吨酮类衍生物抗肿瘤活性研究进展
2012-11-06赵建萍吴玮峰
赵建萍,吴玮峰
(1.上海工会管理职业学院健康安全系,上海 201400; 2.上海市奉贤区中心医院药剂科,上海 201400)
1 概述
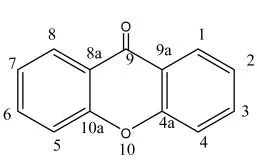
图1 吨酮骨架
2 抗肿瘤活性天然产物

图2 吨酮Xan-1~Xan-18的结构
3 抗肿瘤活性人工合成化合物
人工合成化合物主要包括O-取代化合物[11-12]和N-取代化合物两类。前者的代表药物是5,6-二甲基-4-乙酸基二苯吡酮(DMXAA,Xan-8),具有良好的血管阻断和抗肿瘤活性,已经进入了Ⅲ期临床[13]。后者的代表药物为Xan-9,Xan-10,Xan-11。化合物Xan-9对小鼠白血病细胞L1210的半数抑制浓度为2μmol/L,而对直肠癌细胞HT29的半数抑制浓度为1.7μmol/L,显示了强大的抗肿瘤活性[14]。化合物Xan-10对人口腔表皮样癌细胞 KB 3.1的半数抑制浓度为1.6μmol/L,而对癌细胞直肠MCF-7的半数抑制浓度为1.9μmol/L,显示了强大的抗肿瘤活性[15]。化合物Xan-11对肺癌细胞MDA-MB-231的半数抑制浓度为 16 μmol/L[16]。
4 抗肿瘤作用机制
4.1 抑制肿瘤细胞增殖
4.2 预防相关肿瘤发生
4.3 影响蛋白激酶C(PKC)
蛋白激酶C在肿瘤病理过程中扮演着重要角色,因此影响其表达或抑制其作用均可能产生抗肿瘤作用[24]。一些异戊二烯基取代的吨酮类化合物,包括α和γ曼果斯廷(Xan-12和Xan-13)已被证实对蛋白激酶C有一定的抑制活性[25]。降阿赛里奥(Xan-14)、优中酮(Xan-15)、3,4-二羟基吨酮(Xan-16)和 1-甲酰基-4-羟基 -3-甲氧基吨酮(Xan-17)也被发现对蛋白激酶C有抑制作用。对一些蛋白激酶C亚型来说,上述4种化合物的抑制活性甚至比标准的蛋白激酶C抑制剂白屈菜赤碱和NPC15437[26]还要好。更进一步研究发现,吨酮类化合物能够通过上调蛋白激酶C-α,β,γ,δ等亚型诱导分化肿瘤细胞,不同取代基团的化合物对蛋白激酶C的亚型具有选择性抑制[27]。如3,4-二甲基吨酮可选择性作用于蛋白激酶C-δ,2-羟基-2-甲氧基,3-羟基-4-甲氧基和1,2-二羟基吨酮则对蛋白激酶C-ζ有选择性[28],1,2-二甲氧基吨酮对蛋白激酶C-η有选择性[29]。因此,这些化合物对阐述蛋白激酶C各亚型的生理作用具有重要意义。
4.4 逆转多药耐药性
多药耐药性(MDR)是肿瘤化学治疗失败的主要原因之一。Tcham等[30]发现吨酮类化合物对P-糖蛋白C末端具有较强的亲和力,可调节P-糖蛋白活性,经过结构改造将成为潜在的P-糖蛋白抑制剂。吴秋歌等[31]也发现,P13K/Akt抑制剂吨酮类化合物LY294002能通过增强对信号转导通路的调控,从而增强P13K信号转导通路在多细胞耐药中发挥作用。
4.5 作用于拓扑异构酶
普梭草素(Xan-18)是从非洲植物莽吉柿的根和皮提取分离得到的吨酮类化合物[32]。这一天然产物在体内和体外都有较好的抗白血病活性,并且对乳腺癌、结肠癌、淋巴癌和白血病等多种人类肿瘤细胞有抑制作用[33]。机理学研究证明,普梭草素的作用机理是其在拓扑异构酶Ⅱ的分裂位点进行鸟嘌呤烷基化,从而对插入的DNA分子产生作用[34]。普梭草素的结构类似物及其功能性基团环氧二氢呋喃对体内活性的影响也已被证实[33]。最新研究表明,R构型的普梭草素具有最佳的DNA烷基化和抗肿瘤活性[33]。覃江克等[35]人工合成的酮并吡啶季铵盐类化合物,体外试验显示其对人卵巢癌细胞(A2780)、宫颈癌细胞(Hela)、肺癌细胞(SIC-A)和口腔上皮癌细胞(KB)均有较好的抑制作用,其作用机理也证实与DNA的相互作用有关。
5 结语
[3]El-Seedi HR,El-Ghorab DM,El-Barbary MA,et al.Naturally occurring xanthones;latest investigations:isolation,structure elucidation and chemosystematic significance[J].Curr Med Chem,2009,16(20):2 581-2 626.
[4]Schneider J,Evans EL,Grunberg E,et al.Synthesis and biological activity of acronycine analogs[J].JMed Chem ,1972,15(3):266-270.
[5]Douillard JY,Schiller J.ZD0473 combined with other chemotherapeutic agents for the treatment of solid malignancies[J].Eur J Cancer,2002,38(Suppl 8):25-31.
[6]Sato A,Fujiwara H,Oku H,et al.Alpha-mangostin induces Ca(2+)-ATPase-dependent apoptosis viamitochondrial pathway in PC12 cells[J].M JPharmacol Sci,2004,95(1):33-40.
[7]Kolokythas G,Kostakis IK,Pouli N,et al.Synthesis and cytotoxic activity of some new azapyranoxanthenone aminoderivatives[J].Bioorg Med Chem,2003,11(21):4 591-4 598.
[8]Isaka M,Jaturapat A,Rukseree K,et al.Phomoxanthones A and B,novel xanthone dimers from the endophytic fungus phomopsis species[J].JNat Prod,2001,64(8):1 015-1 018.
[9]Wijeratne EMK,Turbyville TJ,Fritz A,et al.A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity[J].Bioorg Med Chem,2006,14(23):7 917-7 923.
[10]Krick A,Kehraus S,Gerhuser C,et al.Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis[J].J Nat Prod,2007,70(3):353-360.
[11]Wang TC,Zhao YL,Liou SS.Synthesis and cytotoxic evaluation of potential bis-intercalators:tetramethylenebis(oxy)-and hexamethylenebis(oxy)-linked assembliesconsistingof flavones,xanthone,anthraquinone,and dibenzofuran[J].Helv Chim Acta,2002,85(5):1 382-1 389.
[12]Woo S,Jung J,Lee C,et al.Synthesis of new xanthone analogues and their biological activity test-cytotoxicity,topoisomerase Ⅱ inhibition,and DNA cross-linking study[J].Bioorg Med Chem Lett,2007,17(5):1 163-1 166.
[13]McKeagea MJ,Reck M,Jameson MB,et al.PhaseⅡstudy of ASA404(vadimezan,5,6-dimethylxanthenone-4-acetic acid/DMXAA)1800mg/m(2)combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer[J].Lung Cancer,2009,65(2):192-197.
[14]Kostakis IK,Pouli N,Marakos P,et al.Synthesis,cytotoxic activity,NMR study and stereochemical effects of some new pyrano[3,2-b]thioxanthen-6-ones and Pyrano[2,3-c]thioxanthen-7-ones[J].Bioorg Med Chem,2001,9(11):2 793-2 802.
[15]Martine VL,Moreau S,Larrouture S,et al.Synthesis and antiproliferative activity of aryl-and heteroaryl-hydrazones derived from xanthone carbaldehydes[J].Eur JMed Chem,2008,43(6):1 336-1 343.
[16]Kostakis IK,Pouli N,Marakos P,et al.Design,synthesis and cell growth inhibitory activity of a series of novel aminosubstituted xantheno[1,2-d]imidazoles in breast cancer cells[J].Bioorg Med Chem,2008,16(6):3 445-3 455.
[17]Ho CK,Huang YL,Chen CC,et al.Garcinone E,a xanthone derivative,has potent cytotoxic effect against hepatocellular carcinoma cell lines[J].Planta Med,2002,68(11):975-979.
[18]蒲兴祥,江高峰,陈 泳,等.二苯并吨类化合物CY-B12体外抗肿瘤细胞增殖的作用及其机制的初步研究[J].癌症,2005,24(12):1 442-1 447.
[19]Cao Z,Baguley BC,Ching,LM.Interferon-inducible protein 10 induction and inhibition of angiogenesis in vivo by the antitumor agent 5,6-dimethylxanthenone-4-acetic acid(DMXAA)[J].Cancer Res,2001,61(4):1 517-1 521.
[20]Wang LC,Reddy CB,Baguley BC,et al.Induction of tumour necrosis factor and interferon-γin cultured murine splenocytes by the antivascular agent DMXAA and its metabolites[J].Biochem Pharmacol,2004,67(5):937-945.
[21]Murata R,Horsman MR.Tumour-specific enhancement of thermoradiotherapy at mild temperatures by the vascular targeting agent 5, 6-dimethylxanthenone-4-acetic acid[J].Int J Hyperthermia,2004,20(4):393-404.
[22]Ito C,ItoigawaM,FurukawaH,etal.Xanthonesasinhibitorsof Epstein-Barr virus activation[J].Cancer Leters,1998,132(1-2):113-117.
[23]Yoshimi N,Matsunaga K,Katayama M,et al.The inhibitory effects of mangiferin,a naturally occurring glucosylxanthone,in bowel carcinogenesis of male F344 rats[J].Cancer Leters,2001,163(2):163-170.
[24]Saha AK,Liu L,Simoneaux R,et al.Novel triazole based inhibitors of Ras farnesyl transferase[J].Bioorg Med Chem Lett,2005,15(24):5 407-5 411.
[25]Jinsart W,Ternai B,Buddhasukh D,et al.Inhibition of wheat embryo calcium-dependent protein kinase and other kinases by mangostin and gamma-mangostin[J].Phytochemistry,1992,31(11):3 711-3 713.
[26]Saraiva L,Fresco P,Pinto E,et al.Synthesis and in vivo modulatory activity of protein kinase C of xanthone derivatives[J].Bioorg Med Chem,2002,10(10):3 219-3 227.
[27]Mak NK,Lung HL,Wong RN,et al.Expression of protein kinase C isoforms in euxanthone-induced diferentiation of neuroblastma cells[J].Planta Med,2001,67(5):400-405.
[28]Webb BLJ,Hirst SJ,Giembycz MA.Protein kinase C isoenzymes:a review of their structure,regulation and role in regulating airways smooth muscle tone and mitogenesis[J].Br JPharmacol,2000,130(7):1 433-1 452.
[29]Hofmann J.The potential for isoenzyme-selective modulation of protein kinase C[J].Faseb J,1997,11(8):649-669.
[30]Tchamo DN,Dijoux-Franca MG,Mariotte AM,et al.Prenylated xanthones as potential P-glycoprotein Modulators[J].Bioorg Med Chem Lett,2000,10(12):1 343-1 345.
[31]吴秋歌,王 静,蒋军广,等.P13K/Akt抑制剂联合顺铂治疗肺癌的实验研究[J].中国老年学杂志,2009,29(22):2 916-2 918.
[32]Pachuta RR,Cooks RG,Cassady JM ,et al.Antineoplastic agents from higher plants:application of tandem mass spectrometry to xanthones from Psorospermum febrifugum[J].JNat Prod,1986,49(3):412-423.
[33]Heald RA,Dexheimer TS,Vankayalapati H,et al.Conformationally restricted analogues of psorospermin:design,synthesis,and bioactivity of natural-product-related bisfuranoxanthones[J].J Med Chem,2005,48(8):2 993-3 004.
[34]Kwok Y,Hurley LH.TopoisomeraseⅡsite-directed alkylation of DNA by psorospermin and its effect on topoisomeraseⅡ-mediated DNA cleavage[J].JBiol Chem,1998,273(49):33 020-33 026.
[35]覃江克,兰文丽,韩留玉,等.吨酮并吡啶季铵盐的合成及其生物活性[J].应用化学,2010,27(5):528-532.
