Electrocatalytic Activity of Tungsten Carbide and Natural Zeolite Composite in Aqueous Solution*
2012-10-31CHENGYuan程媛XIEWeimiao谢伟淼YAOGuoxin姚国新HUSujuan胡素绢andLIGuohua李国华
CHENG Yuan (程媛), XIE Weimiao (谢伟淼), YAO Guoxin (姚国新), HU Sujuan (胡素绢) and LI Guohua (李国华),2,3,**
1 School of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China 2 State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Hangzhou 310032, China 3 Research Center of Nanoscience and Technology, Zhejiang University of Technology, Hangzhou 310032, China
Electrocatalytic Activity of Tungsten Carbide and Natural Zeolite Composite in Aqueous Solution*
CHENG Yuan (程媛)1, XIE Weimiao (谢伟淼)1, YAO Guoxin (姚国新)1, HU Sujuan (胡素绢)1and LI Guohua (李国华)1,2,3,**
1School of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China2State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Hangzhou 310032, China3Research Center of Nanoscience and Technology, Zhejiang University of Technology, Hangzhou 310032, China
Tungsten carbide and zeolite nanocomposite was prepared by combining a mechanochemical approach with a reduction and carbonization approach, using natural zeolite and ammonia metatungstate as precursors. The sample was characterized by X-ray diffraction and scanning electron microscope. The results showed that the crystal phase of the sample is composed of zeolite, monotungsten carbide and bitungsten carbide. The mass percentage and the crystallite diameter of tungsten carbide change along with the reacted time. Its electrocatalytic activity was measured with a microelectrode system with three electrodes. The results show that its electrocatalytic property is related to its crystal phase and the mass percentage of tungsten carbide, and its electrocatalytic activity is connected with the property of electrolyte, in which it is measured. Synergistic effect between tungsten carbide and zeolite is found during electrocatalysis.
tungsten carbide, zeolite, nanocomposite, electrocatalytic activity, synergistic effect
1 INTRODUCTION
Hydrogen ionization is an important electrochemical reaction in fuel battery [1, 2]. In order to realize the practical application of fuel battery at a large scale, the key problem of hydrogen ionization in fuel battery is to find an approach to solve the problem of catalyst poisoning and to reduce the dosage of rare noble metal, such as platinum [3, 4]. Tungsten carbide bears an analogy of the catalytic capability of platinum [5-7]. In chemical catalysis, it can be used as catalysts in hydrogenation, dehydrogenation, isomerization, and transformation and synthesis of hydrocarbon compounds [8]. In electrocatalysis, it can be used as catalysts in hydrogen ionization and hydrogen engenderation [9, 10]. Monotungsten carbide (WC) owns a promising future in these areas for its typical catalytic property, poisoning resistance, and potential to replace rare noble metal catalyst, such as platinum.Despite of all these, its electrocatalytic activity is much lower than that of platinum, and how to improve its catalytic activity is one of the hot topics.
Composite is one of the efficacious approaches to improve the catalytic activity of catalyst. When metal complexes and clusters bond on oxide or zeolite supports, they may combine the technological advantages of solid catalysts with the selectivity of soluble molecular catalysts [11, 12]. As catalyst supports, the advantages of zeolite and zeolite-type materials are associated with their unique crystal structural geometries and compositions, and the advantages of the composite based on zeolite and zeolite-type materials are also related to metal ions, oxides, clusters and complexes that modify on the surfaces of the materials [13, 14].NiMo catalyst supported on zeolite is active to thiophene hydrodesulfurization [15]. Zeolite composite with a high mass percentage of CeO2H is an excellent selective catalyst for catalytic reduction [16].These results indicate that zeolite is one of the excellent catalyst supports. Stable structure, preserved crystallinity, high electropositivity of the alkai earth oxides, well-defined crystal phases and high accessibility of alkali sites to the reagents are the main characteristic features to an active X zeolite catalyst for its oxidative methylation of toluene with methane [17].The initial isomerization and ring-opening rate of zeolite protonated by platinum are found to be enhanced[18]. These results imply that zeolite can improve the catalytic property of platinum. As tungsten carbide bears an analogy of the catalytic capability of platinum [5-7], it is rationalized to believe that the catalytic capability of tungsten carbide will be enhanced when composited with zeolite.
Based on the inspiration of the above results,WC/zeolite composite was prepared by an impregnation approach, using natural zeolite and ammonia tungstate as precursors, and its electrocatalytic activity for p-nitrophenol in alkali solution was reported by our group [19]. In this paper, the preparation of WC/zeolite nanocomposite, using natural zeolite as a support and ammonia metatungsten as tungsten source was reported, and its electrocatalytic reduction activity for p-nitrophenol was investigated systemically by cyclic voltammetry. The synergistic effect between tungsten carbide and zeolite in the composite was reported for the first time.
2 EXPERIMENTAL
2.1 Precursor preparation
Natural zeolite was milled by a planetary ball miller at a speed of 500 r·min-1for 8 h, and dried at 393 K in air overnight. The zeolite was mixed with ammonia metatungstate at different molar ratios of Si︰W,and the mixture was milled by the miller at a speed of 400 r·min-1for 4 h and dried at 393 K overnight. In this way, WO3/zeolite precursor was prepared mechanochemically.
2.2 Sample preparation
5 g of the precursor was put into a quartz boat,which was sent into a tubal resistance furnace. After pure nitrogen gas passed through the furnace at a flow rate of 100 ml·min-1for 30 min, a mixture gas at a volume ratio of CH4to H21︰9 passed through the furnace for 15 min, and the precursor was reduced and carbonized at 900 °C for different times under the atmosphere of the mixture gas in the furnace. During this process, the exhaust fume was disposed by passing through 5.0 mol·L-1NaOH solution. After the furnace cooled down to room temperature under the protection of pure nitrogen gas, the quartz boat was drawn out from the furnace to get the final product.
2.3 Sample characterization
The crystal phase, morphology and chemical components of the sample were characterized by X-ray diffraction (XRD) and scanning electron microscope (SEM). XRD was performed with a Thermo ARL SCINTAG X’TRA (PANalytical, Netherlands)X-ray at room temperature, using Cu K1radiation source (λ0.154 nm) at a potential of 15 kV and with a current of 40 mA. XRD patterns were recorded from 5◦ to 75◦, or 5◦ to 85◦ with a step size of 0.04° and at a speed of 2.4(°)·min-1. SEM was carried out on a Hitachi S-4700II (Hitachi, Japan) equipped with X-ray energy dispersion spectroscopy analyzer (EDS, Cambridge).
2.4 Electrochemical property measurement
The electrochemical property of the sample was measured with an EG&G M273A potentiostate/galvanostate with a microelectrode system at room temperature (298 K), which was stated in detail in Ref.[20]. A saturated calomel electrode (SCE) was used as the reference electrode and a platinum sheet (1.0 cm2)was used as the counter electrode. Sodium hydrate(A.R.), hydrogen sulfate (A.R.), sodium chloride(A.R.) and p-nitrophenol (A.R.) were distilled with deionized water, which was obtained from a Millipore-Milli-Q system before use.
3 RESULTS AND DISCUSSION
3.1 XRD
Figure 1 shows the XRD results of natural zeolite,the precursor and the samples. The crystal phase of natural zeolite is composed of mordenite (JCPDS: 080-0642),clinoptilolit (JCPDS: 071-1425) and quartz (JCPDS:085-0457), as curve 1 shown in Fig. 1, and that of the precursor is composed of mordenite, clinoptilolit, quartz and tungsten trioxide (WO3) (JCPDS: 087-2385), as curve 2 shown in Fig. 1. The crystal phases of the samples are composed of mordenite, clinoptilolit,quartz, bitungsten carbide (W2C) (JCPDS: 089-2371)and monotungsten carbide (WC) (JCPDS: 089-2371),as curves 3, 4, 5, and 6 shown in Fig. 1. Their mass percentages of W2C and WC, and ratios of W2C to WC were estimated by the interior software of the Thermo ARL SCINTAG X’TRA X-ray based on XRD data, and the results are shown in Table 1. These results show that their percentages of WC and W2C change along with the reduction and carbonization time, and so that the ratios of WC to W2C, as shown in Table 1.
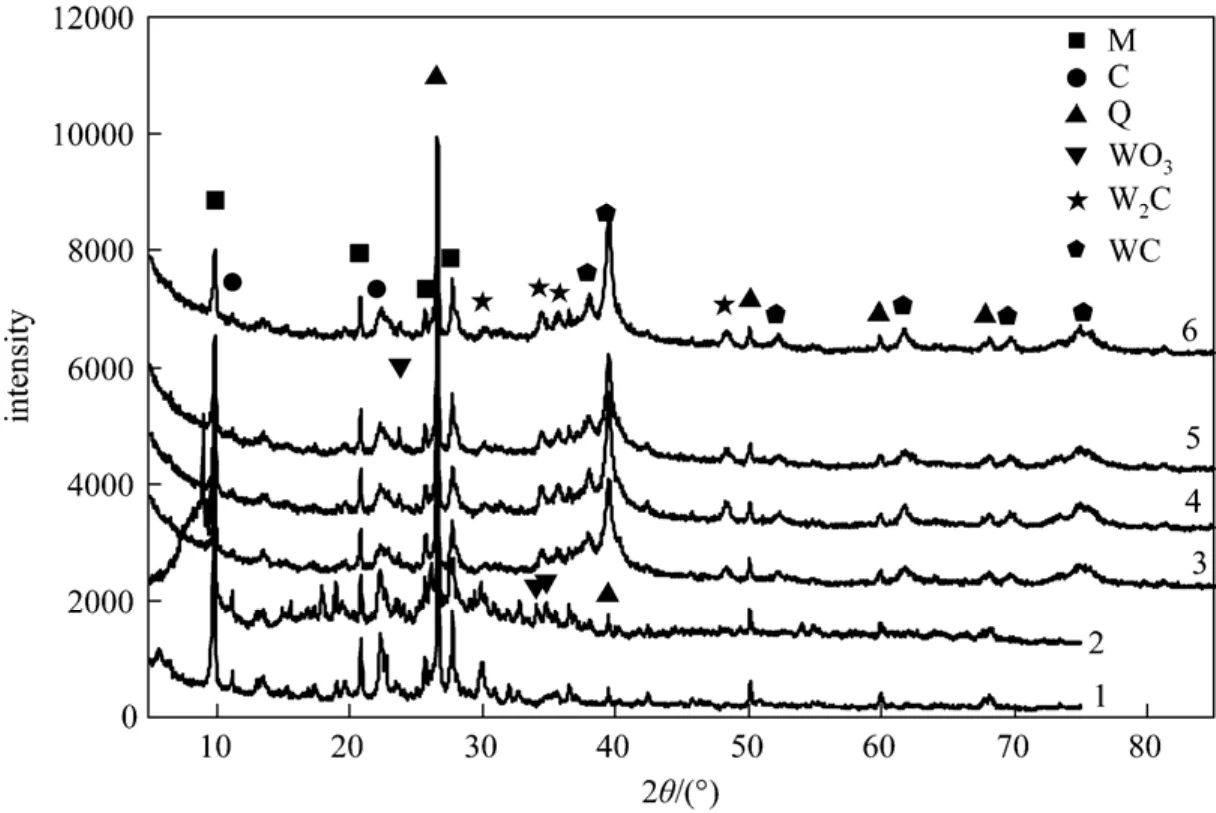
Figure 1 XRD patterns of the samples1—natural zeolite; 2—precursor; the samples reacted for different times: 3 (2 h), 4 (4 h), 5 (6 h), 6 (8 h); M—mordenite; C—clinoptilolit; Q—quartz; WO3—tungsten trioxide; WC—monotungsten carbide; W2C—bitungsten carbide
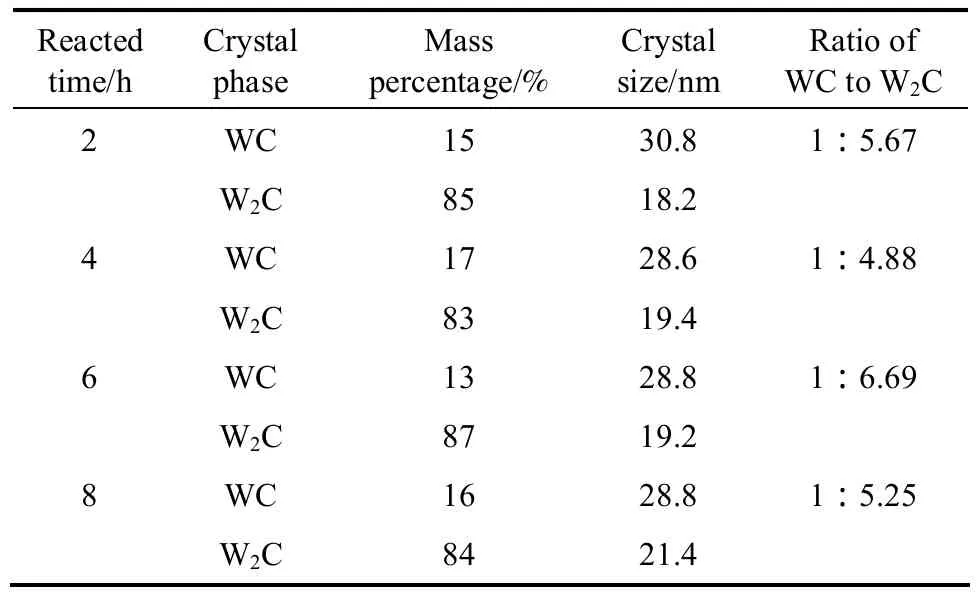
Table 1 The crystal phase, the content and the ratio of WC/W2C, and the crystal size of the samples reacted for different times
The crystallite size estimated from the XRD data is shown in Table 1. The diameter of WC crystallite is 30.8 nm when the reacted time is 2 h, then stabilized at around 28.8 nm as the reacted time increasing from 4 h to 8 h. The diameter of W2C crystallite increases from 18.2 nm to 21.4 nm as the reacted time increasing from 2 h to 8 h.
From Fig. 1, natural zeolite is composed of mordenite, clinoptilolit and quartz, and their diffraction peaks appear in all the curves. These results indicate that natural zeolite is stable during the preparation process, and it is a suitable support for tungsten carbide catalyst.
3.2 SEM
Figure 2 displays images of the surface structure and the morphology of the precursor and the samples.Image 2 (a) is the morphology of the precursor. Some smaller particles decorate on the surface of a larger particle. Compared to its XRD result, the smaller particles is tungsten trioxide, and the larger particle is zeolite. Image 2 (b) shows the morphology of the sample reacted for 4 h. Its morphology is similar to that of the precursor, but their surface structures are different. In image 2 (a), the detailed surface structure of zeolite is clear, while in image 2 (b), the detailed surface structure of the larger particle is not clear,which is covered by a layer with some smaller particles of different diameter. Compared to its XRD result,the smaller particles is tungsten carbide, and the larger particle is zeolite. Image 2 (c) shows the morphology of the sample reacted for 6 h, which is different from that for 4 h. Compared to its XRD result, the smaller particles is tungsten carbide. Image 2 (d) shows that reacted for 8 h. The comparison of the samples reacted for 4 h and 6 h indicates that their morphologies are different, and the diameter of tungsten carbide particle increases apparently, and the congregation exists heavily. The comparison between images 2 (b), 2 (c)and 2 (d), the diameter of tungsten carbide increases along with the reacted time, and congregating phenomenon becomes more serious as the reacted time increases.
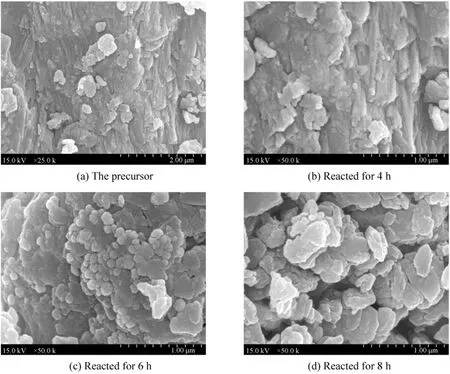
Figure 2 SEM images of the samples
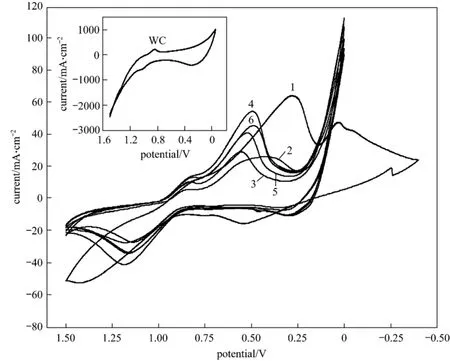
Figure 3 Cyclic voltammograms of the samples in 0.1 mol·L-1 PNP + 0.5 mol·L-1 H2SO4 (scan rate: 100 mV·s-1)1—natural zeolite; 2—WO3 + natural zeolite; 3—sample 1 (2 h); 4—sample 2 (4 h); 5—sample 3 (6 h); 6—sample 4 (8 h)
3.3 Electrocatalytic activity
3.3.1 Acidic environment
The cyclic voltammograms of the samples in 0.1 mol·L-1PNP + 0.5 mol·L-1H2SO4solution is shown in Fig. 3, and that of pure monotungsten carbide (WC) is shown in the inset of Fig. 3. From the inset, except for one strong hydrogenation peak and one strong oxygenation peak, the curve of cyclic voltammogram of WC displays one obvious reduction peak, and one weak oxidation peak. The curve of cyclic voltammogram of natural zeolite, as curve 2 shown in Fig. 3,displays two clear reduction peaks. The curve of cyclic voltammogram of the precursor, WO3+ natural zeolite, displays two reduction peaks: one is a weak peak, another is at a large potential spanning from 0.37 V to 0.60 V, without an evident top. The curves of cyclic voltammogram of the samples show two obvious reduction peaks and one obvious oxidation peak, and their values of potential and current are shown in Table 2.
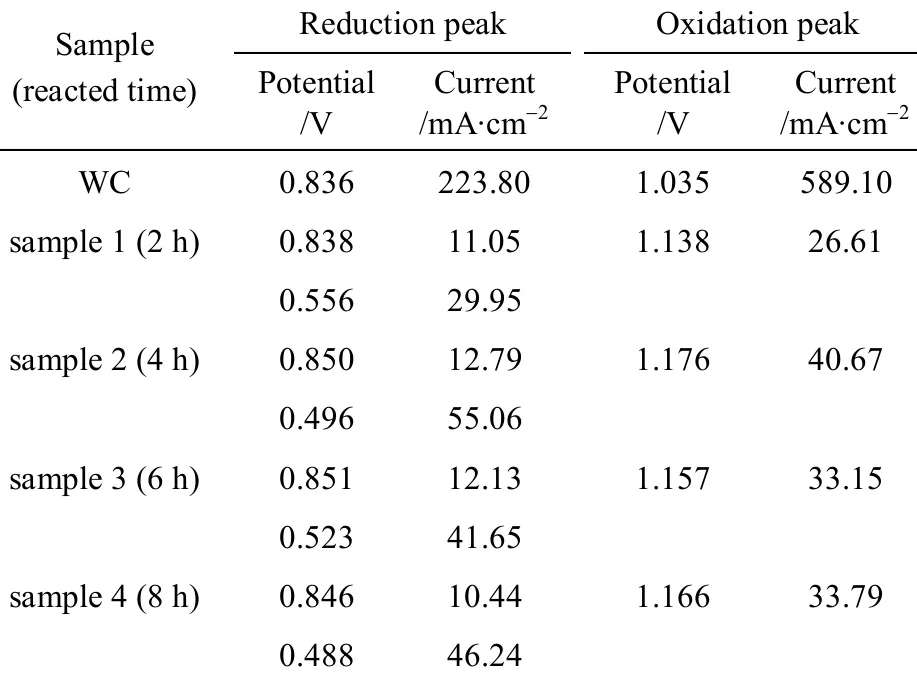
Table 2 The peak potentials and peak currents of the samples in acidic solution
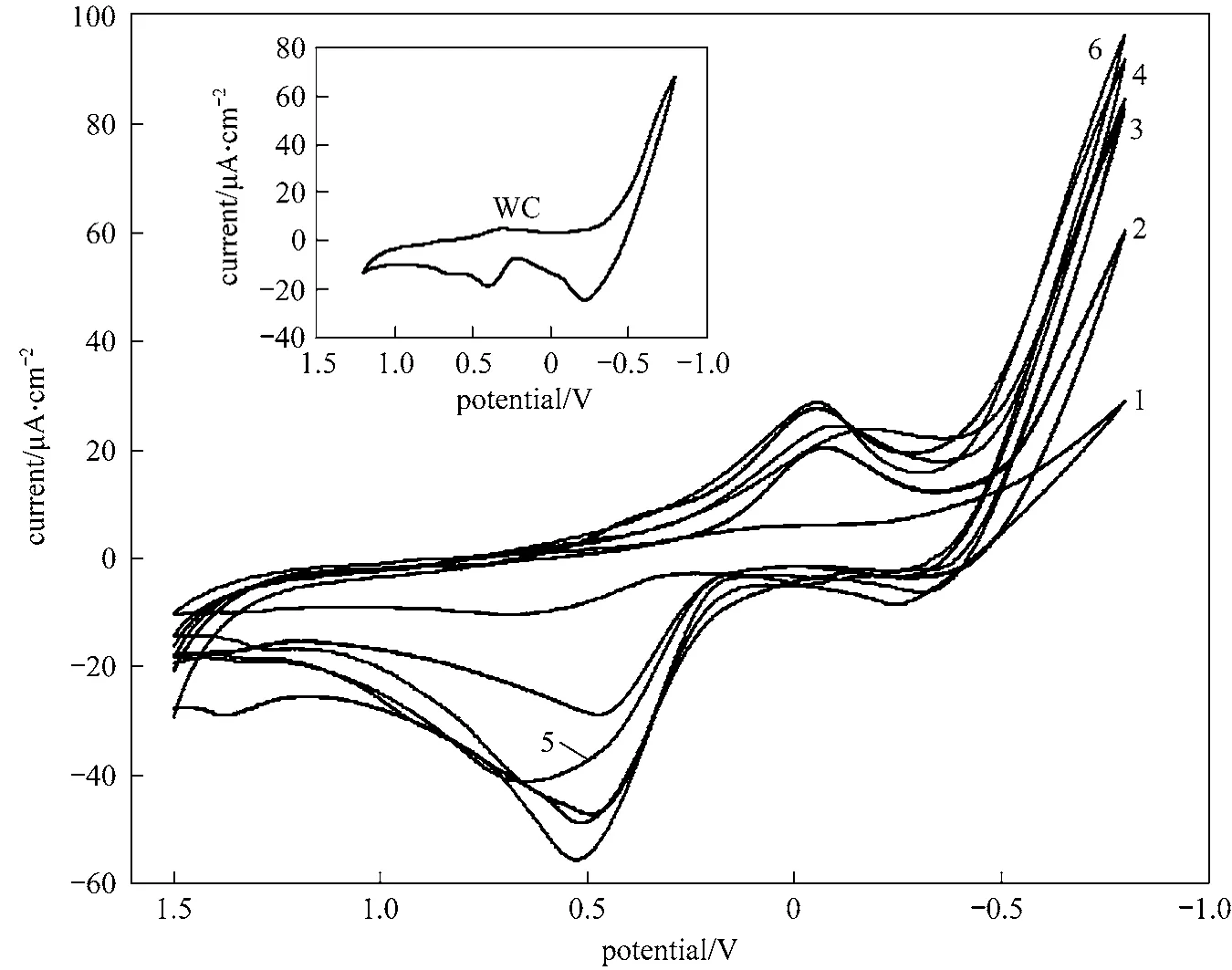
Figure 4 Cyclic voltammograms of the samples in 0.1 mol·L-1 PNP + 0.5 mol·L-1 NaCl (scan rate: 100 mV·s-1)1—natural zeolite; 2—WO3 + natural zeolite; 3—sample 1 (2 h); 4—sample 2 (4 h); 5—sample 3 (6 h); 6—sample 4 (8 h)
From Table 2, the potentials of the first reduction peak of the samples are more positive than that of WC,0.836 V. This result shows that the electrocatalytic activity of the samples is better than that of WC. The currents of the first reduction peak of the samples are much lower than that of WC. This can be attributed to the conductivity of the crystal phase of the samples.The crystal phases of the samples are composed of mordenite, clinoptilolit, quartz, W2C and WC, in which, W2C and WC belong to conductor and their conductivity is good. Mordenite, clinoptilolit and quartz are nonconductor, their conductivities are very weak. After tungsten carbide decorated on the surface of zeolite support, an interface lies between tungsten carbide and zeolite. This interface will increase the resistance of the composite and decrease the conductivity of the composite. These factors will result in the current of the first reduction peak of the samples much lower than that of WC, and the current of the second reduction peak of the samples lower than that of zeolite.
From Table 2, the potentials of the second reduction peak of the samples reacted for 2 h, 4 h, 6 h and 8 h are 0.556 V, 0.496 V, 0.523 V and 0.488 V, respectively, which are more positive than that of natural zeolite and the precursor of the sample. These results indicate that the electrocatalytic activities of the samples are higher than that of natural zeolite and the precursor of the sample.
Based on the above results, it is concluded that the electro-catalytic activities of tungsten carbide and zeolite for p-nitrophenol are improved by decorating tungsten carbide on the surface of zeolite, zeolite is stable and suitable support for tungsten carbide electrocatalytic catalyst. A synergistic effect between tungsten carbide and zeolite exists.
3.3.2 Neutral environment
Figure 4 shows cyclic voltammograms of the samples in 0.1 mol·L-1PNP + 0.5 mol·L-1NaCl solution, and that of pure monotungsten carbide (WC) is shown in the insetted figure of Fig. 4.
From the inset of Fig. 4, except for a strong
hydrogenation peak, the curve of cyclic voltammogram of WC does not show any obvious reduction peak, but shows one obvious oxidation peak at a potential of 0.897 V and with a current of 47.43 μA·cm-2.The curve of cyclic voltammogram of natural zeolite(curve 1 in Fig. 4) does not display any obvious reduction and oxidation peak. The curve of cyclic voltammogram of the precursor, WO3+ natural zeolite,displays one obvious reduction peak at a potential of -0.076 V and with a current of 20.57 μA·cm-2, and one obvious oxidation peak at a potential of 0.468 V and with a current of 29.01 μA·cm-2, as curve 2 shown in Fig. 4. The curves of cyclic voltammograms of the samples reacted for different times show one obvious reduction peak and one obvious oxidation peak, their potentials and currents are shown in Table 3. Beside,the curve of cyclic voltammogram of the sample reacted for 4 h shows another weak reduction peak and another weak oxidation peak, as shown in Table 3.
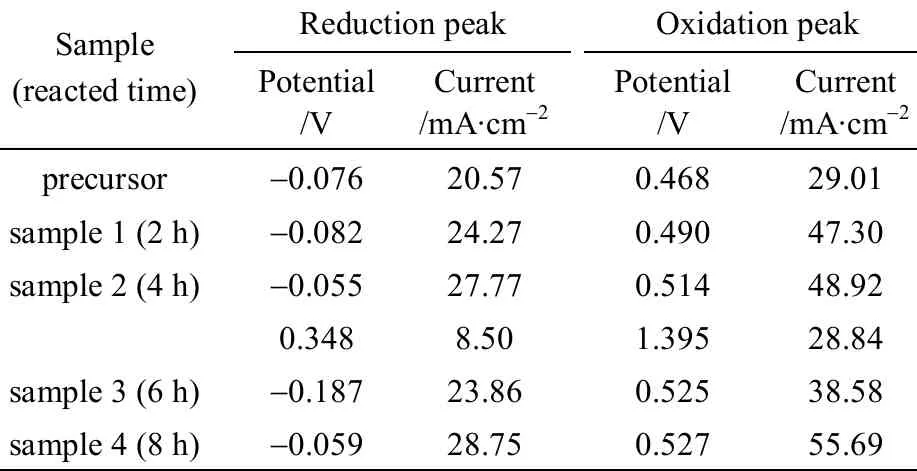
Table 3 The peak potentials and peak currents of the samples in neutral solution
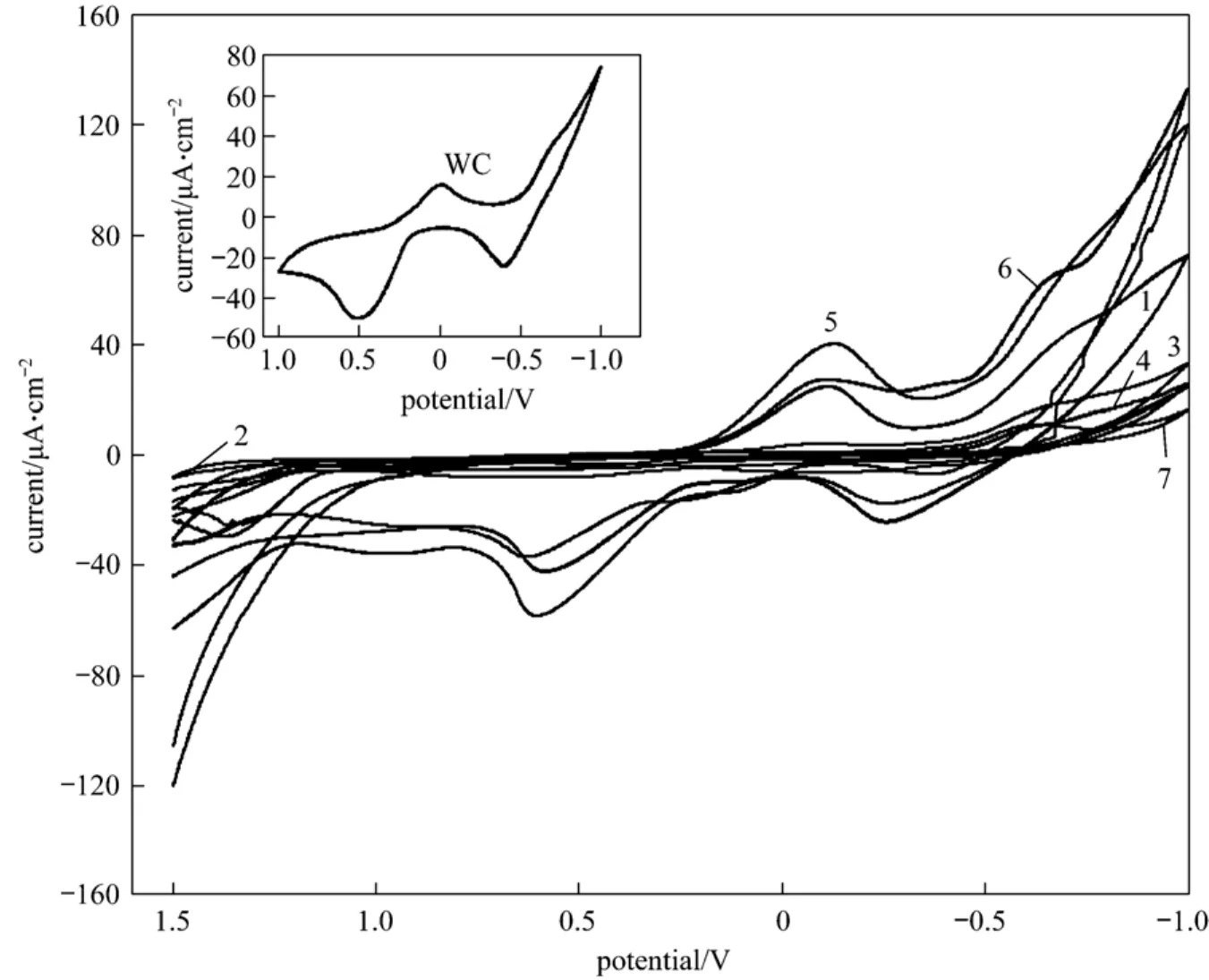
Figure 5 Cyclic voltammograms of the samples in 0.1 mol·L-1 PNP + 0.5 mol·L-1 NaOH (scan rate: 100 mV·s-1)1—hollow porous WC; 2—natural zeolite; 3—WO3 + natural zeolite; 4—sample 1 (2 h); 5—sample 2 (4 h); 6—sample 3 (6 h); 7—sample 4 (8 h)
From the insetted figure of Fig. 4, the curve of the cyclic voltammogram of WC does not display any reduction peak in neutral solution. This indicates that WC does not exhibit any electro-reduction activity for p-nitrophenol in neutral solution. The curves of the cyclic voltammograms of the samples display one or two reduction peaks in neutral solution. This indicates that the samples are electro- reduction active for p-nitrophenol. Based on these results, one conclusion can be drawn that the electro-reduction activity of the composite is higher than that of WC in neutral environment, and a synergistic effect between tungsten carbide and zeolite in the composite exists.
3.3.3 Alkali environment
Figure 5 shows cyclic voltammograms of the samples in 0.1 mol·L-1PNP + 0.5 mol·L-1NaOH solution, and that of pure monotungsten carbide (WC) is shown in the insetted figure of Fig. 5.
From the insetted figure of Fig. 5, beside one strong hydrogenation peak, the curve of cyclic voltammogram of WC shows one obvious reduction peak at a potential of 0.005 V and with a current of 16.23 μA·cm-2, and shows one obvious oxidation peak at a potential of 0.514 V and with a current of 50.44 μA·cm-2.The curve of cyclic voltammogram of hollow porous WC [21] shows one obvious reduction peak at a potential of -0.116 V and with a current of 24.87 μA·cm-2,and shows two obvious oxidation peaks at potentials of -0.256 V and 0.584 V, and with currents of 24.41 μA·cm-2and 42.44 μA·cm-2, as curve 1 shown in Fig. 5. The curve of cyclic voltammogram of natural zeolite, as curve 2 shown in Fig. 5, does not display any obvious reduction peaks and oxidation peaks. The curve of cyclic voltammogram of the precursor, WO3+natural zeolite, does not display any obvious reduction peak and oxidation reduction peak, as curve 3 shown in Fig. 5. The curves of cyclic voltammograms of the samples reacted for 2 h and 8 h do not show any obvious reduction peak and oxidation peak, as curve 4 and 7 shown in Fig. 5. That of the sample reacted for 4 h shows one obvious reduction peak and one oxidation peak, as curve 5 shown in Fig. 5. That of the sample reacted for different 6 h shows one obvious reduction peak and one oxidation peak, as curve 6 shown in Fig. 5. The values of the currents and the potentials of these peaks are shown in Table 4.
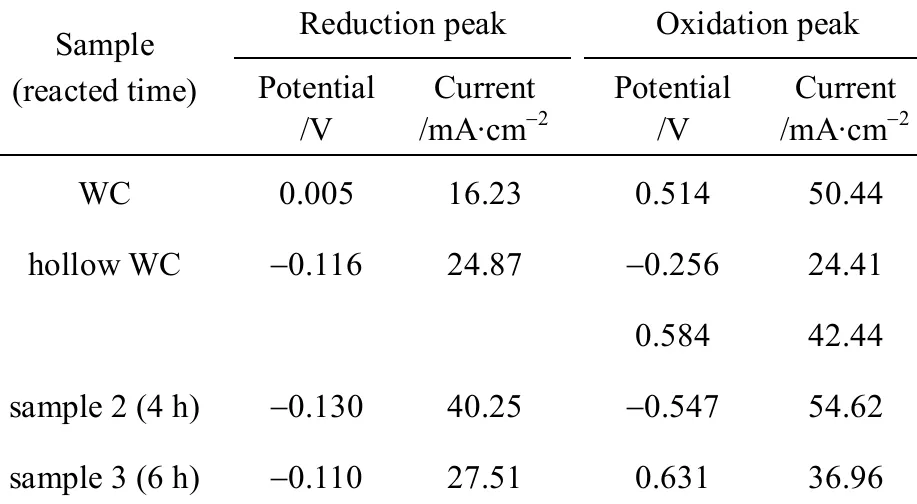
Table 4 The peak potentials and peak currents of the samples in basic solution
From Table 4, the reduction potential of the sample for 4 h is more negative than that of WC and hollow porous WC. This indicates that the electrocatalytic activity of the sample reacted for 4 h is higher than that of WC and hollow porous WC in alkali environment. The reduction potential of the sample reacted for 6 h is more negative than that of WC, and is more positive than of hollow porous WC. This indicates that the electrocatalytic activity of the sample reacted for 6 h is higher than that of WC, but is lower than that of hollow porous WC in alkali solution.
3.3.4 Silica to tungsten ratio
Figure 6 displays the cyclic voltammograms of the samples prepared with different Si︰W molar ratios, 1︰1, 4︰3, 2︰1 and 4︰1. The cyclic voltammograms of the samples for p-nitrophenol in acidic solution show two reduction peaks and one oxidation peak, as shown in Fig. 6 (a). Their peak potentials and currents of the first reduction peak are almost the same, and their peak potentials are more positive than that of WC in acidic solution, 0.836 V. This result indicates that the electrocatalytic activities of the samples are improved by the formation of the composite.The peak potentials and currents of the second reduction peaks of the samples with different Si︰W molar ratios of 1︰1, 4︰3, 2︰1 and 4︰1, are 0.493 V and 29.34 μA·cm-2, 0.495 V and 38.94 μA·cm-2, 0.516 V and 27.05 μA·cm-2and 0.516 V and 27.05 μA·cm-2,respectively. The more positive the peak potential is, the higher the electrocatalytic property. These results show that the electro-catalytic property of the sample with different Si︰W molar ratio is 2︰1 > 4︰1 > 4︰3 >1︰1, which indicates that 2︰1 is the most suitable Si︰W ratio in the composite for p-nitrophenol electro-reduction catalysis in acidic solution.
The cyclic voltammograms of the samples for p-nitrophenol in neutral solution are shown in Fig. 6(b). The sample prepared with the Si︰W ratio of 1︰1 does not display any obvious reduction peak. That with the Si︰W molar ratio of 4︰3 displays one reduction peak with long span. That with Si︰W molar ratios of 2︰1 and 4︰1 display one obvious reduction peak, at the same peak potential of -0.123 V, and with peak currents of 26.56 μA·cm-2and 23.28 μA·cm-2,respectively. These results indicate that only the samples with a Si︰W molar ratio bigger than 2︰1 show electrocatalytic activity for p-nitrophenol in neutral solution evidently.
The cyclic voltammograms of the samples for p-nitrophenol in alkali solution are shown in Fig. 6 (c).The sample prepared with the Si︰W ratio of 1︰1 does not display any obvious reduction peak. That with Si︰W molar ratios of 4︰3 and 4︰1 display one reduction peak with long span. That with the Si︰W molar ratio of 2︰1 displays one obvious reduction peak, at a peak potential at -0.081 V and with a peak current of 30.96 μA·cm-2. These results indicate that only the sample prepared at the Si︰W molar ratio of 2︰1 shows electrocatalytic activity for p-nitrophenol in alkali solution evidently.
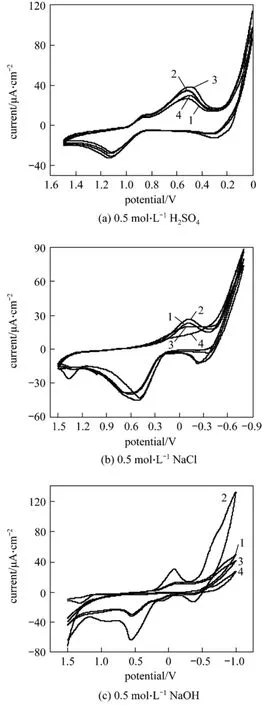
Figure 6 Cyclic voltammograms of the samples with different Si︰W ratios for PNP in 0.5 mol·L-1 H2SO4 (a), 0.5 mol·L-1 NaCl (b) 0.5 mol·L-1 NaOH (c) solutions (scan rate:100 mV·s-1)Si︰W ratio: 1—4︰1; 2—2︰1; 3—4︰3; 4—1︰1
Based on the above results and discussion, one conclusion can be drawn that the Si︰W molar ratio of 2︰1 is the most suitable ratio for electrocatalytic reduction of p-nitrophenol in the composite as our experiments are concerned.
4 CONCLUSIONS
The comparison of cyclic voltammogram of WC in Figs. 3-5, the potential of reduction peak of WC in Fig. 3 is more positive than that in Fig. 5, and there is no obvious reduction peak in Fig. 4. These results indicate that its electrocatalytic activity for p-nitrophenol in acidic solution is higher than that in alkali solution, and it does not show evident electrocatalytic activity for p-nitrophenol in neutral solution.These imply that its electrocatalytic activity is related to the property of electrolyte, in which it is measured.
The comparison of the cyclic voltammograms of the samples reacted for 2 h and 8 h in Figs. 3-5, their potentials of reduction peaks in Fig. 3 are more positive than that in Fig. 4, and there is no obvious reduction peak in Fig. 5. These results indicate that their electrocatalytic activities for p-nitrophenol in acidic solution are higher than in neutral solution, and they do not show evident electrocatalytic activity for p-nitrophenol in alkali solution. These imply that its electrocatalytic activity of the samples is related to the property of electrolyte, in which it is measured. This is consistent with that of WC.
The comparison of the cyclic voltammograms of the sample reacted for 4 h in Figs. 3-5, its potential of reduction peaks in Fig. 3 is the most positive, that in Fig. 4 is the second, and that in Fig. 5 is the most negative. These results indicate that its electrocatalytic activity for p-nitrophenol in acidic solution is the highest, that in neutral solution is the second, and that in alkali solution is the lowest. These imply that its electrocatalytic activity is connected with the property of environment, in which the sample was measured.
The comparison of the cyclic voltammogram of the sample reacted for 6 h in Figs. 3-5, its potential of reduction peaks in Fig. 3 is the most positive, that in Fig. 5 is the second positive, and that Fig. 4 is the most negative. These results indicate that its electro-catalytic activity for p-nitrophenol in acidic solution is the highest, that in alkali solution is the second,and that in neutral solution is the lowest. These imply that its electrocatalytic activity is related to the property of electrolyte, in which the samples was measured.
Based on the above results and discussion, the following conclusion can be drawn that the electrocatalytic activities of WC and the samples are connected with the property of electrolyte, in which they were measured. Furthermore, their electrocatalytic activities for p-nitrophenol are the highest in acidic solution.
In summary, tungsten carbide and zeolite composite can be prepared by combining a mechanochemical approach with a reduction and carbonization approach,using natural zeolite as a support. Its electroreduction property is connected to its crystal phase and mass percentage of tungsten carbide, molar ratio of catalyst to support, and affected by electrolyte property. Natural zeolite is an excellent electrocatalyst support for tungsten carbide. A synergistic effect between tungsten carbide and zeolite exists.
1 Burstein, G.T., Barnett, C.J., Kucernak, R.J., “Anodic oxidation of methanol using a new base electrocatalyst”, Electrochem. Soc, 143(7), 129-134 (1996).
2 Fleischmann, R., Boehm, H., “Hydrogen oxidation on different tungsten carbide materials”, Electrochim. Acta, 20 (10), 1123-1128(1977).
3 Sajo, P.N., Fernandes, J.B., “Ammonolysis of ethanol on pure and zinc oxide modified HZSM-5 zeolites”, Appl. Catal. A Gen., 205,195-199 (2001).
4 Levy, R.B., Boudar, M.T., “Platinum-like behavior of tungsten carbide in surface catalysis”, Science, 181, 547-548 (1973).
5 BÖhm, H., “New non-noble metal anode catalysts for acid fuel cells”, Nature, 227, 484-485 (1970).
6 Xue, H.X., Song, G.X., Wang, L., Chen, J.M., “Studies of n-pentane reaction on tungsten carbides promoted/ZrOsolid super-2 acid catalysts”, Acta Chem., 61, 208-212 (2004). (in Chinese)
7 Zhang, Y.F., Lin, W., Wang, W.F., Li, J.Q., “A first principle study on the phase stability and chemical bonding of the 3d transition metal carbides”, Acta Chem., 62, 1041-1048 (2004). (in Chinese)
8 Xiao, T., Hanif, A., York, A.P.E., Sloan, J., Green, M.L.H., “Conversion of n-heptane to LPG and aromatics over Mo2C and W2C catalysts supported on ZSM-5”, Phys. Chem. Chem. Phys., 4 (14),3522-3529 (2002).
9 Wang, G.J., Liu, R.Z., “New type catalyst-molybdenum carbide and tungsten carbide”, Journal of Qingdao University, 16 (3), 51-53(2001). (in Chinese)
10 Zhu, L.Z., Cheng, Y.F., Zhang, Q.Y., “The catalytic property of(Ni-Co)-WC composite electrode for hydrogen evolution reaction”,Appl. Chem., 16 (4), 52-54 (1999). (in Chinese)
11 Ma, C.A., Yang, W.Z., Zhou, Y.H., Cha, Q.X., “Research on the waterproof gas-diffusion electrode of tungsten carbide”, J. Phys. Chem.,6 (5), 622-627 (1990). (in Chinese)
12 Palanker, V.S., Gajyev, R.A., Sokolsky, D.V., “Electrocatalytic activity of some carbonized nickel, tungsten and molybdenum compounds”, Electrochim. Acta, 22, 133-136 (1977).
13 Baresel, D., Gellert, W., Heidemeyer, J., Scharner, P.A., “Electrocatalytic and corrosion behaviour of tungsten carbide in near-neutral pH electrolytes”, Angew. Chem. Int. Ed., 10, 194-195 (1971).
14 Ramôa, R.F., Alvarez, F., Henrigues, C.F., Lemos, J., Lopes, M.,Ribeiro M.F., “Structure-activity relationship in zeolites”, J. Mol.Catal. A, 96, 245-270 (1995).
15 Sarbak, Z., “Structural properties and HDS activity of NiMo catalysts supported on lanthana modified zeolite type X and Y”, Catal.Today, 65, 293-299 (2001).
16 Krishna, K., Seijger, G.B.F., van den Bleek, C.M., Calis, H.P.A.,“Preparation of ceria-zeolite catalysts by different techniques and its effect on selective catalytic reduction of NO with NH3at high space velocities”, Chem. Comm., 2030-2031 (2002).
17 Kovacheva, P., Predoeva, A., Arishtirova, K., Vassilev, S., “Oxidative methylation of toluene with methane using X zeolite catalyst modified with alkali earth oxides”, Appl. Catal. A Gen., 223, 121-128(2002).
18 Kubička, D., Kumar,N., Mäki-Arvela, P., Tiitta,M., Niemi, V., Karhu,H., Salmi, T., Murzin, D.Y., “Ring opening of decalin over zeolites:II. Activity and selectivity of platinum-modified zeolites”, J. Catal.,227, 313-327 (2004).
19 Li, G.H., Zheng, Y.F., Ma, C.A., Li, M.C., “Preparation of zeolite-supported tungsten carbide and its electrocatalytic property”,Chinese Journal of Catalysis, 26 (6), 443-445 (2005). (in Chinese)
20 Ma, C.A., Huang, Y., Tong, S.P., Zhang, W.M., “The catalytic behavior of tungsten carbide for the electroreduction of p-nitrophenol”,Appl. Chem., 21 (7), 721-724 (2005).
21 Li, G.H., Ma, C.A., Zheng, Y.F., Zhang, W.M., “Preparation and electrocatalytic activity of hollow global tungsten carbide with mesoporosity”, Micropor. Mesopor. Mater., 85, 234-839 (2005).
2010-06-09, accepted 2011-09-06.
* Supported by the National Natural Science Foundation of China (21173193), the Natural Science Foundation of Zhejiang Province (Y4080209, Y406094), and the Science Plan of Zhejiang Province (2007F70039).
** To whom correspondence should be addressed. E-mail: nanozjut@zjut.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
