模式耦合对反常氢键系统中振动频率蓝移的影响
2012-12-05孔祥蕾
孔祥蕾
(南开大学,元素有机化学国家重点实验室,天津300071)
模式耦合对反常氢键系统中振动频率蓝移的影响
孔祥蕾*
(南开大学,元素有机化学国家重点实验室,天津300071)
反常氢键所引起的NH/CH键的键长缩短被普遍地认为必然会引起其振动频率的蓝移.本文的计算结果显示非谐性效应可能会使相应的蓝移大幅度减小.其中模式耦合起着非常重要的作用.在一些例子中,这种强的相互作用可能会导致一些基于谐振子模型计算所预言的气相中的蓝移频率实际上发生红移.尽管这些计算结果有待于通过相应体系中的气相红外(IR)光谱来进行确认,一些已报道的基质隔离红外光谱的实验结果已显示出对这些计算结果的支持.
反常氢键;模式耦合;蓝移;红外光谱;非谐性效应
1 Introduction
Hydrogen bonding interactions are the most important noncovalent interactions in chemical and biological systems.Recently,growing interest has been attracted by the improper blue-shifting H-bond,which is thought to be characterized by a decrease of X-H(X=C,N)bond length and an increase of its stretching frequency,thus can be distinguished from standard H-bond by infrared(IR)experiment or theoretical calculation.1High level calculations have been performed for lots of systems,and it has been predicted that both C-H and N-H can form blue-shifting H-bonds in many complexes.1-3Remarkably,the relationship between the dissociation energy differences and the stretching frequency shift in some weak coupling complexes with both red-and blue-shifting H-bonds has been suggested by Donoso-Tauda et al.4recently.However,it must be realized that most of those frequency calculations were evaluated by the harmonic approximation and the vibrational anharmonicity was neglected.Though the non-diagonal anharmonic mode-mode coupling was known to be very important for the accurate simulation of IR spectra,very few theoretical works that took into account the anharmonic effects included the coupling among normal modes.5In the last twenty years,many different methodologies have been developed to evaluate vibrational wavefunctions including both the diagonal and non-diagonal anharmonicity.Among them,the method of correlationcorrected Vibrational Self-Consistent Field(cc-VSCF)has been one of the most successful methods.6
In this paper,we try to use the cc-VSCF method to study the effect of vibrational anharmonicity on the blue-shifted frequency in the improper H-bonded systems.However,there are still some difficulties in selecting a suitable system.For one thing, though lots of complexes have been predicted to be characterized by one or more improper blue-shifting H-bonds,very few experimental proofs have been gotten in the gas phase;1for another,calculation based on the method of cc-VSCF is still very heavy and can be hardly directly applied to the systems with large sizes.Thus,some complexes with limited sizes and typical improper H-bonds predicted theoretically were selected and studied here,and some results were compared with their corresponding results gotten in matrix isolation IR experiments previously.
2 Computational methods
We used the method of cc-VSCF to re-evaluate the frequencies of NH/CH bonds in some complexes with typical blueshifting H-bonds predicted previously.2(a-e),7,8Complexes of (HNO)2,2(a-c)HNO…CO,2f,(H2CO)2,7and H2CO…HNO2(c-e)were selected as the models to evaluate the effect of anharmonicity, since these complexes were considered to be characterized by their improper H-bonds.2(a-e),7,8The structures used here were based on the most stable isomers found previously2(a-e),7,8and they are shown in Fig.1.The basis set superposition errors (BSSEs)in these systems were investigated in detail previously,and it was found that the effects of BSSE on the predicted blue shifts are typically 2-4 cm-1,or less than 8%of the values of the blue shifts.Thus,the BSSE effects in these systems were neglected here.All structural optimization,harmonic frequency, and VSCF calculation were performed by the GAMESS program.9Standard VSCF calculation was performed based on the default setting of NGRID=16,which means that the calculation added two-dimensional(2D)grids(16×16)along each pair of normal modes besides the 1D grids along each harmonic mode.The bond lengths and harmonic frequencies of N-H and C-H were obtained at the levels of B3LYP/6-311++G(2d, 2p)and MP2/6-311++G(2d,2p),respectively.Standard cc-VSCF method was applied at the level of B3LYP/6-311++ G(2d,2p);while the quartic force field(QFF)was used in the cc-VSCF calculation at the level of MP2/6-311++G(2d,2p)in order to keep the computational cost affordable.6(e)
3 Results and discussion
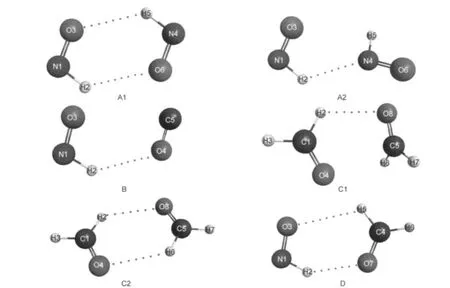
1 The most stable isomers of(HNO)2(A1,A2),HNO···CO(B),(H2CO)2(C1,C2),and H2CO···HNO(D)structures were optimized at the B3LYP/6-311++G(2d,2p)level.Dash lines indicate the improper blue-shifting hydrogen bonds.
Table 1 gives all the computational results and some experimental data.10-15The first example selected is the dimer of HNO,which is characterized by its large blue shifts predicted previously due to the improper hydrogen bonds formed by the process of dimerization.2(a-c)For single molecule of HNO,the harmonic and anharmonic results of NH vibrational frequency give errors of 8.3%(14.9%)and 1.7%(4.6%)based on B3LYP (MP2)methods from experimental results in the gas phase,respectively,10indicating the large intrinsic anharmonic effect on the mode.The most stable isomer of its dimer,A1,has C2hsymmetry.Due to the improper hydrogen bonds,the NH bonds are shortened by ca 0.0005 nm and the corresponding frequencies are blue-shifted by 60-85 cm-1compared with those of the monomer.The result is consistent with the previous study.2(a-c)However,with cc-VSCF method by density functional theory (DFT)calculation,red shifts of 7 and 72 cm-1,instead of blue shifts,have been predicted for two NH stretching modes,respectively.For MP2 calculation,the increased energy difference between the two NH vibrational levels causes one of them red-shifted by 24 cm-1and the other blue-shifted by 57 cm-1.Due to the symmetry restriction,one of them is prohibited and thus the VSCF results predicted that only the red-shifted frequency can be observed in its gas phase IR spectrum.Forcomplex of A2(0.3 kJ·mol-1in energy less stable than A1), both NH bonds shorten by ca 0.0002-0.0003 nm.The blue shifts of 15 and 37 cm-1based on harmonic DFT calculation are adjusted to be 38 and 4 cm-1due to anharmonic effect, while the MP2 results are changed to be red shifts of 34 and 38 cm-1.
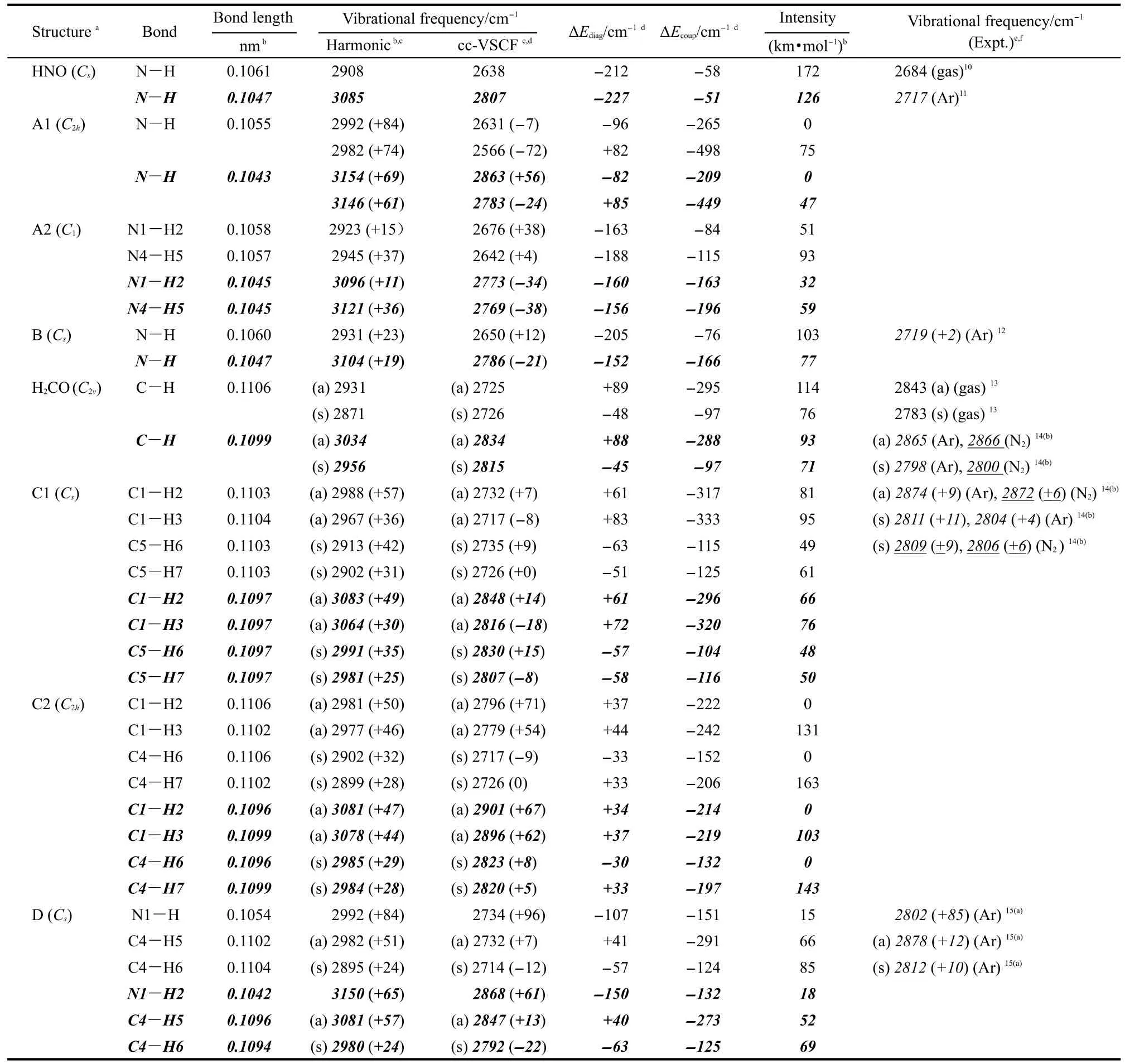
Table 1 Bond lengths,vibrational frequencies,intensities of N-H/C-H bonds,and the contribution of anharmonicties computed for HNO,H2CO,and their complexes
The anharmonic effects can be better understood with considering the intrinsic anharmonicity of the individual mode and the anharmonic coupling between different modes separately. Here the intrinsic contribution,ΔEdiag,is calculated as the difference between the harmonic value and the diagonal potential, and the contribution of anharmonic coupling,ΔEcoup,is calculated as the difference between cc-VSCF value and the diagonal potential.6(d)Those results are shown in Table 1 too.For the single molecule of HNO,the intrinsic contribution is three times greater than the contribution from mode coupling.The high value of ΔEdiagin the monomer indicates the existence of the high delocalization degree of the normal mode.By the process of dimerization,the values of ΔEdiagand ΔEcoupchange very much,indicating that a transfer in energy distribution from delocalization to mode coupling.For A1,the strong mode coupling in the two symmetrical N-H bonds,cause very distinct anharmonic effects and thus induce at least one of the NH frequency red-shifted.While for A2,the asymmetrical conformation weakens the coupling,thus the values of ΔEcoupare much less than those of A1.It should also be noticed that in a recent research by Wang et al.,16the delocalization degree was found to increase significantly from isolated bases to normal H-bonded(red-shifted)dimmers in Watson-Crick base pairs,which is quite different from the results reported here.
Unfortunately,the experimental frequency for the dimer is still unavailable by now,which makes the judgment of both results predicted by harmonic and cc-VSCF methods unperformable.Thus,more examples are selected,studied,and compared with experimental results here.For the example of HNO…CO complex,the weak improper H-bond causes the N-H bond shorten by ca 0.0001 nm,and its frequency blue-shifts by ca 20 cm-1according to the harmonic approximation.The VSCF result with DFT method shows that the increasing coupling interaction induces the blue shift deduced to be 12 cm-1,while that with MP2 method predicts a red-shift by 21 cm-1.Though the direct experimental result in the gas phase for the complex IR is still unavailable,experimental result12of the complex by the matrix isolation method in the solid of argon has been performed previously.The frequencies of NH are reported to be 2717 and 2719 cm-1for the monomer and the complex,respectively,indicating a small blue shift of 2 cm-1due to the H-bonding.Though the interaction with the highly polarizable matrix needs to be studied more carefully(the argon matrix can cause a blue shift of 33 cm-1for the HNO monomer),it is reasonable to suggest that the blue shift value predicted by cc-VSCF method can fit the gas phase experimental result much better than that by harmonic methods.
Formaldehyde has been also predicted to blue shift its CH frequencies by dimerization or complexation with other molecules.8,2(c-e)For single molecule of H2CO,QFF-cc-VSCF based on the level of MP2/6-311++G(2d,2p)can get more accurate results in the frequency calculation,and give only errors of 0.3%-1.0%relative to the gas phase experimental values.12The mode coupling plays important roles in both asymmetric and symmetric vibration modes even for the single molecule of H2CO.By forming C1structure,both CH bonds shorten by ca 0.0001-0.0002 nm.The anharmonicities from mode couplings increase,causing the values of predicted blue shift based on harmonic approximation to decrease from an average of 35(42) cm-1based on MP2(DFT)method to 1(3)cm-1.The IR spectra of formaldehyde monomer and dimer in solid matrix of argon and nitrogen have been also reported by several groups.14,15All results show blue shifts of 4-11 cm-1due to dimerization for the C-H vibrational frequencies observed,which are in good agreement with the VSCF predicted values(Table 1).For the conformation of C2(2.7 kJ·mol-1in energy less stable than C1),the anharmonicities cause the asymmetric and symmetric vibrational frequencies shift in opposite ways.
Interestingly,the complex of H2CO…HNO gives both types of blue-shifting bonds.Though all NH and CH bonds are shortened due to the H-bonding,the anharmonic effects are very different.The blue shift of NH frequency is not affected very much by the anharmonicities;while the blue shifts of CH frequencies are reduced by ca 40 cm-1or thus changed to be red shifts.And the experimental matrix isolation results match those results greatly,which show a big blue shift of 85 cm-1for the NH mode and blue shifts of 12 and 10 cm-1for the CH modes.It should be noticed too that though the calculated frequencies for all examples are different for the two methods used here,the anharmonic effects and the contribution from mode coupling for all examples are very close.
It is important to know which mode in the complex can affect most of the vibration of the blue-shifted bond through the mode-mode coupling.The average absolute value of the coupling potential(I)between modes i and j is:
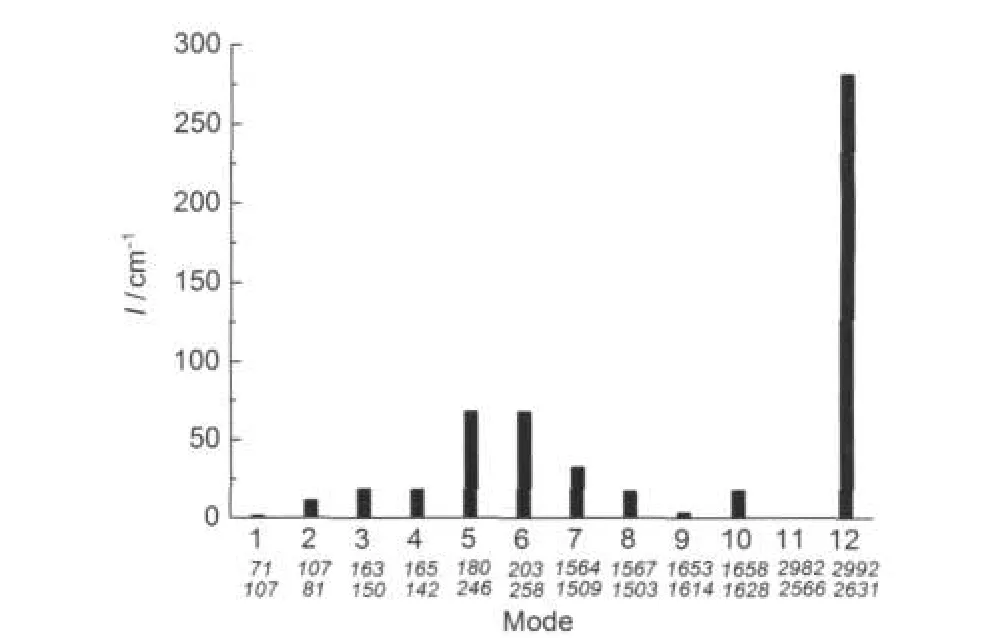
Fig.2 Integrated coupling potential between mode 11 and other modes in complexA1The values shown under the mode numbers are their corresponding frequencies (in cm-1)by harmonic and VSCF calculations based on the method of B3LYP/ 6-311++G(2d,2p)in turn.
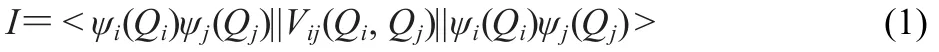
where Qiand Qjare the normal mode coordinates for modes i and j.And this can be calculated by:
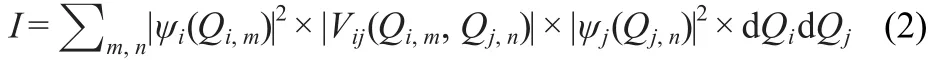
where m,n are the grid points;Qiand dQjare the grid point separations used in the VSCF calculation.6
As an example,the vibration mode 11 in the complex of A1 (N-H vibration with a VSCF frequency of 2566 cm-1in Table 1)was analyzed used the formula(2)and the magnitude of the coupling integral was shown in Fig.2.As we can see from Fig.2,the strongest coupling comes from the mode 12,which is the N-H vibration from the other side of the complex.The coupling among the vibrational models also provides important insights into the nature of vibrational energy flow in its behaviors,and thus those results may be used to help to explain the reason why the dimerization of HNO can induce dehydration to produce nitrous oxide rapidly and irreversibly.17
4 Conclusions
The anharmonic effects on NH and CH bonds are considered for several typical complexes with improper blue-shifting hydrogen bonds.The anharmonicity,especially from the contribution of mode-mode coupling,has a great effect on the frequency.Depending on the structure of the complex,the anharmonic effects may cause the predicted blue shift based on harmonic approximation decreasing very much,or even changed to be a red shift.The example of H2CO···HNO complex also indicates that some blue-shifted frequencies with weak coupling can be less affected and the predicted shift is consistent with harmonic results.Experimental results by matrix isolation IR spectroscopy have proved the great deduction of the blue shift predicted by the harmonic method,typically from 40 to 10 cm-1(though no red shift has been observed in those experiments),supporting that the great anharmonic effects from mode-mode coupling cannot be neglected in those systems.
However,it is noticed that the effects of the matrix in the experiments can cause some differences of frequency shifts compared to the gas phase results.Thus,direct experimental results for the IR spectra of those complexes in the gas phase are needed very much in order to better understand the accuracy of all calculated results and the effects of mode-mode coupling in those improper H-bonded systems.And anharmonic calculations on higher level and different complexes with improper hydrogen bonds are needed.Despite all this,those results strongly suggest that prediction of vibrational frequency of systems including improper H-bonding,strong mode coupling and/or higher symmetry need to be re-evaluated more carefully with considering the anharmonic effects.It may help to answer the question why direct experimental IR spectral proofs for blueshifting H-bonds in the gas phase are much less than those predicted by calculations,and also cause the experiential ways to identify the“improper blue-shifting hydrogen bond”(if we still use the same nomenclature even it is not blue-shifted due to the anharmonic effect)much more difficult.
Acknowledgment:The author thanks Dr.BRAUER Brina from the Hebrew University of Jerusalem very much for the helpful discussion.
(1) (a)Hobza,P.;Spirko,V.;Selzle,H.L.;Schlag,E.W.J.Phys. Chem.A 1998,102,2051.
(b)Gu,Y.;Kar,T.;Scheiner,S.J.Am.Chem.Soc.1999,121, 9411.
(c)Hobza,P.;Havlas,Z.Chem.Rev.2000,100,4253.
(d)Zierkiewicz,W.;Jurecka,P.;Hobza,P.ChemPhysChem 2005,6,609.
(e)Li,X.;Liu,L.;Schlegel,H.B.J.Am.Chem.Soc.2002,124, 9639.
(f)Joseph,J.;Jemmis,E.D.J.Am.Chem.Soc.2007,129,4620.
(2) (a)Liu,L.;Liu,W.;Li,H.;Liu,J.;Yang,Y.J.Phys.Chem.A 2006,110,11760.
(b)Solimannejad,M.;Massahi,S.;Alkorta,I.Chem.Phys. 2009,362,1.
(c)Yang,Y.;Liu,Y.Int.J.Quantum Chem.2010,110,1264.
(d)Trung,N.T.;Hue,T.T.;Nguyen,M.T.J.Phys.Chem.A 2009,113,3245.
(e)Liu,Y.;Liu,W.Q.;Li,H.Y.;Yang,Y.;Cheng,S.Chin.J. Chem.2007,25,44.
(f)Solimannejad,M.;Scheiner,S.J.Phys.Chem.A 2008,112, 4120.
(g)Shirhatti,P.R.;Wategaonkar,S.Phys.Chem.Chem.Phys. 2010,12,6650.
(3) (a)Ni,J.;Li,A.Y.;Yan,X.H.Acta Phys.-Chim.Sin.2008,24, 2000.[倪 杰,黎安勇,闫秀花.物理化学学报,2008,24, 2000.]
(b)Li,A.Y.Sci.China Ser.B-Chem.2008,38,557.[黎安勇.中国科学B辑:化学,2008,38,557.]
(c)Wang,S.W.;Li,A.Y.;Tan,H.W.Chem.J.Chin.Univ. 2007,28,1962.[王素纹,黎安勇,谭宏伟.高等学校化学学报,2007,28,1962.]
(4) Donoso-Tauda,O.;Jaque,P.;Santos,J.C.Phys.Chem.Chem. Phys.2011,13,1552.
(5) Torrent-Sucarrat,M.;Anglada,J.M.;Luis,J.M.Phys.Chem. Chem.Phys.2009,11,6377.
(6) (a)Jung,J.O.;Gerber,R.B.J.Chem.Phys.1996,105,10332.
(b)Chaban,G.M.;Jung,J.O.;Gerber,R.B.J.Phys.Chem.A 2000,104,2772.
(c)Asmis,K.R.;Pivonka,N.L.;Santambrogio,G.;Brummer, M.;Kaposta,C.;Neumark,D.M.;Woste,L.Science 2003,299, 1375.
(d)Adesokan,A.A.;Gerber,R.B.J.Phys.Chem.A 2009,113, 1905.
(e)Yagi,K.;Hirao,K.;Taketsugu,T.;Schmidt,M.W.;Gordon, M.S.J.Chem.Phys.2004,121,1383.
(7) Peters,N.J.S.J.Phys.Chem.A 1998,102,7001.
(8) (a)Forda,T.A.;Glasser,L.J.Mol.Struct.-Theochem 1997, 398-399,381.
(b)Gong,X.L.;Zhou,Z.Y.;Zhang,H.;Liu,S.Z.J.Mol. Struct.-Theochem 2005,718,23.
(9) Schmidt,M.W.;Baldridge,K.K.;Boatz,J.J.;Elbert,S.T.; Gordon,M.S.;Jensen,J.J.;Koseki,S.;Matsunage,N.; Nguyen,K.A.;Su,S.;Windus,T.L.;Dupuis,M.;Mongomery, J.A.J.Comput.Chem.1993,19,1347.
(10) Jacox,M.E.J.Phys.Chem.Ref.Data 1984,13,945.
(11) Jacox,M.E.;Milligan,D.E.J.Mol.Spectrosc.1973,48,536.
(12) Müller,R.P.;Huber,J.R.Reviews of Chemical Intermediates 1984,5,423
(13)Bouwens,R.J.;Hammerschmidt,J.A.;Graeskowiak,M.M.; Stefink,T.A.;Yorba,P.M.;Polik,W.F.J.Chem.Phys.1996, 104,460.
(14) (a)Khoshkhoo,H.;Nixon,E.R.Spectrochim.Acta A 1973,29, 603.
(b)Nelander,B.J.Chem.Phys.1980,73,1034. (c)van der Zwet,G.P.;Allamandola,L.J.;Baas,F.;Greenberg, J.M.J.Mol.Struct.1989,195,213.
(15) (a)Jacox,M.E.J.Phys.Chem.1984,88,3373.
(b)Müller,R.P.;Russegger,P.;Huber,J.R.Chem.Phys.1982, 70,281.
(16)Wang,G.X.;Zhao,J.;Wang,J.P.Science China Chemistry 2011,41,1387.[王桂秀,赵 娟,王建平.中国科学:化学, 2011,41,1387.]
(17) (a)Kohout,F.C.;Lampe,F.W.J.Am.Chem.Soc.1965,87, 5795.
(b)Schafirovich,V.;Lymar,S.V.Proc.Natl.Acad.Sci.U.S.A. 2002,99,7340.
August 31,2011;Revised:November 24,2011;Published on Web:December 1,2011.
Effects of Mode-Mode Coupling on Vibrational Frequency Blue Shift in Improper H-Bonded Systems
KONG Xiang-Lei*
(State Key Laboratory of Elemento-Organic Chemistry,Nankai University,Tianjin 300071,P.R.China)
Bond shortening of NH/CH due to improper hydrogen bonding is always thought to be accompanied by a blue shift in its corresponding frequency.However,results here show that owing to anharmonic effects,especially the contribution of mode-mode coupling,the blue shift could be greatly decreased.The strong interaction may even,in some cases,cause the previously predicted blue-shifting frequencies by harmonic methods to be red-shifted instead in the gas phase.Though these results need to be clearly verified by further infrared(IR)spectrum experiments in the gas phase for the selected systems, comparisons with previous IR results obtained from matrix isolation experiments strongly support the results of the calculations.
Improper hydrogen bond;Mode-mode coupling;Blue shift;Infrared spectrometry; Anharmonic effect
10.3866/PKU.WHXB201112013 www.whxb.pku.edu.cn
*Corresponding author.Email:kongxianglei@nankai.edu.cn;Tel:+86-22-23509564;Fax:+86-22-23502654.
The project was supported by the National Natural Science Foundation of China(21052001).
国家自然科学基金(21052001)资助项目
O641
