慢性乙型肝炎患者血清促肝细胞生长素的临床意义研究
2012-11-07晁春梅韦嘉杨微波
晁春梅,韦嘉,杨微波
慢性乙型肝炎患者血清促肝细胞生长素的临床意义研究
晁春梅1,韦嘉2,杨微波1
(1.昆明医科大学第一附属医院感染病科;2.昆明医科大学第四附属医院云南昆明650032)
目的测定慢性乙型肝炎(CHB)患者不同临床类型组血清促肝细胞生长素(HGF)水平,分析HGF水平与临床类型、肝功能水平、乙肝病毒血清标志物(HBVM)和HBV-DNA的关系,探讨HGF在CHB的意义。方法用酶联免疫吸附试验(ELISA)法检测78例CHB患者不同临床类型组血清HGF水平,与肝功能完全正常的20例健康体检者作对照,同时检测肝功能、乙肝两对半和HBV-DNA,部分病人进行了HbsAg定量测定和PT测定。结果78例CHB患者血清HGF水平为(929.10±1932.15)pg/ml;健康对照组HGF水平分别为(215.1±65.97)pg/ml,两组HGF水平差异有统计学意义。78例慢性乙型肝炎患者中,抗病毒治疗应答组、HbeAg+CHB组、HbeAg-CHB组与肝衰竭组HGF水平分别为(658.20±178.20) pg/ml、(772.60±335.40)pg/ml、(958.80±1005.49)pg/ml与(5912.0±4 497.0)pg/ml,各组HGF水平均高于对照组。4个临床类型组两两比较,肝衰竭组HGF水平最高,与其余各组相比,差异具有统计学意义;HBeAg-CHB组HGF水平>抗病毒组,差异有统计学意义(P<0.01);HbeAg-CHB组HGF水平>HbeAg+CHB组及HbeAg+CHB组>抗病毒组,但差异无统计学意义(P>0.005)。高、低病毒载量组HGF水平分别为(2 141.0±3 115.3)pg/ml与(812.50±639.80)pg/ml,差异无统计学意义(P>0.05)。HbsAg载量高与低组HGF水平分别为(1 258.0±2813.3)与(1794.09±5 033.0)pg/ml,差异无统计学意义。炎症活动CHB组63份病例HGF与ALT、AST、Tbil相互关系经Spearman等级相关检验,相关系数有统计学意义(P<0.05),呈正相关,其中HGF与Tbil呈高度正相关。HGF与HBV-DNA相互关系经Spearman等级相关检验,相关系数无统计学意义(P>0.05)。结论1.HGF在不同临床类型CHB患者血清中都明显升高,说明HGF参与了HBV感染后的免疫反应、肝脏损伤和修复过程;2.肝衰竭组血清HGF明显高于其余各临床类型组,临床使用肝细胞生长素治疗肝衰竭的时机需进一步评价;3.HGF与肝损伤程度有关,与HBV-DNA及HbsAg载量无明显相关性。
乙型肝炎;血清促肝细胞生长素
乙型肝炎病毒(HBV)通过各种途径进入机体后,可出现不同的感染结果,形成复杂的感染疾病谱。HBV感染的结果,很大程度上取决于感染者的年龄,此外,宿主的免疫因素也在HBV感染的过程中起到重要的作用。肝脏病变至肝细胞死亡时可有相应的肝细胞再生,刺激肝再生的体液因子可来自肝脏和肝外组织,肝内的有肝细胞生长因子(HGF)和胰岛素样生长因子[1]。动物实验和人体试验均发现HGF对肝损伤有保护作用[2-4],同时也是肝损伤的判断指标之一。为进一步了解HGF在不同临床类型慢性乙型肝炎患者中的临床意义,我们检测了不同临床类型慢性乙型肝炎患者血清中的HGF含量,探讨HGF在HBV慢性感染、肝衰竭中的意义,以解释临床现象,并为临床判断病情、预后估计和治疗提供一定的理论依据。
材料与方法
一、研究对象
1.CHB组:根据研究目的,选择2009年9月~2010年1月在昆明医学院第一附属医院感染疾病科门诊就诊或住院的病人78例,按标准纳入研究,其中男63例,女15例。年龄9岁~73岁,平均年龄36.0±11.8岁。按照分组标准分为,A组15例,抗病毒治疗完全应答组(简称抗病毒组,HBeAg阳性慢性乙型肝炎患者,治疗后ALT恢复正常,HBV DNA用PCR法检测不出,HBeAg血清学转换;HBeAg阴性慢性乙型肝炎患者,治疗后ALT恢复正常,HBV DNA用PCR法检测不出);B组22例,HBeAg阳性慢性乙型肝炎组(简称HbeAg+CHB组),血清HBsAg、HBV DNA和HBeAg阳性,抗-HBe阴性,血清ALT升高>60U/L或肝组织学检查有肝炎病变;C组22例: HBeAg阴性慢性乙型肝炎组(简称HBeAg﹣CHB组),HBsAg和HBV DNA阳性,HBeAg持续阴性,抗-HBe阳性或阴性,血清ALT异常>60U/L,或肝组织学检查有肝炎病变;D组19例,肝衰竭组。诊断符合中华医学会肝病学分会、中华医学会感染病学分会2005年12月~2006年9月联合制定的慢性乙型肝炎指南、肝衰竭诊疗指南。B组和C组年龄、肝功能损伤水平具可比性。所有病例均排外合并其他肝炎病毒感染、酒精性肝病、药物性肝病、脂肪性肝病、自身免疫性肝病及其他急、慢性疾病。
2.健康对照组:我科门诊就诊体检拟注射乙肝疫苗者20例,男14例,女6例,年龄4岁至39岁,平均年龄25.3±9.97岁(肝功能完全正常,排除甲、乙、丙、丁、戊型肝炎病毒感染,无急、慢性疾病)。
二、研究方法
1.血清HBVM检测:血清HBVM(HBsAg、HBsAb、HBeAg、HBeAb、HBcAb)定性采用酶联免疫吸附试验(ELISA)的方法检测,试剂为上海实业科华公司生产;定量采用时间分辨免疫荧光分析法测定。
2.HBV DNA检测:采用实时荧光定量PCR法检测,试剂为深圳匹基生物工程公司生产。
3.肝功能检测:采用日本生产的OLYMPUSAU-2700型全自动生化分析仪检测。
4.HGF的检测:采用ELISA法检测:HGF试剂盒购自美国R&DSystems。
5.统计学分析:所有数据采用SPSS11.5统计软件包进行处理。正态分布资料采用均数±标准差(x¯±s)表示。偏态分布资料采用中位数±四分位数间距(M±Q)表示。两个样本之间的相关性分析,采用Spearman等级相关分析。采用双侧检验,检验水准а=0.05。
结果
一、CHB组和健康对照组血清HGF水平的比较见表1,CHB组各临床类型组和健康对照组血清HGF水平的比较见表2。
二、CHB各临床类型组相互之间HGF水平的比较(表3)。
三、高及低病毒载量组血清HGF水平比较(表4)。
四、HbeAg+CHB、HBeAg﹣CHB组高及低病毒载量间HGF水平比较(表5)。
五、炎症活动组HbsAg载量高、低间HGF水平比较(表6)。
六、炎症活动CHB组HGF与肝功能各指标、HBV-DNA的相关性分析(表7)。
讨论
1984年,Nakamura等从肝部分切除术的大鼠血清中分离到一种能刺激原代培养的肝细胞生长和DNA合成的肝源性因子,并首次将它命名为肝细胞生长因子(HGF)[5-6];此外,在大鼠的血小板、肝部分切除病人的血清、肝衰竭病人的血浆中也分离到类似物质。在正常肝内,星状细胞是最主要的HGF的来源;急性肝损伤后,窦状上皮细胞、枯否氏细胞也能产生HGF[7];HGF以旁分泌、并可能以内分泌的方式刺激各类上皮细胞的生长[1],是体外试验中肝细胞生长和DNA合成最强有力的刺激因子[8~9]。HGF的促有丝分裂效应也在体内得到证实[10~11],在普通小鼠和肝脏部分切除的小鼠,注射HGF都能刺激肝细胞有丝分裂指数的数倍增加,促进蛋白合成、增加肝脏mRNA的含量和血清白蛋白的水平[1]。
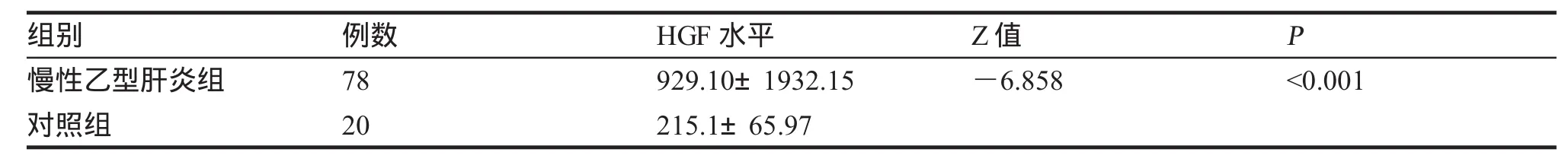
表1 CHB组和健康对照组血清HGF水平的比较(M±Q,pg/ml)
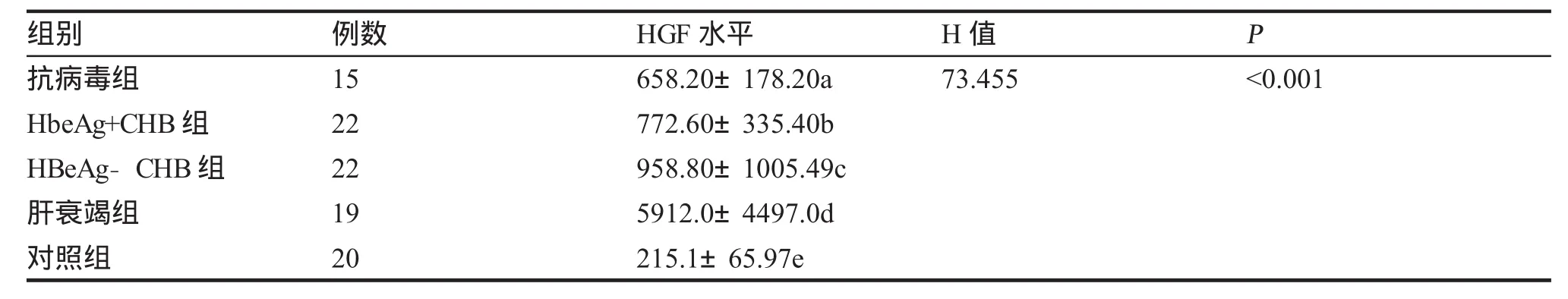
表2 慢性乙型肝炎各临床类型组和健康对照组血清HGF水平的比较(M±Q,pg/ml)

表3 CHB各临床类型组之间HGF水平的比较(M±Q,pg/ml)

表4 高、低病毒载量组HGF水平比较(M±Q,pg/ml)

表5 HbeAg+CHB、HBeAg﹣CHB组高、低病毒载量间HGF水平比较(M±Q,pg/ml)
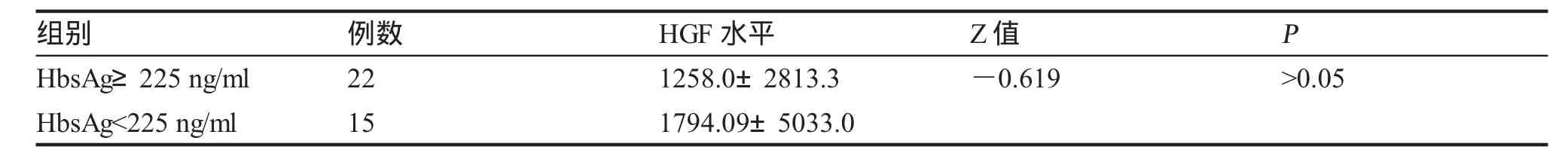
表6 HbsAg载量高、低组HGF水平比较(M±Q,pg/ml)
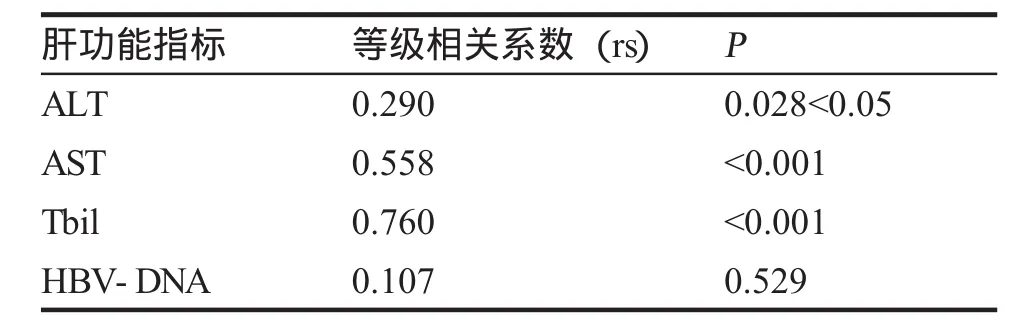
表7 炎症活动组HGF水平与肝功能指标、HBV-DNA水平的相关性分析
本研究中,我们发现,慢性HBV感染各临床类型组血清HGF水平均较正常对照组明显升高,差异有统计学意义,浓度在肝衰竭组>HBeAg-CHB组>HbeAg+CHB组>抗病毒组,肝衰竭组HGF浓度与其余各组比较,差异均有显著性;在炎症活动组(HbeAg+CHB组、HBeAg-CHB组、肝衰竭组),HGF与ALT、AST、TB均呈正相关,特别是与TB相关系数达0.76;HGF与HbsAg载量高、低无相关性;与HBV-DNA载量无相关性。分析原因,考虑为肝脏炎症活动时,在各种炎性因子的刺激下,肝星状细胞被激活,产生大量HGF,刺激肝细胞增殖。此外,肝细胞产生的胰岛素样生长因子(IGF-1)也能刺激星状细胞产生HGF。在肝损伤缓解后,激活的星状细胞可自发逆转,HGF水平降低。因此,临床上可把血清HGF作为肝损伤的指标之一[12],张迁[13]等人的研究也提示HGF值的变化不仅与血清TbiL值呈正相关,而且亦可作为判断病情及估计预后的参考指标。
本研究中,抗病毒组HGF水平低于HBeAg-CHB组,差异有显著性。分析原因,可能为抗病毒治疗后,炎症缓解,产生HGF的间质细胞减少,从而表现为血清HGF水平的下降。在动物肝损伤模型中,不论是D-半乳糖还是硫代乙酰胺诱导的肝损伤,携带HGF基因的转基因鼠肝损伤都要比对照组轻,HGF的保护作用也许是通过PGE2的合成增多而实现的[2-3]。但是Barreiros[14]等也认为,由HGF产生的肝细胞生存信号的加强也许是HBV持续感染的危险因素。
肝衰竭在我国曾命名为重型肝炎,其病理变化特点为肝细胞发生广泛坏死,病死率高达60%以上,肝衰竭确切发病机制尚未完全清楚。本研究肝衰竭组HGF水平高达5912.0±4497.0 pg/ml,分析其急剧增高的原因为血清中的HGF清除主要依靠有活性的肝实质细胞,肝衰竭患者由于肝脏的代谢功能减低,不能有效地将血液中的HGF清除,加之一些炎性因子的浓度升高,刺激非实质肝细胞不断合成HGF,因此导致血清HGF的含量升高,而且随着病情的发展而进行性升高[14]。提示我们,肝衰竭病人使用HGF的时期仍需进一步研究。
综上所述,伴随者肝损伤的发生和发展,肝脏间质细胞活化,产生HGF,修复肝损伤。HGF、多种细胞因子互相作用,与机体、病毒因素一起共同影响着慢性HBV感染的过程和结局。如何利用HGF预防和治疗肝损伤,应用的时机,以及HGF与临床的关系都值得进一步研究。
[1]骆抗先.乙型肝炎基础和临床[M].第三版.北京:人民卫生出版社,2007:203-207.
[2]JUN-ICHIOKANO,GOSHISHIOTA,HIRONAKA KAWASAKI.Protectiveaction ofhepatocytegrowth factor for acute liverinjurycaused by D-galactosamine in transgenic mice[J].Hepatology,1997,26(5):1241-1249.
[3]TAE-HOHWANGA,BYUNG-CHEOL YOONA,JIN-SOOK JEONGB,et al.A single administration of adenoviral-mediated HGF cDNA permits survival of mice from acute hepatic failure[J].Life Sciences,2003,72: 851-861.
[4]王军,于红.促肝细胞生长因子治疗肝炎后肝硬化的效果[J].实用医药杂志,2005,22(4):312.
[5]NAKAMURA T,NAWA K,ICHIHAR A.Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats[J].Biochem Biophys Res Commun,1984,122:1450-1459.
[6]RUSSELL WE,MCGOWAN JA,BUCHER NLR.Biological properties ofa hepatocyte growth factor from rat platelets [J].J Cell Physiol,1984,119:183-192.
[7]MAHER J.Cell-specific expression of hepatocyte growth factor in liver:Upregulation in sinusoidal endothelial cells after carbon tetrachloride[J].J Clin In vest,1993,91: 2244-2252.
[8]MATSUMOTOK,NAKAMURA T.Hepatocyte growth factor:molecular structure,roles in liver regeneration,and other biological functions[J].Crit Rev Oncog,1992,3: 27-54.
[9]RUBIN JS,BOTTARO DP,AARONSON SA.Hepatocyte growth factor/scatter factor and its receptor,the c-met proto-oncogeneproduct[J].BiochimBiophyActa, 1993,1155:357-371.
[10]ISHIKI Y,OHNISHI H,MUTO Y,et al.Direct evidence that hepatocyte growth factor is a hepatocytotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo[J].Hepatology,1992,16:1227-1235.
[11]FUJIWARAK,NAGOSHI S,OHNOA,et al.Stimulation of liver growth by exogenous human hepatocyte growth factor in normal and partially hepatectomized rats[J].Hepatology, 1993,18:1443-1449.
[12]SCHIMACHER P,GEERT A,PIETANGELO A.Hepatocyte growth factor/hepatopoietin A is expressedin fatstoring cells from rat liver but not myofibroblast-like cellsdeprivedfromfatstoringcells[J].Hepatology, 1992,15:5-11.
[13]张迁,万谟彬,李成忠,等.慢性重型肝炎患者血清肝细胞生长因子浓度变化的动态观察[J].临床肝胆病杂志,2003,19(1):18~19.
[14]PAULA B,SPRINZL M,ROSSET S,et al.EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes[J].Int J Cancer, 2009,124:120-129.
The study of the clinical significance of HGF of serum in chronic hepatitis B.
CHAO Chun-mei,WEI Jia,YANG Wei-bo(1.Dept.of Infectious Diseases,The 1st Affiliated Hospital of Kunming Medical College;2.The Fourth Affiliated Hospital ofKunmingMedical College,KunmingYunnan 650032,China)
ObjectiveTo measure the levels of hepatocyte growth factor(HGF)in serum of different clinical types of chronic hepatitis B and analyze them trying to find a relationship with different clinical types,the levels of liver function,HBVMand HBVDNA,probing the clinical significance of HGF in the treatment of chronic hepatitis B. MethodsThe levels of HGF in serum of 78 subjescts with chronic hepatitis had been measured with ELISA.20 with normal liver function healthy subjects whose HGF also had been measured were chosen as the contrast group. Meanwhile,all the subjects had been measured about their liver function,HBsAg,HBsAb,HBeAg,HBeAb,HbcAb and HBV-DNA,and some patients'HbsAg levels and PT also had been measured.ResultsThe levels of HGF in chronic hepatitis B group were(929.10±1932.15)pg/ml,while in the healthy control group,the levels of HGF were (215.1±65.97)pg/ml.These two data had statistically significant difference.In Anti-virus group,HbeAg+CHB group,HBeAg﹣CHB group and liver failure group,the levels of HGF were(658.20±178.20)pg/ml,(772.60±335.40) pg/ml,(958.80±1005.49)pg/ml and(5912.0±4497.0)pg/ml.The levels of HGF in these groups were all higher than that ofhealthycontrol group(P<0.0125).The level ofHGF in the liver failure group was the highest compared with the other four groups,and the difference was statistically significant(P<0.008).The level of HGF in HBeAg-CHB group>the level of HGF in Anti-virus group and the differences were statistically significant(P<0.008).The level of HGF in HBeAg﹣CHB group>the level in HbeAg+CHB group and the level of HGF in HbeAg+CHB group>the level of HGF in Anti-virus group,but the difference was not statisticallysignificant(P>0.008).The levels ofHGF in high and low viral loadgroups were(2 141.0±3115.3)pg/ml and(812.50±639.80)pg/ml respectively,there was no statistically significant difference(P>0.05).The level of HGF of the load of HbsAg in the high and lowgroups were (1 258.0±2813.3)and(1 794.09±5 033.0)pg/ml,there was no different statistical significance.HGF and Tbil had a high degree of positive correlation.In the relationship between HGF and HBV-DNA tested by the Spearman rank correlation,the correlation coefficient was not statistically significant(P>0.05).The HGF and inflammatory activity of chronic HBV infection showed a high degree of positive correlation,and the correlation coefficient was statistically significant(P<0.05).Conclusion1.HGFs in different types ofchronic hepatitis B are significantlyhigher in serum, indicatingthat HGF has involved in the immune response,liver injuryand reconditioningtoHBV infection process;2. The levels of HGF in hepatic failure group are significantly higher than that of the rest of the clinical types,which showthat abnormal increasing of the levels of HGF might be related to one of the reasons of hepatic failure.In addition,the role of HGF in the treatment of liver failure needs further evaluation;3.HGFs are related to the degree of liver injury,and have nosignificant correlation with the loads ofHBV-DNAand HbsAg.
Chronic hepatitis B;Hepatocyte growth factor
R512.6
A
1006-4141(2012)04-342-05
2012-02-17
2012-03-21
晁春梅(1971~)女,云南禄丰人,医学硕士,主治医师,主要从事感染性疾病的临床和教学工作。
韦嘉,E-mail:wejia@yahoo.cn
