Effect of the Interference Instant of Zeolite HY Catalyst on the Pyrolysis of Pubescens*
2012-10-31LIUWenwu刘文武HUChangwei胡常伟YANGYu杨宇ZHULiangfang祝良芳andTONGDongmei童冬梅
LIU Wenwu (刘文武), HU Changwei (胡常伟)**, YANG Yu (杨宇), ZHU Liangfang (祝良芳)and TONG Dongmei (童冬梅)
Key Laboratory of Green Chemistry and Technology, Minister of Education, College of Chemistry, Sichuan University, Chengdu 610064, China
Effect of the Interference Instant of Zeolite HY Catalyst on the Pyrolysis of Pubescens*
LIU Wenwu (刘文武), HU Changwei (胡常伟)**, YANG Yu (杨宇), ZHU Liangfang (祝良芳)and TONG Dongmei (童冬梅)
Key Laboratory of Green Chemistry and Technology, Minister of Education, College of Chemistry, Sichuan University, Chengdu 610064, China
A laboratory reactor was designed to test the effect of the interference instant of HY on the pyrolysis of pubescens. The time instant for intermediate species from pyrolysis to contact HY was controlled by varying the position of the catalyst bed relative to the pyrolytic cell. It was found that the effect of the interference instant was significant on the variation of different intermediate species, and the yield and quality of the products. The results also showed that, with the increase in the distance between the pyrolytic cell and the catalyst bed, the yield of liquid and relative content of the organics such as aldehyde, phenols, etc., decreased, while the yield of residue and relative content of acetic acid increased. The deoxygenation of the intermediate species was favored when the catalyst exerted its performance on them immediately after their formation.
zeolite HY, catalytic pyrolysis, pubescens, acetic acid, intermediate conversion
1 INTRODUCTION
Biomass can be converted into fuels, useful chemicals and high quality hydrocarbon products through pyrolysis [1, 2], and is considered as a promising energy resource. The liquid products from the pyrolysis of biomass, called bio-oil, is a complex mixture of water and organic chemicals, including acids, alcohols, aldehydes, ketones, esters, heterocyclic derivatives, and phenolic compounds. The properties of bio-oil are strongly dependent on the feedstock and production conditions, or in other words, the organic components of bio-oil vary with the raw materials and the catalysts used. Many catalysts such as zeolites Y, ZSM-5, (Me)-Al-MCM-41 and some inorganic compounds [3-6] were tested for biomass conversion.Usually these catalysts were mixed directly with the biomass or isolated from the biomass by inert materials such as glass wool. Under the operating conditions the distance between biomass and catalyst was fixed.Acetic acid is often one of the main products from the pyrolysis of biomass [7, 8], especially for bamboos which had low content of lignin. However, the bio-oil with high acetic acid content could not be used as a fuel due to its low heat value. The reduction of the content of acetic acid in the liquid products from pyrolysis of biomass might increase the potential heat value and make the bio-oil more valuable.
In the present work, the catalytic pyrolysis of pubescens, a kind of bamboo in Sichuan Province of China, was studied with zeolite HY to improve the quality of the liquid products. The dependence of product distribution and yields on the interference instant (which is defined as the time interval for the pyrolytic products to begin the contact with catalyst)of HY, as manipulated by adjusting the position of the catalyst bed in the pyrolyzer, was investigated in detail.
2 METHODS
2.1 Materials
Zeolite HY was purchased from Nanjing Ruiyan Petrochemical Company, and its particle size was about 149 μm (100 mesh). The air-dried pubescens was smashed into powder and then screened to give fraction with the particle size of 360 μm (40 mesh).
2.2 Apparatus
The sketch of the reactor is shown in Fig. 1. The reactor was composed of two glass tubes. The outer tube was 68 cm high with an internal diameter of 4.5 cm. The inner tube was 15.5 cm high with an internal diameter of 2 cm, acting as the pyrolytic cell. The bottom of the inner tube was sealed to load the test sample. Movable catalyst containers made of copper gauze 74 μm (200 mesh) were used as the catalyst bed.It could be placed inside the inner tube or between the inner and outer tubes. The position of the catalyst bed was marked by its distance from the outlet of the inner tube, as shown in Fig. 1. A furnace was used to heat the reactor to the set temperature with a heating rate of 10 K·min−1. The temperature was monitored with a thermal couple. As the reactor was small, the wall of glass tube was thin, and the reactor was placed in the constant temperature zone of the furnace, the temperature of the catalyst bed was considered constant.

Figure 1 The sketch of the reactor for the pyrolysis of pubescens
2.3 Operating conditions
Pyrolysis experiments were performed at 420 °C for 2.5 h under N2atmosphere using 2 g sample of pubescens as the raw material. The decomposition temperature was firstly probed by TG-DTG (thermogravimetry-differential thermal gravimetric analysis)technique using a TG-TGA92 type thermogravimetric analyzer made by Setaram. Co., France (samples of 60 mg each being tested). As shown in Fig. 2, the temperature at the end of mass loss was at about 420 °C,which was selected as the reaction temperature. The pyrolysis time of 2.5 h was determined by performing the experiment with different time durations and determining the time when the liquid yield did not increase any more. The volatiles from the pyrolytic cell initially ascended to the outlet of the inner tube, then were swept down by N2with a flow rate of 40 ml·min−1. 0.5 g of zeolite HY was mixed directly with pubescens or placed on the movable catalyst bed. The liquid product was collected in an ice trap (0 °C), and the gaseous products were collected using a bag and then analyzed by gas chromatography (GC).
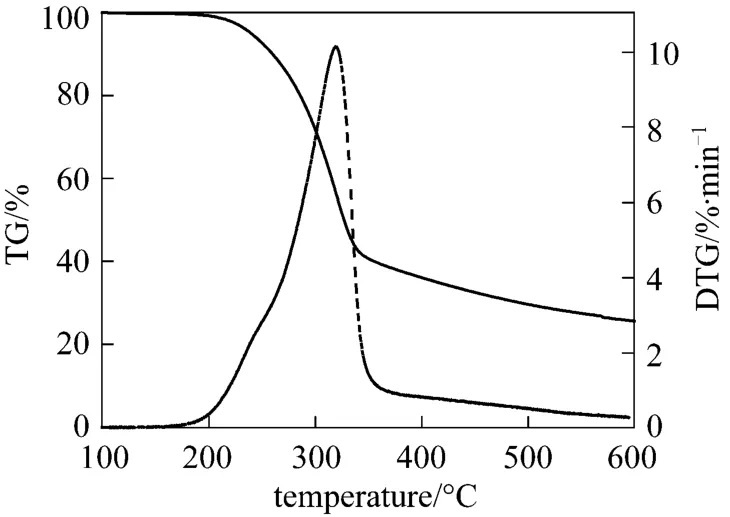
Figure 2 TG and DTG curves of pubescens (heating rate:10 K·min−1)
2.4 Product analysis
The liquid product and the residue were weighted directly. The yield of liquid product, the percentages of residue and that of gas plus losses were calculated as follows:
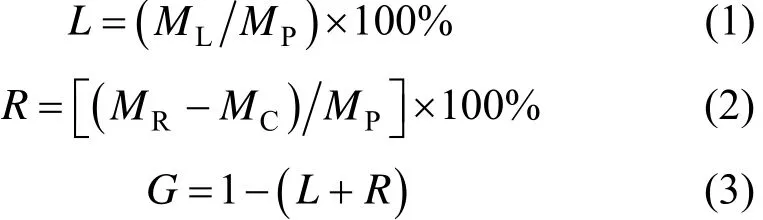
The composition and relative content of the liquid products were analyzed using Agilent 6890 series GC system supplied with 5973 mass selective detector(GC-MS, USA). The GC program parameters were:injector temperature, 250 °C; column temperature,100-180 °C; carrier gas, helium; split ratio, 10︰1; total flow rate, 16 ml·min−1.
The composition of the gas products was analyzed by a thermal conductivity detector (TCD) in temperature programming by gas chromatography(SP-6800A, Chemical Instrument Factory, South Shandong, Jinan) using a Porapak Q column (2 m × 3 mm). The peaks corresponding to different gas species were identified and calculated by comparing retention times with standard samples with known molar percentages of reference compounds, excluding nitrogen and water from gas composition.
3 RESULTS AND DISCUSSION
In the as-designed reactor, the variation of the location of the catalyst bed was deemed to alter the interference time of the catalyst HY on the pyrolytic intermediate species.
3.1 Effect of the interference instant of catalyst HY on the yield of products
The variation of the percentage of liquid, gas and residue (the solids deposited on walls included) with the position of catalyst HY are shown in Table 1. The results showed that the highest liquid yield was gotten when the zeolite HY was mixed directly with the pubescens sample. While the catalyst was put on the movable catalyst bed in the inner tube, the liquid yield decreased with the increase of the distance between the pyrolytic cell and the catalyst bed. Although the yield of liquid product in the presence of HY catalyst did not change much when the catalyst was moved to between the two tubes, it was all higher than that obtained by simple thermal pyrolysis, similar to what some researchers had obtained previously [3, 8]. The percentage of gaseous product reduced significantly in the presence of catalyst HY, but little change was observed with the variation of the position of the catalyst.The percentage of residue was 36% in the absence of HY, while it decreased to near 34% when HY was mixed directly with pubescens or placed in the inner tube. It was interesting to note that when HY was placed between the two tubes the percentage of residue became even greater (38%-39%) than that obtained in the thermal pyrolysis (36%). This implies the variation of the intermediates under different condition.
The facts mentioned above implied that the pyrolysis of pubescens might be generally divided into two stages: firstly, the direct pyrolysis producing primary intermediate species by thermal conversion;secondly, the thermal and catalytic conversion of these variable intermediate species. This was in accordance with the results reported previously [9, 10]. The variation of the location of the catalyst bed influenced the instant of the intermediate species to contact with the catalyst, thus affecting the yield of gas, liquid and solid residue.
3.2 Effect of the interference instant of catalyst HY on liquid product
The results in Table 2 showed that, when the catalyst HY was mixed directly with pubescens, the relative content of acetic acid and phenols were found to be 40.4% and 3.2%, respectively (the minima in all runs) and that of furans was 22.3% (the maxima in all runs). Furthermore, with the increase of the distance between the pyrolytic cell and the catalyst bed, the relative content of acetic acid increased, while that of other organics such as aldehyde and phenols decreased gradually. The results demonstrated that the later the intermediate species contacted the catalyst,the more intermediate species, potentially being converted into other organics, were converted into acetic acid through multiple thermal and catalytic reactions.It was well known that the properties of the liquid products were strongly dependent on the conditions such as reaction temperature, feedstock and the catalyst used [6]. Though different raw materials, different catalysts and techniques [11, 12] had been used in the pyrolysis of biomass in the literatures, the thermal conversion of the preliminarily formed intermediate species, as well as the effect of catalytic interference on these variable species had not been addressed. The present work revealed that the quality of the liquid products was also affected by the time interval for intermediate species to contact the catalyst.
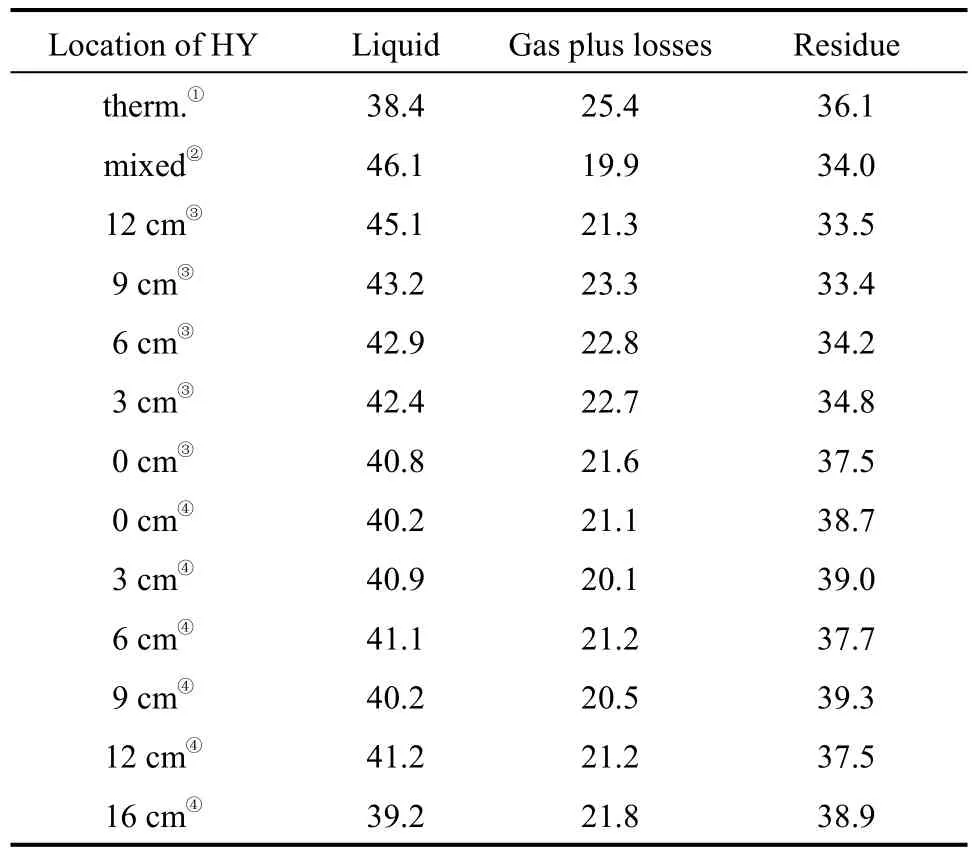
Table 1 The effect of the location of catalyst HY on the yields of products (%, by mass)[N2 (40 ml·min−1), 420 °C, 2.5 h]
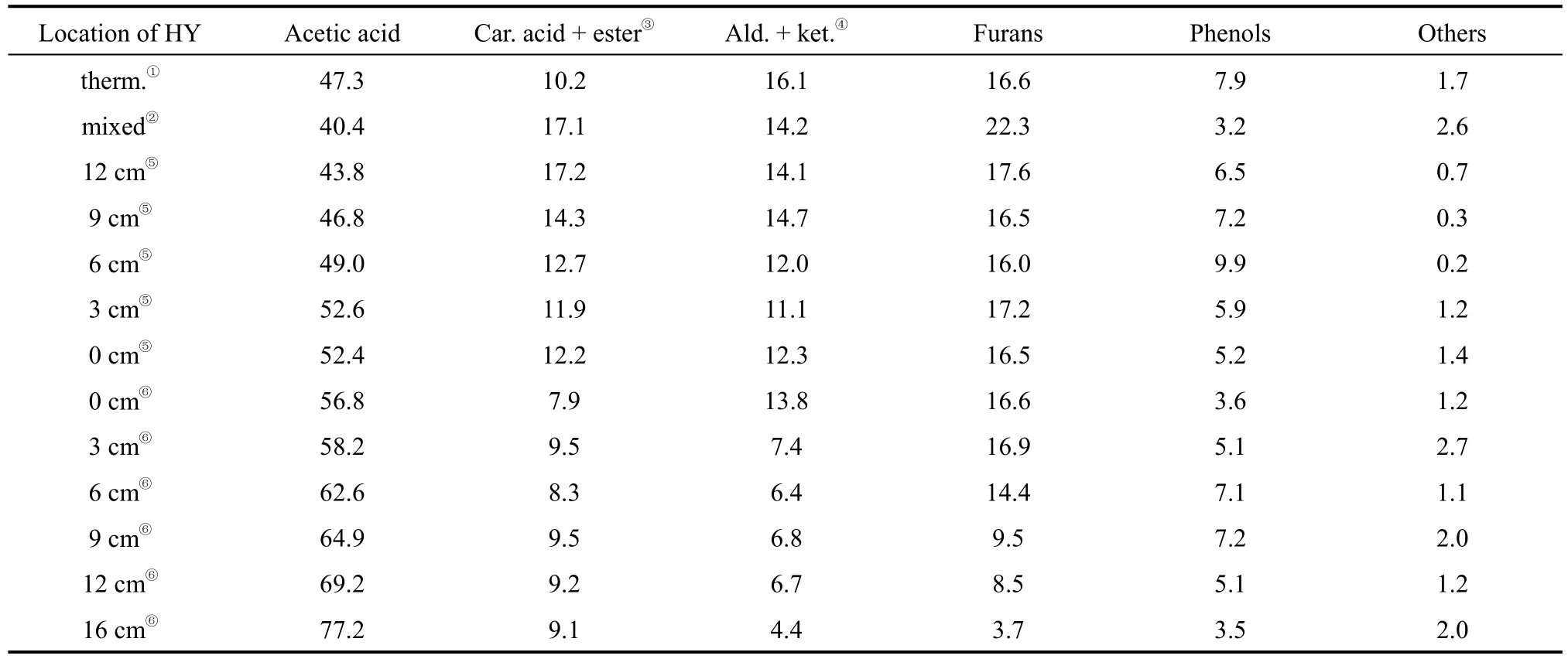
Table 2 The effect of the location of catalyst HY on the distribution and relative content of liquid products (%, by mass)[N2 (40 ml·min−1), 420 °C, 2.5 h]
3.3 Composition of gases from pyrolysis of pubescens
Table 3 showed the composition of gases from non-catalytic and catalytic pyrolysis by HY at different positions in nitrogen atmosphere. The main gases produced in all runs were CH4, CO2and CO, while the yields of H2, C2H4and C2H6were relatively small.There was little influence of HY at different positions on the volume percentage of CH4, so it might be reasonable to deduce that CH4was formed mainly at the initial stage of thermal pyrolysis. It was noteworthy that when zeolite HY and pubescens were mixed together the concentration of H2was found to be 0.8%(by volume, the minimum yield of hydrogen in all runs), while that of CO2was 39.7% (by volume, the maximum yield of CO2in all runs). The fact indicated that the deoxygenation in the form of CO2was favored when directly mixing HY catalyst and pubescens together. And this would provide a bio-oil with better combustion characteristics. This was consistent with the composition of the corresponding liquid phase (the relative content of acetic acid was 40.4%,the minimum in all runs).
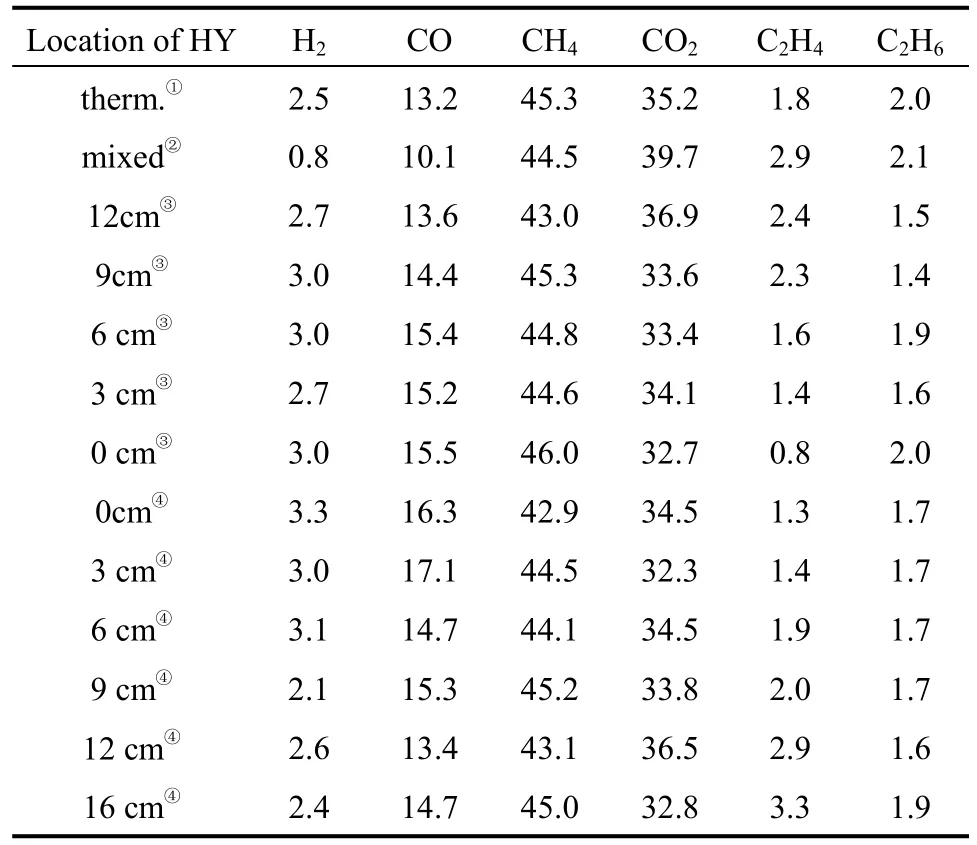
Table 3 The composition of pyrolysis gases catalyzed by HY at different positions (%, by volume)[N2 (40 ml·min−1), 420 °C, 2.5 h]
As mentioned above, the time instant for the gaseous intermediate species to contact HY was also an important factor affecting the distribution of gaseous products from the pyrolysis. The volume percentage of CO2obtained at 16 cm was relatively lower(32.8%, by volume) than that at other positions. This might be relevant to the relatively highest (77.2%)content of acetic acid in liquid obtained at the same position. This implied that it became hard for the intermediate species to be deoxygenated in the form of CO2after thermal conversion firstly and then encountering the catalyst. The derivatives from biomass had been upgraded by direct deoxy-liquefaction [13], in an air-proof stainless steel reactor at different temperatures and high pressure, in order to preserve hydrogen and remove oxygen in the form of CO2from biomass and further produce long-chain alkanes and aromatic hydrocarbons. The present work indicated that, when HY catalyst was directly mixed with pubescens together, the deoxygenation in the form of CO2from pubescens was favored at low temperature and normal atmosphere, so this might provide useful information on the production of bio-oil and chemicals from biomass, especially on catalytic process design.
NOMENCLATURE
G mass percentage of gaseous products plus losses, %
L yield of liquid, % (by mass)
MCmass of catalyst, g
MLmass of liquid, g
MPmass of pubescens, g
MRmass of remains in inner tube, g
R mass percentage of residue, %
1 Aclkgpz, C., Onay, O., Kockar, O.M., “Fast pyrolysis of linseed:Product yields and compositions”, J. Anal. Appl. Pyrol., 71, 417-429(2004).
2 Xu, X.B., Lin, J.P., Cen, P.L., “Advances in the research and development of acrylic acid production from biomass”, Chin. J. Chem.Eng., 14 (4), 419-427 (2006).
3 Adam, J., Antonakou, E., Lappas, A., “In situ catalytic upgrading of biomass derived fast pyrolysis vapours in a fixed bed reactor using mesoporous materials”, Micropor. Mesopor. Mater., 96, 93-101(2006).
4 Nilsen, M.H., Antonakou, E., Bouzga, A., Lappas, A., Mathisen, K.,Stöcker, M., “Investigation of the effect of metal sites in Me-Al-MCM-41 (Me Fe, Cu or Zn) on the catalytic behavior during the pyrolysis of wooden based biomass”, Micropor. Mesopor.Mater., 105, 189-203 (2007).
5 Peng, J., Chen, P., Lou, H., Zheng, X.M., “Catalytic upgrading of bio-oil by HZSM-5 in sub- and super- critical ethanol”, Bioresour.Technol., 100, 3415-3418 (2009).
6 Yang, H.P., Yan, R., Chen, H.P., “Influence of mineral matter on pyrolysis of palm oil wastes”, Combust. Flame, 146, 605-611 (2006).
7 Demirbas, A., “The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis”, Fuel Process. Technol., 88, 591-597 (2007).
8 Qi, W.Y., Hu, C.W., Li, G.Y., Guo, L.H., Yang, Y., Luo, J., Miao, X.,Du, Y., “Catalytic pyrolysis of several kinds of bamboos over zeolite NaY”, Green Chem., 8, 183-190 (2006).
9 Baumlin, S., Browt, F., Bazer-Bachi, F., Bourdeaux, T., Herbinet, O.,Ndiaye, F.T., Ferrer, M., Lédé, J., “Production of hydrogen by lignin fast pyrolysis”, Int. J. Hydrogen Energy, 31, 2179-2192 (2006).
10 Rath, J., Staudinger, G., “Cracking reactions of tar from pyrolysis of spruce wood”, Fuel, 80, 1379-1389 (2001).
11 Jing, Q., Lü, X.Y., “Kinetics of non-catalyzed decomposition of glucose in high-temperature liquid water”, Chin. J. Chem. Eng., 16,890-894 (2008).
12 Chen, J.Y., Zhang, H., Zheng, H.F., Zhu, X., Zeng, Y.S., “In situ visualization of transformation of organic matter in water at high pressures and temperatures”, J. Anal. Appl. Pyrol., 76, 260-264 (2006).
13 Wu, L.B., Guo, S.P., Wang, C., Yang, Z.Y., “Production of alkanes(C7-C29) from different part of poplar tree via direct deoxy-liquefaction”, Bioresour. Technol., 100, 2069-2076 (2009).
2009-06-13, accepted 2009-11-09.
* Supported by the State Key Development Program for Basic Research of China (2007CB210203) and the Special Research Fund for the Doctoral Program of Higher Education of China (20050610013).
** To whom correspondence should be addressed. E-mail: Changweihu@scu.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- Application of Choline Chloride·xZnCl2 Ionic Liquids for Preparation of Biodiesel*
- Inhibiting Effect of Ciprofloxacin, Norfloxacin and Ofloxacin on Corrosion of Mild Steel in Hydrochloric Acid*
- Experimental and Numerical Study on Heat Transfer Enhancement of a Rectangular Channel with Discontinuous Crossed Ribs and Grooves*
- Translocation of Polymer Through a Nanopore Studied by Langevin Dynamics: Effect of the Friction Coefficient*
- Electrocatalytic Activity of Tungsten Carbide and Natural Zeolite Composite in Aqueous Solution*
