves for extraction were collected from matureM.charantia(after the fruiting stage). The leaves were cut into pieces and extracted with methanol at room temperature (29~31 ℃, 24 h). The extract was filtered and subsequently concentrated to dryness under reduced pressure with a rotary evaporator. The methanol extract was dissolved in water and successively partitioned with hexane and chloroform. A portion of the chloroform extract was dried under reduced pressure.
Bioassays of botanical insecticides
All bioassays were conducted in the laboratory at 30±1 ℃ and 16 h ∶8 h (L ∶D) regime.
ActivityofbotanicalinsecticidestoadultsofA.dispersusbyspraying
A 10 mL sample of each test solution (concentration 500 mg·L-1with 50% acetone in water) was prepared. Bioassays were carried out on freshly cut leaves of youngT.catappa, which had not been subjected to any prior chemical treatment. Single leaves were placed onto moistened filter paper in Petri dishes (9 cm diameter), with the undersides of the leaves facing upward. After anaesthetizing theA.dispersusadults (gt;24 h old) on theT.catappawith ether, thirty anaesthetized adults ofA.dispersuswere gently introduced onto each leaf in the Petri dishes with a fine brush. When most of the insects had recovered and resumed activity, dead and weak insects were removed before starting the exposure. One mL of the 500 mg·L-1test solution was sprayed onto each leaf, by means of a spray tower (Potter, Burkard Company). The solvent was quickly removed by air-drying and the lid of Petri dish was covered by a plastic wrap. Control dishes were treated similarly with 50% acetone in water. Each test was replicated three times. The treated adults were examined for mortality at 12 h intervals after treatment. Adults that did not show any responses when probed with a small brush were considered dead. In the case of pyrethrum, rotenone, azadirachtin and BM extracts,A.dispersusadults were examined for mortality at 24 h after treatment of five concentrations gradient, respectively.
Log-probit regression equations were established for pyrethrum, rotenone, azadirachtin and BM extracts and their 24 h median lethal concentrations (LC50) were calculated, respectively.
Activityofbotanicalinsecticidesappliedasdryfilmstoadults
Bioassays were carried out on freshly cut leaves of youngT.catappawhich had not been subjected to any chemical treatment. The leaves were placed individually on moistened filter paper in Petri dishes (9 cm diameter) with the underside of the leaf facing upward. One mL of the 500 mg·L-1test solution was sprayed onto each leaf using a spray tower, and the solvent was quickly removed by air-drying. Thirty adults ofA.dispersus(gt;24 h old) were anaesthetized and gently introduced onto the treated leaves in the Petri dishes. When most of the adults were awake and active, inactive individuals were eliminated, and the Petri dish lid was closed. Controls were treated similarly with 50% acetone in water. Each test was replicated three times. Insects were examined for mortality at 12 h intervals after treatment. Adults that did not show any activity when probed with a small brush were considered dead. Mortalities were compared statistically.
ActivityofpyrethrumtotheeggsandnymphsofA.dispersusbyspraying
Ten mL samples of pyrethrum dissolved in 50%
acetone in water were diluted to concentrations of 12.5, 25, 50, 100, 200 and 400 mg·L-1. The assays were performed on freshP.guajavaleaves heavily infested with a natural population of eggs and various stages of nymphs ofA.dispersus. The leaves were placed individually on moistened filter paper in Petri dishes (9 cm diameter) with the eggs and nymphs on the undersides of the leaves facing upward. The number of eggs and nymphs in each Petri dish were counted with a stereo dissecting microscope. A 1 mL aliquot of the 500 mg·L-1test solution was applied to the eggs and nymphs on the leaf in each Petri dish using a spray tower. The solvent was quickly removed by air-drying and the lid of Petri dish was then covered. Control leaves were treated similarly with 50% acetone in water. Each test was replicated six times. The eggs and nymphs ofA.dispersuswere examined for mortality after five days. Eggs that exhibited shrinkage and darkening were considered dead. Nymphs that showed activity when probed with a small brush were considered to be survivors.
Testswithmixedextracts
The synergistic effect of the mixtures of azadirachtin or rotenone with pyrethrum on adults ofA.dispersuswere determined by the co-toxicity coefficient (CTC) method in the laboratory (Sun amp; Johnson,1960; Zhangetal.,2008). Five concentrations of two different mixtures were applied by a spray tower to treat thirtyA.dispersusadults incubated in Petri dishes. The control was distilled water. Each treatment was replicated five times. The number of mortality was recorded at 24 h after treatment.CTCof the mixtures were calculated as (Zhangetal.,2008):
CTC=[(1/LC50M)/(PA/LC50A+PB/LC50B)]×100%
Where:LC50A,LC50BandLC50Mmean 50% lethal concentration of pesticide A, B and mixture, respectively.PAandPBmean percentage of pesticide A and B in the active constituent of mixture respectively. WhenCTCis 100, it indicates a probability of similar action. WithCTCgt;gt;100, a synergistic action can be assumed, while atCTClt;lt;100 indicates antagonism.
Statistical analysis
Mean values were given with the standard error (SE). Differences in mortalities among treatments were analyzed by one-way ANOVA of arcsine square root transformed values, using Duncan′s Multiple Range Test for post-hoc comparison of means.LC50values of the tested botanical insecticides and confidence limits were calculated forA.dispersuswith Log dosage-mortality probit regression equations. All statistical analyses were performed using SAS 9.0 software (SAS Institute Inc.2005).
Results
ToxicityofbotanicalinsecticidestoadultsofA.dispersusbyspraying
Mortalities ofA.dispersusadults onT.catappaleaves, sprayed with different botanical insecticides under laboratory conditions showed significant differences (Table 1). The maximum adult mortality was recorded for pyrethrum and rotenone (mortality of 98.33% and 90.98% at 12 h and 100% and 98.89% at 24 h, respectively. Celangulin, azadirachtin and BM extracts were also relatively effective with mortality at 36 h between 58.8% and 71.5%. There were no significant differences between control and matrine, sanguinarine, osthole and stemonine (Table 1).
Table1ToxicityoftheninetestedbotanicalinsecticidestoadultsofA.dispersusbyspraying
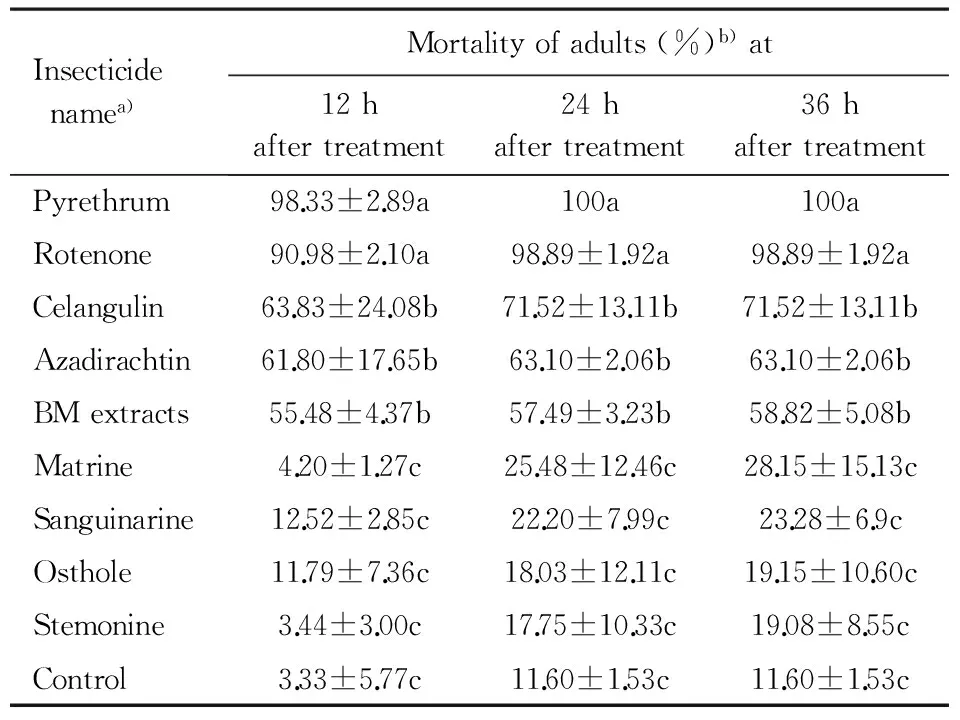
Insecticidenamea)Mortalityofadults(%)b)at12haftertreatment24haftertreatment36haftertreatmentPyrethrum98.33±2.89a100a100aRotenone90.98±2.10a98.89±1.92a98.89±1.92aCelangulin63.83±24.08b71.52±13.11b71.52±13.11bAzadirachtin61.80±17.65b63.10±2.06b63.10±2.06bBMextracts55.48±4.37b57.49±3.23b58.82±5.08bMatrine4.20±1.27c25.48±12.46c28.15±15.13cSanguinarine12.52±2.85c22.20±7.99c23.28±6.9cOsthole11.79±7.36c18.03±12.11c19.15±10.60cStemonine3.44±3.00c17.75±10.33c19.08±8.55cControl3.33±5.77c11.60±1.53c11.60±1.53c
a)The concentration was 500 mg·L-1.b)Mean±SEin the same column followed by different letters indicate significant difference at 0.05 level (Duncan′s multiple range test).
Based on the Log-probit regression equations (Table 2)A.dispersusadults were the most sensitive to pyrethrum and the least sensitive to BM extracts.LC50of pyrethrum was 1.56 mg·L-1, which was significantly lower than that of rotenone, azadirachtin and BM extracts.

Table 2 Comparative toxicity of nine botanical insecticides to A.dispersus adults after 24 h
a)Yis the logarithm of the treatment concentration (mg·L-1), andXis the mortality converted to probit.
TheeffectsofspraysofazadirachtinorrotenonemixedwithpyrethrumonadultA.dispersus
The effects of two different mixtures on the adults ofA.dispersuswere similarly synergistic. Bioassay results revealed that the synergism was significant, and theirCTCwere 193.11 and 224.35, respectively (Table 2).
ToxicityofbotanicalinsecticidesappliedasdryfilmstoadultsofA.dispersus
When adultA.dispersusresiding onT.catappaleaves were treated with dry films of different botanical insecticides under laboratory conditions (Table 3), pyrethrum caused the highest mortalities througout the experiment, followed by azadirachtin (34.8% mortality at 24 h, increasing to 81.8% at 36 h). Matrine, rotenone and sanguinarine were also effective, but they exhibited significantly lower mortalities than the first two (Table 3). None of the other botanical insecticides caused significantly greater mortality than the control. Rotenone, BM extracts and celangulin, when applied as dry films against adults ofA.dispersushad reduced effects compared to the effects of spraying.
ToxicityofpyrethrumtotheeggsandnymphsofA.dispersusbyspraying
Log-probit regression equations of pyrethrum to eggs and nymphs ofA.dispersuswere shown in Table 4.LC50of pyrethrum to the eggs and nymphs were 77.39 mg·L-1and 61.42 mg·L-1, respectively. Toxicity of pyrethrum toA.dispersusadults was higher than that to nymphs or eggs. The fourth instar nymph was the least sensitive to pyrethrum.
Table3ToxicityoftheninetestedbotanicalinsecticidesappliedasdryfilmstoadultsofA.dispersus
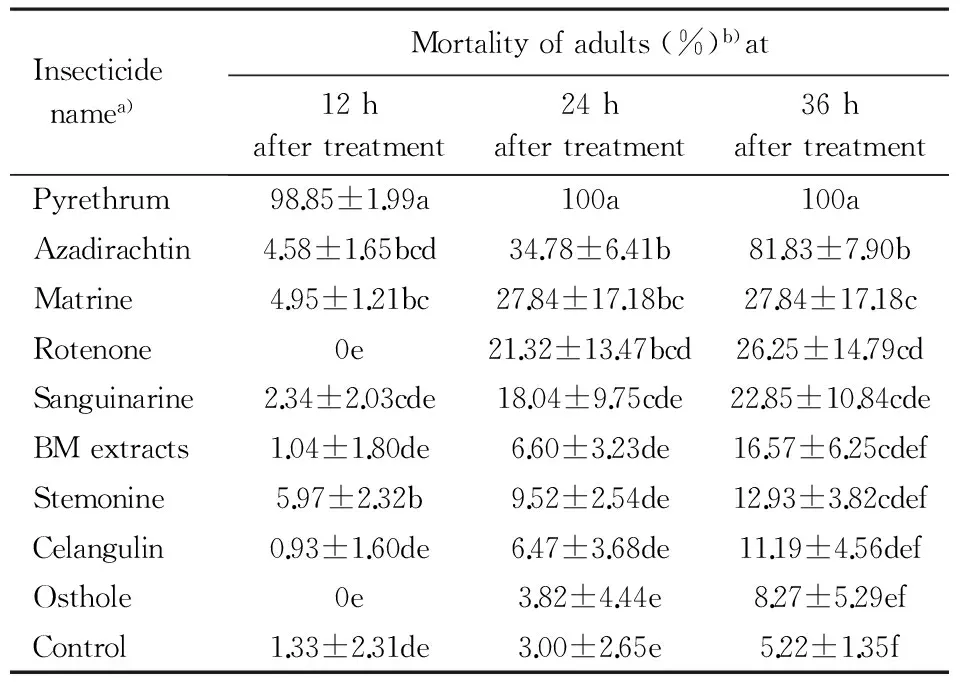
Insecticidenamea)Mortalityofadults(%)b)at12haftertreatment24haftertreatment36haftertreatmentPyrethrum98.85±1.99a100a100aAzadirachtin4.58±1.65bcd34.78±6.41b81.83±7.90bMatrine4.95±1.21bc27.84±17.18bc27.84±17.18cRotenone0e21.32±13.47bcd26.25±14.79cdSanguinarine2.34±2.03cde18.04±9.75cde22.85±10.84cdeBMextracts1.04±1.80de6.60±3.23de16.57±6.25cdefStemonine5.97±2.32b9.52±2.54de12.93±3.82cdefCelangulin0.93±1.60de6.47±3.68de11.19±4.56defOsthole0e3.82±4.44e8.27±5.29efControl1.33±2.31de3.00±2.65e5.22±1.35f
a)The concentration was 500 mg·L-1.b)Mean±SEin the same column followed by different letters indicate significant difference at 0.05 level (Duncan′s multiple range test).

Table 4 Toxicity of pyrethrum to eggs and nymphs of A.dispersus by spraying
a)Yis the logarithm of the treatment concentration (mg·L-1), andXis the mortality converted to probit.
Discussion
Chemicals derived from pyrethrum (TanacetumcinerariifoliumTrev.), neem (AzadirachtaindicaJuss), and other plant species are traditionally used in many crop pest control operations (Atkinsonetal.,2004; Liangetal.,2003). Neem products, tobacco extracts and rosin soap had been found effective againstA.dispersusin several countries (Dubey amp; Sundararaj,2004; Kambrekaretal.,2003; Lüetal.,2009; Singhetal.,2005). Zhongetal.(2009) observed that ethanol extracts ofCelosiaargenteaL.andEupatoriumodoratumL.had high bioactivities againstA.dispersuswithLC50being 753.40 mg·L-1and 999.81 mg·L-1, respectively. Our results indicated that pyrethrum, rotenone, azadirachtin, BM extracts, and celangulin at 500 mg·L-1concentration exhibited significant insecticidal action onA.dispersusadults with mortality above 57.5%, while matrine, sanguinarine, osthole and stemonine showed little insecticidal action onA.dispersusadults by spraying. Among the nine botanical insecticides, the maximum mortality of adults was recorded for pyrethrum, whether applied by spraying or as a dry film (Tables 1, 2 and 3). Acute toxicity of pyrethrum toA.dispersusadults was the highest and the most rapid than that to the eggs and nymphs (Tables 2 and 4). This may be releated to the thicker waxy layer covering the insect body at these stages. Consequently, we recommend that pyrethrum should be applied during the peak occurrence of adultA.dispersus.
The second effective botanical insecticide spray was rotenone. Although the toxicity of azadirachtin toA.dispersusadults was lower than that of pyrethrum and rotenone, its toxicity when applied as a dry film was higher with a longer duration of toxicity (Table 3). Dubey amp; Sundararaj (2004) reported that fortnightly and three-weekly applications of neem effectively controlled nymphal populations ofA.dispersus, causing 62.2% mortality even at 21 days after treatment. Formulatoin does matter: Neemark retained its toxicity (from an initial mortality of 55% to 45% on day 15) better than Neemazal (45% to 25%mortality, Kambrekaretal.,2003). The different results of toxicity tests might be due to the formulation effect and the response ofA.dispersusto other toxic components of the neem.
The results of this study showed that the synergistic effects of pyrethrum mixed with azadirachtin or rotenone to the adults ofA.dispersuswere significant (Table 2). There are different mechanisms of action onA.dispersusbetween each insecticide. The pyrethrum is to block voltage-gated sodium channels in nerve axons (Xu,2001). Rotenone is a mitochondrial poison, which blocks the electron transport chain and prevents energy production (Goyal amp; Srivastava,1990). In contrast to action mechanisms of pyrethrum and rotenone, azadirachtin has diverse biological effects on insects, demonstrating antifeedant, oviposition deterrent, repellent, insect growth inhibitor, mating disruptor, and toxic properties (Xu,2001). Mixed use of insecticides with different action mechanisms can enhance the synergism and also delay the development of resistance inA.dispersus.
Extracts ofM.charantialeaves were evaluated not only for oviposition deterrence, antifeedant effect and inhibition of development but also for toxic action against many phytophagous insect pests (Lietal.,2001; Lingetal.,2008,2009; Mekuriaetal.,2005). Our study confirmed that chloroform extracts ofM.charantialeaves had insecticidal effect toA.dispersusadults. The acetone extracts of fresh leaves ofM.charantiaare toxic to the adults ofLiriomyzasativaeBlanchard, causing up to 50.48% mortality (Lietal.,2001). The methanol extracts of the leaves ofM.charantiahad insecticidal activity on larvae ofCulexquinquefasciatusSay withLC50of 465.85 mg·L-1(Prabakar amp; Jebanesan,2004). Methanol extracts ofM.charantiahave ovicidal action inLeucopteracoffeella(Guérin-Mèneville) (Alvesetal.,2011). The extracts ofM.charantialeaves contain a variety of cucurbitane triterpenoid compounds, such as momordicin Ⅰ, momordicin Ⅱ, 23-dihydroxy-3-O-malonycucurbita-5, 24-dien-19-al, (19S, 23E)-5β, 19-epoxy-19-methoxy-cucurbita-6, 23-diene-3β, and 25-diol, (19R, 23E)-5β, 19-epoxy-19-methoxycucurbita-6, 23-diene-3β, 25-diol, which deter oviposition ofLiriomyzatrifolii(Burgess) andL.sativaeadults, and inhibit feeding and development ofPlutellaxylostellaL.(Lingetal.,2008,2009; Mekuriaetal.,2005). The study have established the insecticidal potential ofM.charantiaextracts onA.dispersus, further research is required to determine the active components and formulate them.
Abe M and Matsuda K. 2000. Feeding deterrents fromMomordicacharantialeaves to cucurbitaceous feeding beetle species.ApplyEntomologyandZoology, 35: 143-149.
Alves D S, Oliveira D F and Carvalho G A. 2011. Plant extracts as an alternative to controlLeucopteracoffeella(GuérinMèneville) (Lepidoptera: Lyonetiidae ).NeotropicalEntomology, 40: 123-128.
Atkinson B L, Blackman A J and Faber H. 2004. The degradation of the natural pyrethrins in crop storage.JournalofAgriculturalFoodChemistry, 52: 280-287.
Dubey A K and Sundararaj R. 2004. Evalution of neem products againstAleurodicusdispersesRussell (Homoptera: Aleyrodidae) onBauhiniavariegateandMicheliachampaca.IndianJournalofPlantProtection, 32: 126-128.
Goyal N and Srivastava V M L. 1990. Mitochondrial NADH oxidase activity ofSetariacervi.VeterinaryParasitology, 37: 229-236.
Han D Y, Liu K, Chen W, Fan Z W, Peng Z Q, Huang W R, Yu G Y, Zhang G L and Fu R G. 2008. Distribution and host plants of the spiraling whitefly,Aleurodicusdisperses, in Hainan.ChineseBulletinEntomology, 45: 765-770.
Isman M B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world.AnnualReviewsEntomology, 51: 45-66.
Kambrekar D N, Awaknavar J S and Kulkarni K A. 2003. Insecticidal toxicity against spiraling whitefly,AleurodicusdispersesRussell on Acalypha.JournalEntomologyResearch, 27: 77-80.
Lambkin T A. 1999. A host list forAleurodicusdispersesRussell (Homoptera: Aleyrodidae) in Australia.AustraliaJournalofEntomology, 38: 373-376.
Li S Q, Den X W and Zhang Q D. 2001. The killing action and growth influence ofMomordicacharantiaextracts onLiriomyzsativae.JournalofHuazhongAgriculturalUniversity, 20: 539-543.
Liang G M, Chen W and Liu T X. 2003. Effects of three neem-based insecticides on diamondback moth (Lepidoptera: Plutellidae).CropProtection, 22: 333-340.
Lin Z F, Xiao T B, Xie S H, Chen M C and Wang S Y. 2007. Effects of eight insecticides on control of spiraling whitefly.Agrochemicals, 46: 630-632.
Ling B, Wang G C, Ya J, Zhang M X and Liang G W. 2008. Studies on antifeeding activity and active ingredients againstPlutellaxylostellafromMomordicacharantialeaves.ScientiaAgriculturaSinica, 41: 1466-1473.
Ling B, Xiang Y L, Wang G C, Cheng S H and Zhang M X. 2009. Antifeedant and antioviposition activities ofMomordicacharantialeaf ethanol extract againstLiriomyzasativae.ChineseJournalofAppliedEcology, 20: 836-842.
Liu K, Yao G, Fu R G, Zhang F P, Qin H F, Wen L N, Liu J and Chen R. 2007. Effectiveness of normal pesticides against new invasive insectAleurodicusdispersesRussell.ChineseAgriculturalScienceBulletin, 23: 333-337.
Lü C J, Zhong B Z, Sun X D, Qin W Q, Han C W, Fu R G and Ma Z L. 2009. Bioactivities of several botanical pesticides against spiraling whitefly (AleurodicusdispersusRussell).ChineseJournalofTropicalCrops, 30: 1865-1869.
Martin J H and Lucas G R. 1984.AleurodicusdispersesRussell (Homoptera: Aleyrodidae), a whitefly species new to Asia.ThePhilippineScientist, 21: 168-171.
Mekuria D B, Kashiwagi T, Tebayashi S and Kim C S. 2005. Cucurbitane triterpenoid oviposition deterrent fromMomordicacharantiato the leafminer,Liriomyzatrifolii.BioscienceBiotechnologyBiochemistry, 69: 1706-1710.
Neuenschwander P. 1994. Spiraling whitefly,Aleurodicusdisperses, a recent invader and new cassava pest in Africa.AfricanCropScienceJournal, 2: 419-421.
Prabakar K and Jebanesan A. 2004. Larvicidal efficacy of some cucurbitacious plant leaf extracts againstCulexquinquefasciatus(Say).BioresourceTechnology, 95: 113-114.
Ramani S, Poorani J and Bhumannavar B S. 2002. Spiraling whitefly,Aleurodicusdispersesin India.BiocontrolNewsandInformation, 23(2): 55-62.
Russell L. M. 1965. A new species ofAleurodicusDpuglas and two close relatives (Homoptera: Aleyrodidae).FloridaEntomologist, 48: 47-55.
Singh R N, Maheshwari M and Saratchandea B. 2005. Biocoenology and control of whiteflies in sericulture.InsectScience, 12: 401-412.
Srinivasa M V. 2000. Host plants of the spiraling whitefly,Aleurodicusdisperses(Homoptera: Aleyrodidae).PestManagementinHorticulturalEcosystem, 6: 79-105.
Sun Y P and Johnson E R. 1960. Analysis of joint action of insecticides against house flies.JournalofEconomicEntomology, 53: 887-892.
Xu H H. 2001.TheInsecticidalPlantsandBotanicalInsecticides. Beijing: China Agriculture Press.
Yu G Y. 2011.SpiralingWhiteflyandItsNaturalInsectEnemies. Beijing: Science Press.
Yu G Y, Zhang G, Liang P, Zheng Q, Liu K and Fu Y G. 2007. The spiraling whitefly,Aleurodicusdispersus,invaded Hainan Island of China.ChineseBulletinEntomology, 44: 428-431.
Zhang Z X, Cheng M D, Hu S and Xu H H. 2008. It is unreasonable to use co-toxicity coeffieient to evaluate the synergism of pesticides.PlantProtection, 34: 34-36.
Zheng L Y, Fu Y G, Han D Y, Bi R J, Huang H and Su Z G. 2008. Selectivity ofAleurodicusdispersesRussell to ten host plants of Hainan.EntomologicalJournalofEastChina, 17: 200-204.
Zhong B Z, Lü C J, Han C W, Qin W Q and Ma Z L. 2009. Bioactivities of extracts of several plants against spiraling whitefly (AleurodicusdispersusRussell).ChineseJournalofTropicalCrops, 30: 1009-1012.
(责任编辑:杨郁霞)
植物性杀虫剂及其混剂对螺旋粉虱的毒力测定
王健明, 凌 冰, 曹 溪, 包淑琳, 张茂新*
华南农业大学昆虫生态研究室,广东 广州 510642
【背景】螺旋粉虱是新入侵海南省的严重为害经济作物及园林苗木的害虫。目前,植物源杀虫剂因具有高效和环境友好等特性而被广泛用于害虫防治中。【方法】采用喷雾法和药膜接触法分别测定了9种植物性杀虫剂对螺旋粉虱的毒力。【结果】在供试的9种植物性杀虫剂中,除虫菊素和鱼藤酮对螺旋粉虱成虫的毒力最强,24 h 的LC50分别为2.56和34.15 mg·L-1;印楝素和苦瓜叶提取物的毒力次之,LC50分别为158.36和311.02 mg·L-1。除虫菊素对螺旋粉虱若虫和卵也有一定的触杀作用,LC50分别为61.42和77.39 mg·L-1。将印楝素和鱼藤酮分别与除虫菊素以1∶1的比例混合,对螺旋粉虱成虫的毒力表现出明显的增效作用,其共毒系数(CTC)分别为193.11 和224.35。【结论与意义】除虫菊素、鱼藤酮、印楝素和苦瓜叶提取物对螺旋粉虱均具有较强的毒性;印楝素或鱼藤酮与除虫菊素(1∶1)的混合物不仅能增强触杀效果,而且能延缓害虫抗药性的产生。
除虫菊素; 印楝素; 鱼藤酮; 螺旋粉虱; 杀虫活性
10.3969/j.issn.2095-1787.2012.02.011
2012-03-13Accepted2012-04-09
FoundprojectThis work was supported by the Nonprofit Sector (Agriculture) for Special Research of the Ministry of Agriculture of China (200803023-02, 201103026-04)
*Author for correspondence, E-mail: mxzhang@scau.edu.cn
