吩噻嗪-Corrole镓(III)配合物的合成、荧光及其光断裂DNA性质
2012-12-05江焕峰汪华华惠张计亮年刘海洋
史 蕾 江焕峰 尹 伟 汪华华 王 惠张 雷 计亮年 刘海洋,*
(1广东第二师范学院化学系,广州510303; 2华南理工大学化学与化工学院,广州510641; 3中山大学光电材料与技术国家重点实验室,广州510275)
吩噻嗪-Corrole镓(III)配合物的合成、荧光及其光断裂DNA性质
史 蕾1,2,*江焕峰2尹 伟1汪华华2王 惠3张 雷2计亮年3刘海洋2,*
(1广东第二师范学院化学系,广州510303;2华南理工大学化学与化工学院,广州510641;3中山大学光电材料与技术国家重点实验室,广州510275)
合成了吩噻嗪(PTZ)-corrole二元体1-3及其镓(III)配合物4-6.采用稳态吸收与稳态发射及时间分辨的瞬态光谱技术研究了这几种化合物的光物理特性.结合荧光量子产率和荧光寿命计算得到它们的辐射和无辐射速率常数.稳态吸收光谱表明:几种二元体中,corrole镓(III)单元表现出更强的Soret带和Q带.化合物1-3的荧光量子产率分别是0.156、0.134和0.139,辐射速率常数分别为4.02×107、3.47×107和2.89×107s-1.化合物4-6的荧光量子产率分别是0.502、0.443和0.494,辐射速率常数分别为20.90×107、16.78×107和21.11× 107s-1.可见,化合物4-6的荧光量子产率和辐射速率常数均高于化合物1-3.然而,化合物4-6的荧光寿命分别是2.40、2.64和2.34 ns,低于自由corrole 1-3.琼脂糖凝胶电泳实验表明:在光照的条件下,这些吩噻嗪-corrole镓(III)二元体化合物能够把超螺旋DNA(form I)切割成缺刻型DNA(form II).
Corrole;吩噻嗪;镓(III);荧光;DNA
1 Introduction
Metallocorroles have been applied in catalysis,1,2medicinal applications,3-5and recently in photophysics.6-10Since the computational investigations suggest that gallium(III)may fit perfectly into the coordination core of corroles,11gallium(III)corroles have been received interest in recent ten years.The first reported corrole gallium(III)complex was synthesized by Gross,12,13its X-ray structures,electrochemical and photophysical properties were also determined.Facile synthetic methodologies and high fluorescence yield allowing for the preparation of more gallium(III)corroles and more extensively properties have been determined.14-16Amphiphilic gallium(III)corroles could form tightly bound noncovalent conjugates with human serum albumin.17In addition,it has been discovered recently that these gallium(III)corroles could also be explored for tumor detection and elimination.18Phenothiazine(PTZ)is an interesting chromophore recognized pharmaceutical compound, which has shown diverse biological activities such as neuroleptic,antiemeic,antihistamine and anthelmintic activities.19,20Recently,we21found that phenothiazine-corrole dyads 1-3 exhibited enhanced DNA photocleavage properties,high fluorescence quantum yield,and DNA binding activities.Although Ga(III)corroles exhibited potential application in medicinal chemistry,17,18no report was found on the interaction between Ga(III) corrole complexes and DNA so far.In this paper,we report the fluorescence property and DNA photocleavage activity of orth-,meso-,and para-phenthiazine-corrole gallium(III)complexes 4-6,which are derived from free base corrole dyads 1-3,respectively.
2 Experimental
2.1 Materials and methods
Tetraphenylporphyrin(TPP)was synthesized by Adlerʹs method.22Calf thymus deoxyribonucleic acid(CT DNA)was purchased from Sigma-Aldrich Corporation and pBR 322 plasmid DNA was purchased from TaKaRa Biotechnology Co., Ltd.(TaKaRa Dalian,China).All other reagents and solvents were reagent grade and used without further purification.Silica gel(100-200 mesh)were used for column chromatography. Reactions were monitored by thin layer chromatography and spectrophotometry.Mass spectra were obtained using a Bruker Esquire HCT PLUS mass spectrometer(Bruker Company, USA).1H-NMR spectra were recorded with a VARIAN 300 MHz NMR spectrometer in CDCl3(Varian Company,USA). Absorption spectra of all samples were measured by a Perkin Elmer Lambda 850 UV-Vis Spectrometer(PE Company, USA).Fluorescence spectra were recorded by a Perkin Elmer LS55 Luminescence Spectrometer(PE Company,USA).The fluorescence decay curves were measured by a time-resolved fluorescence spectroscopic experimental setup.A Nd:YAG laser(EKSPLA PL2143)andanOPGsystem(EKSPLA PG401SH/DFG2-10)generated the laser pulse(420 nm,10 Hz)with a full width at half maximum(FWHM)of 22 ps as a light source(EKSPLA Company,Lithuania).The fluorescence was collected with a pair of lenses with big caliber.After passing through a monochromator,it was recorded by a streak camera(Hamamatsu C1587)and a CCD(Hamamatsu C4742-95). The fluorescence lifetime can be determined with a 30 ps resolution by the deconvolution procedure.Photoirradiation was carried out using a simple system consisting of an 11 W fluorescence lamp placed 10 cm away from a sample compartment thermostatted in a water jacket at 25°C.Supercoiled pBR 322 DNA(0.1 μg)was treated with phenothiazine-corrole gallium(III)complexes in 50 mmol·L-1tris-HCl,18 mmol·L-1NaCl buffer,pH=7.2,and the solutions were incubated for 1 h in the dark,then irradiated.The samples were analyzed by electrophoresis for 2 h at 50 V and 30 mA in tris-HCl buffer containing 1%(mass fraction)agarose gel.The gel was stained with 1 μg mL-1ethidium bromide and then photographed under UV light(365 nm).All measurements were carried out at room temperature.
2.2 Synthesis
2.2.1 Synthesis of the phenothiazine-corrole dyads
Phenothiazine-corrole dyads 1-3 were prepared previously.21
2.2.2 Preparation of phenothiazine-corrole gallium(III) complex(4)
A solution of phenothiazine-corrole dyad 1(19.9 mg,21 μmol)in pyridine(10 mL)was added to a flask that contained a large excess(about 0.2 g)of flame-dried GaCl3,and the reaction mixture was heated to reflux for 1 h under N2,followed by evaporation of the solvent.The inorganic salts were separated by column chromatography on silica(Vhexane:VCH2Cl2:Vpyridine=100: 50:0.5,volume ratio),affording 17.0 mg(16.8 μmol,80.0% yield)of the pyridine gallium(III)complex of 4.1H-NMR(CDCl3,300 MHz):δ,3.18-3.19(m,2H,pyridine-H),5.48-5.49 (m,2H,pyridine-H),6.30-6.32 (m,1H,pyridine-H), 7.16-7.21(m,2H,Ph),7.28-7.33(m,2H,Ph),7.36-7.40(m, 2H,Ph),7.55-7.60(m,3H,Ph),7.64-7.66(m,1H,Ph), 7.75-7.80(m,1H,Ph),8.01-8.04(m,1H,Ph),8.62-8.64(m, 2H,Ph),8.82-8.84(m,4H,pyrrole-H),9.21-9.23(m,2H,pyrrole-H);19F-NMR(CDCl3,380 MHz):δ,-162.43--162.24 (m,4F),-153.91(t,J=44.1 Hz,2F),-138.06--137.95(m, 4F);UV-Vis(toluene),λmax,unit in nm,(relative intensity): 288.0(0.230),423.0(2.45),574.0(0.251),598.0(0.335);Atmosphericpressurechemicalionization MS (APCI-MS): 1014.1[M-pyridine+H+].

Fig.1 Structure and synthesis of phenothiazine-corrole gallium(III)complexes
2.2.3 Preparation of phenothiazine-corrole gallium(III) complex(5)
A solution of phenothiazine-corrole dyad 2(19.9 mg,21 μmol)in pyridine(10 mL)was added to a flask that contained a large excess(about 0.2 g)of flame-dried GaCl3,and the reaction mixture was heated to reflux for 1 h under N2,followed by evaporation of the solvent.The inorganic salts were separated by column chromatography on silica(Vhexane:VCH2Cl2:Vpyridine=100: 70:0.5),affording 19.1 mg(18.9 μmol,90.0%yield)of the pyridine gallium(III)complex of 5.1H-NMR(CDCl3,300 MHz): δ,3.10-3.22(m,2H,pyridine-H),5.87-5.92(m,2H,pyridine-H),6.63-6.70(m,1H,pyridine-H),7.18-7.23(m,2H, Ph),7.30-7.35(m,2H,Ph),7.38(d,J=8.4 Hz,2H,Ph),7.56 (d,J=9.1 Hz,1H,Ph),7.67-7.75(m,3H,Ph),7.96(d,J=8.4 Hz,2H,Ph),8.77-8.82(m,6H,pyrrole-H),9.20-9.21(m,2H, pyrrole-H);19F-NMR(CDCl3,380 MHz):δ,-162.68--162.38 (m,4F),-154.16(t,J=44.1 Hz,2F),-138.15--137.92(m, 4F);UV-Vis(toluene),λmax,unit in nm,(relative intensity): 293.0(0.209),424.0(2.59),574.0(0.245),602.0(0.366); APCI-MS:1014.1[M-pyridine+H+].
2.2.4 Preparation of phenothiazine-corrole gallium(III) complex(6)
A solution of phenothiazine-corrole dyad 3(19.9 mg,21 μmol)in pyridine(10 mL)was added to a flask that contained a large excess(about 0.2 g)of flame-dried GaCl3,and the reaction mixture was heated to reflux for 1 h under N2,followed by evaporation of the solvent.The inorganic salts were separated by column chromatography on silica(Vhexane:VCH2Cl2:Vpyridine=100: 70:0.5),affording 19.1 mg(18.9 μmol,90.0%yield)of the pyridine gallium(III)complex of 6.1H-NMR(CDCl3,300 MHz): δ,2.88-2.98(m,2H,pyridine-H),5.81-5.89(m,2H,pyridine-H),6.62-6.70(m,1H,pyridine-H),7.29-7.32(m,2H, Ph),7.40-7.45(m,2H,Ph),7.48-7.51(m,2H,Ph),7.54(d,J= 8.4 Hz,2H,Ph),7.82-7.85(m,2H,Ph),8.10(d,J=8.4 Hz, 2H,Ph),8.77-8.82(m,6H,pyrrole-H),9.20-9.22(m,2H,pyrrole-H);19F-NMR(CDCl3,380 MHz):δ,-162.59--162.45 (m,4F),-154.18(t,J=44.1 Hz,2F),-138.28--138.02(m, 4F);UV-Vis(toluene),λmax,unit in nm,(relative intensity): 293.0(0.192),424.0(2.58),574.0(0.238),603.0(0.365); APCI-MS:1014.1[M-pyridine+H+].
3 Results and discussion
Phenothiazin-corrole dyads 1-3 was prepared according to previous published procedure in the literature.21Phenothiazine-corrole gallium(III)complexes 4-6 could efficiently be obtained(Fig.1)in yields ranging from 80.0%to 90.0%according the method reported by Gross.12Fig.2 shows the absorption spectra of phenothiazine-corrole dyad 3,PTZ,and their gallium complexes 4-6 in toluene.The absorption spectra of 4-6 reveal a band at 290 nm corresponding to the phenothiazine entity,a Soret band and a Q band related to corrole unit.The S0→S2(Soret band)and S0→S1(Q band)transition of 4-6 are obviously enhanced compared to their free base corroles.This may be explained by the fact that the corrole macrocycle intends to be more planar or the changed acidity when the gallium is introduced,12resulting in the increase of the matrix element of the π-π*electronic transitions and stronger absorption.23This phenomenon is similar to the previously reported gallium(III) corroles.13,24While the molar absorption coefficient(ε)of the PTZ is nearly identical to the phenothiazine entity of 3,4-6, which means that the phenothiazine unit can be introduced without affecting the absorption characteristics of these gallium(III)corroles.

Fig.2 UV-Vis absorption spectra of PTZ (phenothiazine-10-carbonyl chloride),3 and 4-6 in toluene
In our reported studies,21we found that the phenothi-azine-corrole dyads exhibited higher fluorescence quantum yields compared to their corrole units.During the metallation by gallium,we noted a stronger red fluorescence.The fluorescence spectra of 3,4-6,and TPP in toluene at room temperature upon excitation at the Q band(560 nm)are displayed in Fig.3 and the most relevant photophysical values are collected in Table 1.The luminescence peaks of our synthesized gallium complexes are all shifted to higher energies(ca 45 nm)as compared to their free base corroles,which maybe attributed to the larger energy between HOMO and LUMO of gallium(III)corroles.23The major points of interest are that three gallium(III) complexes exhibit higher fluorescence quantum yields than phenothiazine-corrole dyads,which can be explained by the more planar structure of metallic corroles.16,24,25Whatʹs more, sample 4 exhibits the highest fluorescence quantum yields among the reported gallium(III)complexes we can find.Their lifetimes were also determined by the method described in experimental section.Samples were excitated at 420 nm and the fluorescences were focused into the spectrometer before being collected by a streak camera and the collected wavelength was 608 nm.The resultant decay profiles for all samples could be explained satisfactorily in terms of a single exponential fit (Fig.4)and the calculated lifetimes(τ)are summarized in Table 1.The fluorescence lifetime decreases when the gallium is introduced.The emission rate constant(kf)and nonemission rate constant(knr)constant can be determined from kf=Φf/τ and knr= (1-Φf)/τ.26For phenothiazine-corrole gallium(III)complexes 4-6,the values of the kfare 20.90×107,16.78×107,and 21.11× 107s-1.Note that the kfof 4 and 5 are about 5-fold more than their free base corroles,and the kfof 6 is about 7-fold more than that of 3,while the value of knrisalmost identical.

Fig.3 Fluorescence emission spectra of TPP,complexes 3 and 4-6 in toluene at room temperature
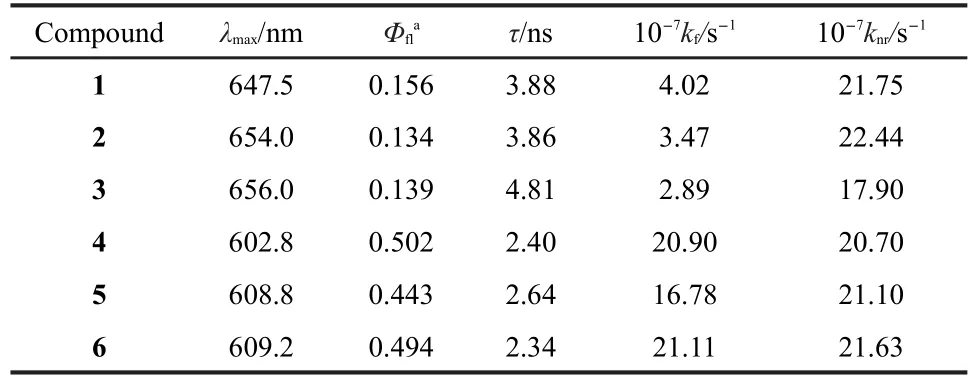
Table 1 Fluorescence emission peak(λmax),quantum yields(Φfl), life time(τ),radiative rate constant(kf),and nonradiative rate constant(knr)data of complexes 1-6 in toluene at 295 K

Fig.5 Stability of supercoiled pBR 322 DNAwith irradiationReaction mixtures(10 μL)contained 0.1 μg of plasmid DNAand 5%DMF. lanes 1-8:samples with 0,30,60,90,120,150,200,250 min irradiation, respectively.c(form II):conversion of form I to form II

Fig.6 Agarose gel electrophoresis pattern for the cleavage of supercoiled pBR 322 DNAReaction mixtures(10 μL)contained 0.1 μg of plasmid DNA,400 μmol·L-1 samples,and 5%DMF.lane 1:DNAalone(no hν);lane 2:DNA+4(no hν); lanes 3-5:complexes 4-6 with DNA,respectively(2 h hν).

Fig.4 Fluorescence decay curves of complexes 4-6 in toluene
The DNA photocleavage activities were examined using supercoiled pBR 322 DNA.A mixture of corrole in DMF and the plasmid DNA in tris-HCl buffer(pH=7.2)was illuminated for 2 h at room temperature in a system consisting of an 11 W fluorescent lamp light source placed 10 cm away.The stability of supercoiled pBR 322 DNA with irradiation and agarose gel electrophoresis patterns for the photocleavage of DNA are shown in Fig.5 and Fig.6,respectively.Lane 1 is the control DNA.Without illumination,all phenothiazine-corrole dyads or their gallium complexes exhibited no DNA cleavage activity (exampled by lane 2).Phenothiazine-corrole dyads 1-3 exhibited 85%-100%conversion of supercoiled DNA(form I)to nicked-circular DNA(form II)at the concentration of 100 μmol·L-1under illumination.12In contrast,their gallium complexes could cleave form I DNA to form II at the concentration of 400 μmol·L-1and the DNA photocleavage activity follows an order of 4<5=6.The descendent DNA photocleavage activities of 4-6 maybe explained by the reduction of singlet oxygen quantum yield(ФΔ)photosensitized by corroles.Phenothiazine-corrole dyads 1-3 show the ФΔof 0.89-0.93,while we can not detect the singlet oxygen luminescence spectra of 4-6 in the same experimental conditions,which maybe because of the amazing radiative transition of 4-6.
4 Conclusions
In summary,we synthesized three phenothiazine-corrole dyads 1-3 and their novel gallium(III)complexes 4-6.The corrole unit exhibits stronger Soret band and Q band.The steady-state emission spectra and the temporal fluorescence decay profiles reveal that the fluorescence quantum yield and radiative decay constant are enhanced when the gallium is introduced.The quantum yields are 0.502,0.443,and 0.494 for 4-6,respectively.To our knowledge,the quantum yield of sample 4 is the highest among the reported gallium(III)complexes.The radiative and nonradiative rate constants were determined using a kinetic scheme:the values of radiative rate constant are 20.90×107,16.78×107,and 21.11×107s-1for 4-6, respectively,which are obviously higher than their free base corroles,but the nonradiative rate constant is almost identical. Agarose gel electrophoresis shows that these gallium(III)corrolescould photocleave supercoiled DNA (form I)to nicked-circular DNA(form II)at the concentration of 400 μmol·L-1,which are the first observation of DNA photocleavage by corrole gallium(III)complexes.This information is of importance for potential utilization of corroles in photophysical and therapeutic applications.
(1) Fang,H.F.;Ling,Z.;Brothers,J.P.;Fu,X.F.Chem.Commun. 2011,47,11677.
(2) Nigel-Etinger,I.;Mahammed,A.;Gross,Z.Catal.Sci.Technol. 2011,1(4),578.
(3)Zhai,Q.Q.;Xu,L.;Ge,Y.S.;Tian,T.;Wu,W.D.;Yan,S.Y.; Zhou,Y.Y.;Deng,M.G.;Liu,Y.;Zhou,X.Chem.Eur.J.2011, 17(32),8890.
(4)Aviv,I.;Gross,Z.Chem.Commun.2007,1987 and references therein.
(5)Liu,H.Y.;Yam,F.;Xie,Y.T.;Li,X.Y.;Chang,C.K.J.Am. Chem.Soc.2009,131,12890.
(6) Flamigni,L.;Gryko,D.T.Chem.Soc.Rev.2009,38,1635.
(7) Botoshansky,M.;Palmer,J.H.;Durrell,A.C.;Gray,H.B.; Gross,Z.J.Am.Chem.Soc.2011,133(33),12899.
(8)Tasior,M.;Gryko,D.T.;Cembor,M.;Jaworski,J.S.;Venturac B.;Flamigni,L.New J.Chem.2007,31,247.
(9) Tasior,M.;Gryko,D.T.;Shen,J.;Kadish,K.M.;Becherer,T.; Venturac,B.;Flamigni,L.J.Phys.Chem.C 2008,112,19699.
(10) He,C.L.;Ren,F.L.;Zhang,X.B.;Han,Z.X.Talanta 2006, 70,364.
(11) Ghosh,A.;Jynge,K.Chem.Eur.J.1997,3,823.
(12) Simkhovich,L.;Goldberg,I.;Gross,Z.J.Inorg.Biochem. 2000,80(3-4),235.
(13)Bendix,J.;Dmochowski,I.J.;Gray,H.B.;Mahammed,A.; Simkhovich,L.;Gross,Z.Angew.Chem.Int.Edit.2000,39 (22),4048.
(14)Liu,X.;Mahammed,A.;Tripathy,U.;Gross,Z.;Steer,R.P. Chem.Phys.Lett.2008,459(1-6),113.
(15) Saltsman,I.;Mahammed,A.;Goldberg,I.;Tkachenko,E.; Botoshansky,M.;Gross,Z.J.Am.Chem.Soc.2002,124(25), 7411.
(16) Sorasaenee,K.;Taqavi,P.;Henling,L.M.;Gray,H.B.; Tkachenko,E.;Mahammed,A.;Gross,Z.J.Porphyr. Phthalocyanines 2007,11(3-4),189.
(17) Mahammed,A.;Gray,H.B.;Weaver,J.J.;Sorasaenee,K.; Gross,Z.Bioconjugate Chem.2004,15(4),738.
(18)Agadjanian,H.;Ma,J.;Rentsendorj,A.;Valluripalli,V.;Hwang, J.Y.;Mahammed,A.;Farkas,D.L.;Gray,H.B.;Gross,Z.; Medina-Kauwe,L.K.Proc.Nat.Acad.Sci.U.S.A.2009,106 (15),6105.
(19) Motohashi,N.AntitumorActivities of Phenothiaiznes.In Phenothiazines and 1,4-Benzothiazines,Chemical and Biological Aspects,Bioactive Molecules;Gupta,R.R.Ed.; Elsevier:Amsterdam,1988;Vol.4,pp 705-770.
(20) Viola,G.;DallʹAcqua,F.Current Drug Targets 2006,7,1135.
(21)Shi,L.;Liu,H.Y.;Peng,K.M.;Wang,X.L.;You,L.L.;Zhang, L.;Wang,H.;Ji,L.N.;Jiang,H.F.Tetrahedron Lett.2010,51, 3439.
(22) Adler,A.D.;Longo,F.R.;Finarelli,J.D.;Goldmacher,J.; Assour,J.;Korsakoff,L.J.Org.Chem.1967,32(2),476.
(23)Ghosh,A.;Wondimagegn,T.;Parusel,A.B.J.J.Am.Chem. Soc.2000,122,5100.
(24)Peng,K.M.;Shao,W.L.;Wang,H.H.;Ying,X.;Wang,H.;Ji, L.N.;Liu,H.Y.Acta Phys.-Chim.Sin.2011,27,199.[彭开美,邵文莉,汪华华,应 晓,王 惠,计亮年,刘海洋.物理化学学报,2011,27,199.]
(25) Gross,Z.;Galili,N.;Simkhovich,L.;Saltsman,I.;Botoshansky, M.;Bläser,D.;Boese,R.;Goldberg,I.Org.Lett.1999,1,599.
(26)Kowalska,D.;Liu,X.;Tripathy,U.;Mahammed,A.;Gross,Z.; Hirayama,S.;Steer,R.P.Inorg.Chem.2009,48(6),2670.
October 13,2011;Revised:November 21,2011;Published on Web:November 29,2011.
Synthesis,Fluorescence and DNA Photocleavage Activity of Phenothiazine-Corrole Gallium(III)Complexes
SHI Lei1,2,*JIANG Huan-Feng2YIN Wei1WANG Hua-Hua2WANG Hui3ZHANG Lei2JI Liang-Nian3LIU Hai-Yang2,*
(1Department of Chemistry,Guangdong University of Education,Guangzhou 510303,P.R.China;2School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510641,P.R.China;3State Key Laboratory of Optoelectronics Materials and Technologies,Sun Yat-Sen University,Guangzhou 510275,P.R.China)
Phenothiazine(PTZ)-corrole dyads 1-3 and their gallium(III)complexes 4-6 have been synthesized and characterized.The steady-state absorption and emission spectra and the time-resolved fluorescence decay profiles have been measured in toluene.The radiative and nonradiative rate constants have been obtained from the fluorescence quantum yields and monoexponential fluorescence lifetimes. The absorption spectra revealed that the gallium(III)corrole dyads exhibit stronger Soret bands and Q bands than free base corrole dyads.The fluorescence quantum yields of 1-3 are 0.156,0.134,and 0.139, and the radiative rate constants are 4.02×107,3.47×107,and 2.89×107s-1,respectively.The fluorescence quantum yields of 4-6 are 0.502,0.443,and 0.494,and the radiative rate constants are 20.9×107,16.78× 107,and 21.11×107s-1,which are obviously higher than those of the corresponding free base corroles.The lifetimes of 4-6 are 2.40,2.64,and 2.34 ns,respectively,which are somewhat shorter than those of the corresponding free base corroles.Agarose gel electrophoresis shows that these gallium(III)corrole dyads could cleave supercoiled DNA(form I)to give nicked-circular DNA(form II)under irradiation.
Corrole;Phenothiazine;Gallium(III);Fluorescence;DNA
10.3866/PKU.WHXB201111291www.whxb.pku.edu.cn
*Corresponding authors.SHI Lei,Email:shil@gdei.edu.cn;Tel:+86-20-34113254.LIU Hai-Yang,Email:chhyliu@scut.edu.cn; Tel:+86-20-22236805.
The project was supported by the National Natural Science Foundation of China(20971046,21171057,61178037,11004256),Natural Science Foundation of Guangdong Province,China(10351064101000000),Open Fund of the State Key Laboratory of Optoelectronic Materials and Technologies(Sun Yat-Sen University),China,andAppropriative Researching Fund for Professors and Doctors,Guangdong University of Education,China(10ARF14).
国家自然科学基金(20971046,21171057,61178037,11004256),广东省自然科学基金(10351064101000000),光电材料与技术国家重点实验室(中山大学)开放基金及广东第二师范学院教授博士科研专项经费研究项目(10ARF14)资助
O644
