Application of Choline Chloride·xZnCl2 Ionic Liquids for Preparation of Biodiesel*
2012-10-31LONGTao龙涛DENGYuefeng邓岳锋GANShucai甘树才andCHENJi陈继
LONG Tao (龙涛), DENG Yuefeng (邓岳锋), GAN Shucai (甘树才) and CHEN Ji (陈继),**
1 College of Chemistry, Jilin University, Changchun 130026, China 2 State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Science, Changchun 130022, China
Application of Choline Chloride·xZnCl2Ionic Liquids for Preparation of Biodiesel*
LONG Tao (龙涛)1,2, DENG Yuefeng (邓岳锋)2, GAN Shucai (甘树才)1and CHEN Ji (陈继)2,**
1College of Chemistry, Jilin University, Changchun 130026, China2State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Science, Changchun 130022, China
The inexpensive and moisture-stable Lewis-acidic ionic liquids were prepared and applied for transesterification of soybean oil to biodiesel. The influences of molar ratio of methanol to soybean oil, reaction temperature and amount of ionic liquids were investigated. The transesterification of soybean oil to biodiesel catalyzed by choline chloride·xZnCl2ionic liquids showed many advantages such as mild conditions and lower cost. On the other hand, the non-ideal yield and complicated separation between biodiesel and soybean oil were also investigated and analyzed. The improvement on the systems of choline chloride·xZnCl2was proposed for further investigation.
ionic liquids, biodiesel, transesterification, Lewis acid
1 INTRODUCTION
In recent years, room-temperature ionic liquids(ILs) have attracted much attention for synthetic and catalytic application because of their important attributes such as wide liquid range, negligible vapor pressure, high catalytic activity, excellent chemical and thermal stabilities, potential recoverability, design possibilities, and ease of separation of the products from reactants [1]. ILs, up to now investigated, can be broadly divided into two types: one based on chloro-metallate anions such as andthe other onnon-metal-containing anions such as . The Lewis acidity of the ILs that composed of imidazole cation and chlorometallate anion increased in the order:CuCl<FeCl3<ZnCl2<AlCl3[2]. Therefore, chloroaluminate ILs have been studied extensively for use in acid catalyzed reactions such as alkylation [3], esterification [4], Baylis-Hillman reactions [5], Friedel-Crafts [6],Diels-Alder [7], electrochemical polymerization [8]and Henry reactions [9]. However, the cost of imidazolium ILs is relatively high for bulk application,whilst chloroaluminate ILs should be used under anhydrous and oxygen-free condition due to their low tolerance to moisture. Based on these reasons, choline chloride·xZnCl2have been prepared and investigated because of their easy preparation, moisture stable and relatively cheap [10]. Choline chloride·xZnCl2have been applied for electrolytic deposition [11], regiospecific Fischer indole reaction [12], protection of carbonyls reaction [13] and Diels-Alder reaction [14].In seeking new application for choline chloride·xZnCl2, some recent publication on biodiesel in ILs have attracted our attention. Earle et al. [15] used ILs ([emim][HSO4], 4-(3-methylimidazolium) butanesulfonic acid cation ILs and quaternary-ammonium-salt alkali ILs, etc.) as solvents and catalysts for the preparation of biodiesel. Zhang et al. [16] prepared composite catalyst from alkali or acidic IL and other alkali(sodium hydroxide, etc.) or acid (concentrated sulfuric acid, etc.) to catalyze soybean oil to biodiesel. Wu et al.[17] catalyzed biodiesel from cottonseed with Brønsted acidic ILs. These ILs above-mentioned were mostly belonged to imidazole and pyridines ILs. In contrast,choline chloride·xZnCl2ILs have many merits [10]such as generally accessible, easy to handle and relatively cheap. In this paper, the application of choline chloride·xZnCl2as efficient catalysts for preparation of biodiesel under mild conditions is reported.
2 EXPERIMENTAL
2.1 Preparation of choline chloride·xZnCl2
Choline chloride was purchased from Sinopharm Chemical Reagent Co., Ltd. ZnCl2was from Beijing chemical industrial factory. 0.1 mol choline chloride was mixed with zinc chloride (0.1 mol, 0.2 mol, 0.3 mol) and heated to 100 °C in air with stirring until a clear colorless liquid was obtained. The parallel controlled trial was conducted with 2︰1 molar ratio of ZnCl2to choline chloride under sealed nitrogen. The structures of the ILs are illustrated in Scheme 1.
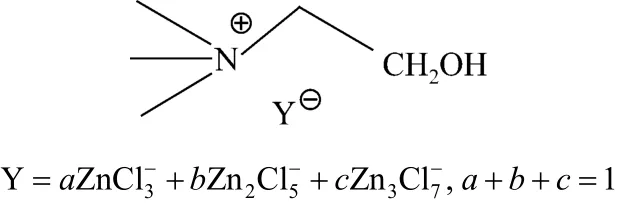
Scheme 1 Structures of choline chloride·xZnCl2
2.2 Characterization of choline chloride·xZnCl2
The structures of the ILs were analyzed by nuclear magnetic resonance (NMR) and Fourier transform infrared (FT-IR).1H NMR and13C NMR spectra were obtained on a BRUKER AV600 nuclear magnetic resonance spectrometer. Chemical shifts were reported in parts per million. IR spectra were recorded on a BRUKER Vertex 70 FTIR spectrometer using KBr to form a liquid film. Thermogravimetric analysis (TGA) was determined by a thermal analysis instrument (SDTQ600,TA Instruments, USA) from room temperature to 800°C in air at a heating rate of 10 °C·min−1. Viscosity of choline chloride·2ZnCl2was determined by a rotary rheometer (MCR300, CP 25-2, Australia) at 25 °C.
2.3 Determination of the Lewis acidity of choline chloride·xZnCl2
All IR samples were prepared by mixing probe liquids and choline chloride·xZnCl2in a given molar ratio of pyridine/ILs=1︰1, and then spreading into liquid films on KBr windows.
2.4 Preparation of biodiesel
Soybean oil was obtained from Heilongjiang Jiusan Oil Co., Ltd. and was used without further treatment. Methanol was obtained from Shandong Yuwang Co., Ltd. The transesterification reaction was carried out in a 100 ml round-bottom flask in reflux for the desired time. Soybean oil, methanol, and choline chloride·xZnCl2with different molar ratios were quantitatively introduced into the reactor, and choline chloride·xZnCl2was dissolved in methanol firstly, and then soybean oil was added. The reaction with magnetic stirring and heating at the desired temperature was allowed to proceed for 24, 48 and 72 h, respectively. After reaction, the reactor was cooled to room temperature and two phases were formed. The upper phase consisted of the produced methyl esters, and the lower phase contained choline chloride·xZnCl2and excess methanol. The upper phase containing the methyl esters was simply separated from the lower phase by decantation. The biodiesel products were analyzed by HPLC equipped with a pump (515 HPLC Pump, Waters), an ultraviolet detector (Waters 2487),and a SunFire C18analytical column (250 mm length×4.6 mm i.d.; 5 μm particle size). The detection wavelength was 211 nm. The solvents were filtered through a 0.45 μm Millipore filter prior use. The mobile phase was methanol at a flow rate of 1.0 ml·min−1.The biodiesel samples were diluted with acetone(HPLC grade). The parallel controlled trial was conducted under sealed nitrogen.
3 RESULTS AND DISCUSSION
3.1 Characterizations of choline chloride·xZnCl2
3.1.1 NMR analyses of choline chloride·xZnCl2
Choline chloride·xZnCl2are analyzed by1H NMR and13C NMR spectroscopies. The NMR spectral data of choline chloride·xZnCl2are as follows.
choline chloride·ZnCl2(1︰1):1H NMR (600 MHz, DMSO-d6): δ 3.106 (s, 9H), 3.385-3.401 (t, 2H,J 4.8 Hz), 3.831 (s, 2H), 5.266 (s, 1H).13C NMR (150 MHz, DMSO-d6): δ 53.15, 53.18, 53.20, 55.13, 66.94.
choline chloride·2ZnCl2(1︰2):1H NMR (600 MHz, DMSO-d6): δ 3.101 (s, 9H), 3.381-3.398 (t, 2H,J 4.8 Hz), 3.828 (s, 2H), 5.241 (s,1H).13C NMR (150 MHz, DMSO-d6): δ 53.21, 53.23, 53.20, 55.16, 66.97.
choline chloride·3ZnCl2(1︰3):1H NMR (600 MHz, DMSO-d6): δ 3.098 (s, 9H), 3.378-3.394 (t, 2H,J 4.8 Hz), 3.825 (s, 2H), 5.234 (s,1H).13C NMR (150 MHz, DMSO-d6): δ 53.34, 53.37, 53.38, 55.25, 66.06.
The NMR spectral data of the ILs agree with their designed structures (Scheme 1) [10]. As can be seen from the spectra of these catalysts, there is no impurity peak in1H NMR. The band of 3.31 indicates the presence of water, which implies the ILs moisture insensitive.
3.1.2 IR Analyses of choline chloride·xZnCl2
Infrared spectra of choline chloride and choline chloride·xZnCl2are shown in Fig. 1. Observation of a broad band in 3430 cm−1and a band near 1620 cm−1[Figs. 1 (a-c)] indicate the presence of water in these ILs, which is different from choline chloride [Fig.1(d)], and both of the bands are found to increase to a certain degree with an increase of x for chloride·xZnCl2.The bands near 3300-3500, 1090 and 1050 cm−1could be assigned to the OH part or C C O stretching vibration associated with N C2H4OH group of choline cation. The infrared spectra of choline chloride·xZnCl2also agree with their designed structures (Scheme 1).

Figure 1 FT-IR spectra of (a) choline chloride·ZnCl2, (b)choline chloride·2ZnCl2, (c) choline chloride·3ZnCl2, and (d)choline chloride
3.1.3 Lewis acidity of choline chloride·xZnCl2
Pyridine, a strongly basic molecule, can react with different type of acid to form pyridinium cation or stable complex. Thus, pyridine has been used as a probe molecule for determination of Lewis and Brønsted acidity of ILs by monitoring the bands of 1400-1700 cm−1arising from its ring vibration modes[18]. The bands in ranges of 1445-1460 cm−1and 1602-1640 cm−1are indicative of pyridine coordination to Lewis acid sites, whilst the bands in ranges of 1530-1550 cm−1and 1631-1640 cm−1are the indication of the formation of pyridinium ions resulting from the presence of Brønsted acidic sites [19]. Pyridine is added to choline chloride·xZnCl2to estimate the Lewis acidities of ILs followed by FT-IR scanning.As shown in Fig. 2, neat pyridine represents two well resolved single bands at 1437 cm−1and 1581 cm−1[Fig. 2 (a)]. In Figs. 2 (b-d), bands shift from 1437 to 1449, 1450, 1451 cm−1, respectively and 1581 to 1608 cm−1indicating the coordination of pyridine to Lewis acid sites. The band shift of pyridine molecule could estimate the intensity of acidity [20]. Anionic clusters of choline chloride·xZnCl2are reported to contain[11]. The relative proportions of each species have been quantified using potentiometry [21]. The acidic strength is found to increase in the following order:according to the slight band shifts from 1449 to 1450 and 1451 cm−1. Therefore, the acidity increases with increasing the mole ratios of ZnCl2to choline chloride from 1 to 3. This is in agreement with those catalytic activities obtained in transesterification of soybean oil to biodiesel (Fig. 3). The band of 1537cm−1in Fig. 2 (d) indicates the presence of Brønsted acidic sites due to hydrolysis of part anion by the absorbed water mentioned above, which is consistent with [C4mim]Cl/AlCl3[22]. However, the hydrolysis is not reported in all the metal chloride systems because of different experimental conditions such as time,temperature and pH, and thus the explanation couldn’t be available in the similar study.

Figure 2 FT-IR spectra of (a) pure pyridine, (b) pyridine +choline chloride·ZnCl2, (c) pyridine + choline chloride·2ZnCl2,and (d) pyridine + choline chloride·3ZnCl2(pyridine/IL=1︰1 by molar ratios in b-d)

Figure 3 TG curve of choline chloride·ZnCl2, choline chloride·2ZnCl2 and Choline chloride·3ZnCl2
3.1.4 Thermal stability and viscosity of choline chloride·xZnCl2
The thermogravimetric analysis is illustrated in Fig. 3. The decomposition temperatures of choline chloride·ZnCl2, choline chloride·2ZnCl2and choline chloride·3ZnCl2are 320.01 °C, 322.82 °C and 328.89°C, respectively. There is a mass loss on the TG curve near 100 °C which results from the evaporation of water. Therefore, choline chloride·xZnCl2exhibit high thermal stabilities.
The freezing points of choline chloride·xZnCl2vary between 65 °C (1︰1), 25 °C (1︰2) and 45 °C(1︰3) [10]. Therefore, choline chloride·2ZnCl2is liquid at room temperature while choline chloride·ZnCl2and choline chloride·3ZnCl2are solid. The viscosity of choline chloride·2ZnCl2is determined to be 281 Pa·s at 298 K, close to the calculated viscosity value about 242 Pa·s at 298 K [21].
3.2 Preparation of biodiesel and analysis
3.2.1 Effect of different molar ratios of choline chloride to ZnCl2
The reaction was conducted while the molar ratio of methanol to soybean oil is 16︰1 at 70 °C for 24 h,48 h, 72 h and 84 h, respectively (see Fig. 4). It is found that choline chloride·xZnCl2efficiently promoted the transesterification. The transesterification is promoted by the Lewis acidic speciesin the catalysts. The yield of biodiesel is slightly enhanced with increasing x from 1 to 3. The conversion of soybean oil increases markedly with time, but there is no significant enhancement after 72 h.Hence, 72 h is chosen as the optimized reaction time.Choline chloride·2ZnCl2is chosen as a typical example of catalyst.
3.2.2 Effect of the amount of choline chloride·2ZnCl2A series of experiments were carried out using the choline chloride·2ZnCl2with different dosages(see Fig. 5). The amount of choline chloride·2ZnCl2is denoted by the IL/oil mass ratio. The results show that with an increase in the relative amount of choline chloride·2ZnCl2, the rate of transesterification reaction is obviously enhanced before 10% and then decreased.The highest conversion is achieved at 10% choline chloride·2ZnCl2. It is very likely that the amount of choline chloride·2ZnCl2higher than 10% would result in a decrease in the catalytic activity and the reason needs to be further investigated.

Figure 5 Effect of choline chloride·2ZnCl2 amount on the transesterification reaction[IL, n(methanol)︰n(oil)=16︰1, 70 °C]● 24 h; ■ 48 h; ▲ 72 h
3.2.3 Effect of the molar ratio of methanol to soybean oil
It is shown that the biodiesel content rapidly increases with increasing molar ratio of methanol to oil(see Fig. 6). Since biodiesel production by transesterification is a reversible reaction, the production yield could be elevated by introducing excess amount of the reactant methanol to change the equilibrium. When the ratio is less than 16, the molar ratio of methanol to oil has a significant effect on the catalytic activity.When methanol is further increased, the concentration of catalyst is diluted at a fixed amount of choline chloride·2ZnCl2and soybean oil, and the amount of methanol has a slight effect on the catalytic performance after 16. Moreover, a higher molar ratio of methanol to oil will cause the separation problem during recycling. Therefore, the optimal molar ratio of methanol to soybean oil of 16 is preferable.
3.2.4 Effect of the reaction temperature
In general, the reaction temperature can influence the reaction rate and biodiesel yield. In present work,the reaction temperature varies within a range from 50 to 90 °C. The experimental results are shown in Fig. 7.The biodiesel yield increases with increasing temperature every 10 °C from 50 to 90 °C. The increasing rates are 13.21%, 7.16%, 2.84% and 4.38%, respectively. The temperature intervals of the increase are equal, but the correspondingly increased biodiesel content is reduced gradually. These results show that the influence of reaction temperature on the transesterification reaction becomes smaller with an increase in temperature. Furthermore, the reaction temperature consumedly exceeds the boiling point of methanol such as 80 and 90°C, and the methanol will quickly vaporize and form a large number of bubbles,which inhibits the reaction on the two-phase interface.Moreover, in order to save energy, it is necessary to choose the relative low temperature. Therefore, the optimum reaction temperature for the transesterification of soybean oil to biodiesel is considered to be around 70°C.
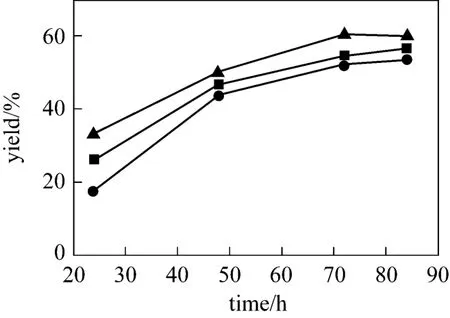
Figure 4 Effect of different molar ratios of choline chloride to ZnCl2[catalyst 10%, n(methanol)︰n(oil)=16︰1, 70 °C]● 1︰1; ■ 1︰2; ▲ 1︰3
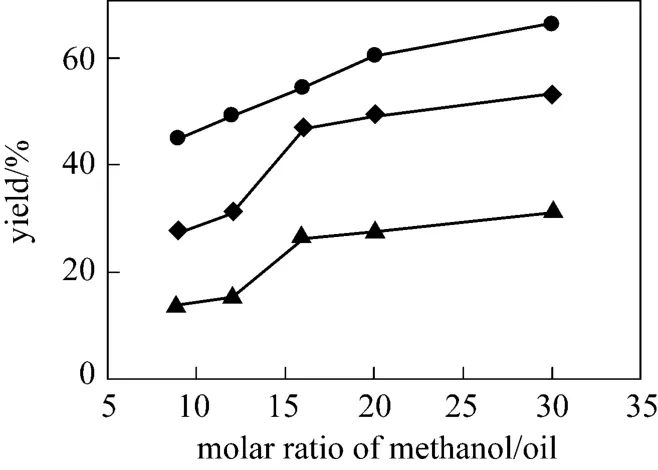
Figure 6 Effect of methanol/soybean oil molar ratio on the transesterification reaction (IL 10%, 70 °C)▲ 24 h; ◆ 48 h; ● 72 h

Figure 7 Effect of reaction temperature on the transesterification reaction[n(methanol)︰n(oil) =16︰1, IL 10%]● 24 h; ■ 48 h; ▲ 72 h
3.2.5 Effect of moisture
The parallel experiment was carried out with and without sealed nitrogen with 16︰1 molar ratio, 70 °C and 72 h. The conversations are 54.52% and 56.14%without and with nitrogen protection, respectively.Therefore, choline chloride·2ZnCl2is insensitive to moisture.
3.3 Problems and analyses
The conversion rate of transesterification using choline chloride·2ZnCl2as catalyst under molar ratio of methanol to oil of 16︰1 with addition of 10%catalyst at 70 °C for 72 h is 54.52%. The reaction mechanism of transesterification is shown in Scheme 2.is the predominant species in IL so that thetransesterification is mainly catalyzed by. The non-ideal yield of biodiesel predominately attributesto the weak acidity of . Therefore, the main method increasing the conversion is to improve the acidity of catalyst. The recycling utilization of choline chloride·2ZnCl2plays an important role in preparation procedure. Choline chloride·2ZnCl2exists in methanol phase with glycerol. Glycerol was separated from ILs by vacuum distillation in the reported literatures[23-27]. However, the high boiling point and high viscosity of glycerol make the separation between ILs and glycerol difficult. Furthermore, the requirement for equipment is high in vacuum distillation, which has not been realized in industry. Therefore, the separation between glycerol and ILs may become the bottleneck problem. Thus, the ILs immobilized on some solid materials such as resin to catalyze transesterification to biodiesel or purification of ILs by solvent extraction are proposed, which will be further studied.
4 CONCLUSIONS
Choline chloride·xZnCl2were applied as Lewis acidic catalysts for transesterification of soybean oil,which extended their application. FT-IR investigation demonstrated that Lewis acid strength of ILs increased with the increase of ZnCl2, which was in agreement with the activities observed in the preparation of biodiesel. Because of the weak acidity of catalyst, the yield of biodiesel was lower than other ILs.
Choline chloride·xZnCl2is a new system with many excellent advantages such as simple preparation and low prices. The preparation process of biodiesel using choline chloride·xZnCl2as Lewis acidic catalysts is effective.
1 Seddon, K.R., “ Ionic liquids for clean technology”, J. Chem. Technol.Biotechnol., 68, 351 (1997).
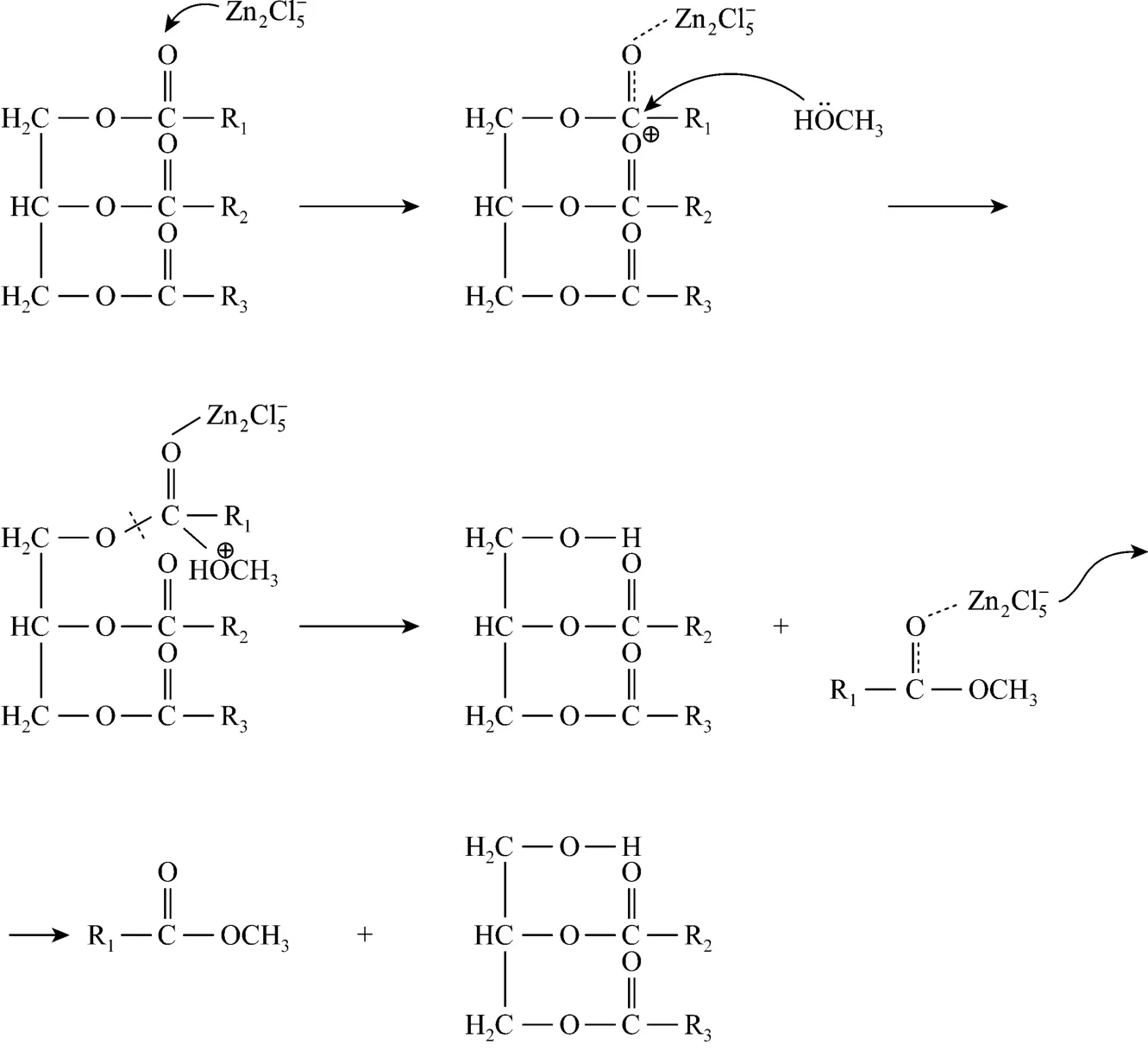
Scheme 2 The reaction mechanism of transesterification
2 Acevedo, O., “Determination of local effects for chloroaluminate ionic liquids on Diel-Alder reactions”, J. Mol. Graphics Modell., 28,95-101 (2009).
3 DeCastro, C., Sauvage, E., Valkenberg, M.H., Hölderich, W.F.,“Immobilised ionic liquids as Lewis acid catalysts for the alkylation of aromatic compounds with dodecene”, J. Catal., 196, 86-94 (2000).
4 Shen, Z.L., He, X.J., Mo, W.M., Xie, Y., Hu, B.X., Sun, N., “Synthesis of α-hydroxy esters by glyoxylate-ene reaction in lewis acid chloroaluminate ionic liquids”, Chin. J. Catal., 27 (3), 197-199 (2006).
5 Kumar, A., Pawar, S.S., “The DABCO-catalysed Baylis-Hillman reactions in the chloroaluminate room temperature ionic liquids: rate promoting and recyclable media”, J. Mol. Catal. A: Chem., 211, 43-47(2004).
6 Boon, J.A., Levisky, J.A., Pflug, J.L., Wilkes, J.S., “Friedel crafts reactions at ambient-temperature molten-salts”, J. Org. Chem., 51(58), 480-483 (1986).
7 Lee, C.W., “Diels-Alder reactions in chloroaluminate ionic liquids:acceleration and selectivity enhancement”, Tetrahedron Lett., 40,2461-2464 (1999).
8 Tang, J., Osteryoung, R., “Formation and electrochemistry of polyaniline in ambient-temperature molten salts”, Synth. Met., 45 (11),1-13 (1991).
9 Kumar, A., Pawar, S.S., “Catalyzing Henry reactions in chloroaluminate ionic liquids”, J. Mol. Catal. A: Chem., 235, 244-248 (2005).
10 Abbott, A.P., Capper, G., Davies, D.L., Munro, H.L., Rasheed, R.K.,Tambyrajah, V., “Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains”, Chem. Commun., 2010-2011 (2001).
11 Abbott, A.P., Capper, G., Mckenzie, K.J., Ryder, K.S., “Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride”, J. Electroanal. Chem., 599, 288-294 (2007).
12 Morales, R.C., Tambyrajah, V., Jenkins, P.R., Davies, D.L., Abbott,A.P., “The regiospecific Fischer indole reaction in choline chloride·2ZnCl2with product isolation by direct sublimation from the ionic liquid”, Chem. Commun., 158-159 (2004).
13 Duan, Z.Y., Gu, Y.L., Deng, Y.Q., “Green and moisture-stable Lewis acidic ionic liquids(choline chloride·xZnCl2) catalyzed protection of carbonyls at room temperature under solvent-free conditions”, Catal.Commun., 7, 651-656 (2006).
14 Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., Tambyrajah,V., “Quaternary ammonium zinc-or tin-containing ionic liquids: water insensitive, recyclable catalysts for Diels-Alder reactions”, Green Chem., 4, 24-26 (2002).
15 Earle, M.J., Seddon, K.R., Plechkova, N.V., “Production of bio-diesel”, Eur. Pat., EP1866086 (2006).
16 Zhang, S.J., Sun, J., Zhang, J.M., “The preparation of biodiesel based on ionic liquid”, CN Pat., 200510082972.0 (2005).
17 Wu, Q., Chen, H., Han, M.H., Wang, D.Z., Wang, J.F., “Transesterification of cottonseed oil catalyzed by Brønsted acidic ionic liquids”,Ind. Eng. Chem. Res., 46, 7955-7960 (2007).
18 Parry, E.P., “An infrared study of pyridine adsorbed on acidic solids,characterisation of surface acidity”, J. Catal., 2, 371-379 (1963).
19 Wu, Q., Dong, B.Q., Han, M.H., Xin, H.L., Jin, Y., “Studies on acidity of chloroaluminate ionic liquids using pyridine as infrared spectroscopic probe” , Chin. J. Anal. Chem., 9 (34), 1323-1326 (2006).
20 Bourne, K.H., Cannings, F.R., Pitkethly, R.C., “Structure and properties of acid sites in a mixed-oxide system (I) Synthesis and infrared characterization”, J. Phys. Chem., 74 (10), 2197-2205 (1970).
21 Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., “Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures”, Inorg. Chem., 43, 3447-3452 (2004).
22 Wang, X.H., Tao, G.H., Wu, X.M., Kou, Y., “Investigation of the acidity of ionic liquids by IR spectroscopy”, Acta Phys. Chim. Sin.,21 (5), 528-533 (2005). (in Chinese)
23 Wu, Q., Chen, H., Han, M.H., “Preparation of biodiesel oil from cottonseed oil catalyzed by ionic liquids”, Petrochem. Technol., 35(6), 583-586 (2006). (in Chinese)
24 Wang, W.K., Bao, Z.H., “Preparation of biodiesel from soybean oil catalyzed by aluminum chloride-based ionic liquid”, China Oils and Fats, 32 (9), 51-53 (2007). (in Chinese)
25 Yi, W.L., Han, M.H., Wu, Q., Jin, Y., “Preparation of biodiesel from waste oil catalyzed with Brønsted acid ionic liquid”, Chin. J. Process. Eng., 8 (6), 1144-1148 (2007). (in Chinese)
26 Li, H.P., Wang, Q.Y., Lan, X.Q., Wang, X., Song, H., “Preparation of biodiesel from rapeseed oil catalyzed by ionic liquid [Hmim]HSO4”,China Oils and Fats, 33 (4), 57-59 (2008). (in Chinese)
27 Han, M.H., Yi, W.L., Wu, Q., Hong, Y.C., Wang, D.Z., “Preparation of biodiesel from waste oils catalyzed by a Brønsted acidic ionic liquid”, Bioresour. Technol., 100, 2308-2310 (2009).
2009-07-14, accepted 2009-11-11.
* Supported by the National High Technology Research and Development Program of China (2007AA06Z202), the National Key Technology Research and Development Program of China (2006BAC02A10), and the Distinguished Young Scholars Foundation of Jilin Province (20060114).
** To whom correspondence should be addressed. E-mail: jchen@ciac.jl.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
