Inhibiting Effect of Ciprofloxacin, Norfloxacin and Ofloxacin on Corrosion of Mild Steel in Hydrochloric Acid*
2012-10-31PANGXuehui庞雪辉RANXiangbin冉祥滨KUANGFei匡飞XIEJiandong解建东andHOUBaorong侯保荣SchoolofChemistryandChemicalEngineeringUniversityofJinanJinan00ChinaInstituteofOceanologyChineseAcademyofSciencesQingdao66071ChinaResearchCenterfor
PANG Xuehui (庞雪辉)**, RAN Xiangbin (冉祥滨), KUANG Fei (匡飞), XIE Jiandong(解建东) and HOU Baorong (侯保荣) School of Chemistry and Chemical Engineering , University of Jinan, Jinan 00, China Institute of Oceanology, Chinese Academy of Sciences, Qingdao 66071, China Research Center for Marine Ecology, First Institute of Oceanography, SOA, Qingdao 66061, China School fo Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 67001, China Shandong Jianzhu University, Jinan 001, China
Inhibiting Effect of Ciprofloxacin, Norfloxacin and Ofloxacin on Corrosion of Mild Steel in Hydrochloric Acid*
PANG Xuehui (庞雪辉)1,2,**, RAN Xiangbin (冉祥滨)3, KUANG Fei (匡飞)4, XIE Jiandong(解建东)5and HOU Baorong (侯保荣)21School of Chemistry and Chemical Engineering , University of Jinan, Jinan 250022, China2Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China3Research Center for Marine Ecology, First Institute of Oceanography, SOA, Qingdao 266061, China4School fo Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 637001, China5Shandong Jianzhu University, Jinan 250014, China
The inhibiting effect of ciprofloxacin, norfloxacin and ofloxacin on the corrosion of mild steel in 1 mol·L−1HCl and the mechanism were studied at different temperatures using mass loss measurement, electrochemical method, and X-ray photoelectron spectroscopy (XPS). Effective inhibition was shown by mass loss, potentiodynamic polarization and impedance spectroscopy measurement. The corrosion rate of the metal in the mass loss measurement, and the corrosion reaction on cathode and anode in the electrochemical measurement were accelerated when temperature was increased. XPS results showed that the inhibitors adsorbed effectively on the metal surface.
corrosion, inhibition, electrochemical impedance spectroscopy, potentiodynamic polarization, X-ray photoelectron spectroscopy
1 INTRODUCTION
In the corrosion of a metal in its environment,chemical or electrochemical reactions occur and some metal is lost [1]. Inhibitors are used for controlling the corrosion of metals and alloys in severe environment in practice [2-6], which are added to many systems, such as cooling systems, refinery units, chemicals, oil and gas production units, and boiler, to prevent the corrosion.
Many organic compounds [7-16], especially those with N, S, P, O and π bond, are studied and being studied for their corrosion inhibition potential. Some compounds show good anti-corrosive ability, but most of them are highly toxic to both human beings and environment during their synthesis or applications.Some environment-friendly and body-friendly corrosion inhibitors, especially those not containing heavy metals and nutrition salts, have been investigated,such as eco-friendly natural products [17-19] or rare earth elements [20-22].
Pharmacokinetics, toxicology, drug resistance,residual in the body, and teratogenicity of ciprofloxacin, norfloxacin and ofloxacin have been tested in the animals’ body. They are widely used as the components of common antibiotic medicine for human beings because of the low toxicity, high efficiency,wide-spectrum antiseptic, and the ability to inhibit the population of bacteria [23-25].
In this work, based on our previous work [26], we investigate the mechanism and inhibitory effect of ciprofloxacin, norfloxacin and ofloxacin at different temperatures by mass loss experiment and electrochemical experiment. X-ray photoelectron spectroscopy (XPS) measurement is used to investigate the adsorption of inhibitors on the metal surface.
2 MATERIALS AND METHODS
2.1 Materials
Ciprofloxacin, norfloxacin and ofloxacin (Fig. 1)were purchased from Aldrich. 1 mol·L−1HCl solution without inhibitor was used as blank control. Mild steel specimens had composition (%, by mass) of 0.017 C,0.78 Si, 0.85 Mn, 0.0047 P, 0.017 S, and Fe for the rest.

Figure 1 Molecule structure
2.2 Mass loss experiment
The mild steel specimens were polished with SiC emery papers (400, 600, 800 grit), rinsed in water and acetone, dried in a desiccator, weighed by an analytic balance (±0.0001 g), and immersed in the test solutions for 4 h at 293, 303, 313, and 323 K in triplicate.Then the specimens were removed, rinsed in water and absolute ethyl alcohol, dried in a desiccator, and weighed. The difference of specimen mass before and after the immersion is the corrosion mass loss. The inhibition efficiency IE and corrosion rate R from mass loss are calculated by:

where W0and W are blank and inhibited mass loss,respectively, Δ W= W0−W , S is the area of specimen,and t is the corrosion time.
2.3 Electrochemical measurement
Working electrodes with 0.01 m×0.01 m exposed area used in the electrochemical measurement were covered with araldite epoxy, polished with SiC emery papers (400, 600, 1000 grit), rinsed with distilled water, and degreased ultrasonically in acetone. An IM6e electrochemical measurement system (ZAHNER,Germany) was used for polarization curve and electrochemical impedance spectroscopy (EIS) measurements. Electrochemical experiments were performed in a three-electrode cell with a mild steel working electrode, a platinum foil (0.015 m×0.015 m) as a counter-electrode and a saturated calomel electrode(SCE) with a Luggin capillary as reference electrode.Before the experiment, the working electrodes were immersed in the test solution for 30 min. The steady-state open circuit potential (OCP) was disturbed using an ac sine wave with 0.005V amplitude,the frequency range was 20 mHz-100 kHz, and the parameters were obtained by SIM program. IE is calculated from EIS measurements by:

where Rt′ and Rtare charge transfer resistance values for blank and inhibited solution, respectively.
Potentiodynamic curves were obtained fromanalysis software. IE is from potentiodynamic curves,

2.4 XPS measurement
XPS measurement was performed at a power of 100W using a PHI Quantera Scanning X-ray Microscope (SXM) with an Al Kαmonochromated source(hν1486.6 eV) with a beaming diameter of 100 μm.All spectra were collected using a suitable takeoff angel modified by the instrument with respect to the sample surface. The energy analyzer was operated in the fixed retard ratio mode with 0.5 eV each step for survey spectra. The Al 2p peak (74.75 eV) of aluminum oxide was used as internal reference for calibration of binding energy scale. The data were fitted by using Origin 7.0 with peak fitting module (PFM) pack.A Salvztsky Golay function was applied for each spectrum. The peak area was measured by fitting the individual region scans with Gaussian peaks.
3 RESULTS AND DISCUSSION
3.1 Mass loss experiment
IE and R at different concentrations and temperatures are shown in Table 1 and Fig. 2, respectively.The values of R decrease markedly with increasing concentration at 303 K, and reach the lowest values of 0.059 (ciprofloxacin), 0.075 (norfloxacin), and 0.139(ofloxacin) mg·cm−2·h−1at 3.16×10−3mol·L−1, which are slower than other heterocyclic inhibitors [25, 27-31]under the same condition. R increases with temperature,since the desorption of inhibitors increases at higher temperature and the metal surface is not protected effectively. However, Table 1 shows that IE does not reduce, though higher temperature accelerates the corrosion rate. The results indicate that the three compounds are excellent acid inhibitors, and ciprofloxacin is the best.

Table 1 The inhibition efficiency from mass loss experiment for mild steel
3.2 Electrochemical impendence spectroscopy
A typical set of Nyquist plots for mild steel electrode at 293, 303, 313, and 323 K for inhibitor of 3.16×10−4mol·L−1are shown in Fig. 3. They display single capacitive semi-loop [26], and can be interpreted in terms of the usual equivalent circuitcalculated as described in Ref. [34]. Impedance parameters are given in Table 2. Fig. 3 shows that the inhibition mechanism does not change when T is increased, which is the depression of charge transfer by the compact inhibitor adsorption film [26]. In Fig. 3 and Table 2, 3 and Table 2, Rtvalues decrease and Cdlvalues increase as temperature increases. The decrease in Rtindicates that the charge transfer resistance, resulted from the inhibitor, between the metal surface and electric double layer decreases when T increases.The increase of Cdlsuggests that the protection is weakened by the thermal motion of inhibitor at higher T, so that the electrode reaction is accelerated and the charge number is increased on metal surface and in the double layer. Thus higher temperature reduces the inhibitory effect. However, the inhibitory ability does not disappear as temperature increases. Table 2 shows that the inhibitor enhances the corrosion resistance at higher temperature, compared with the blank control.At 3.16×10−4mol·L−1of inhibitor and in the temperature range, ciprofloxacin shows the highest Rtand ofloxacin the lowest Rt. The inhibitory ability is:ciprofloxacin>norfloxacin>ofloxacin, as deduced in Ref. [26]. The result is consistent with that from mass loss experiment.
3.3 Potentiodynamic polarization curves
Polarization curves for the mild steel in the acidic solution with 3.16×10−4mol·L−1inhibitor are shown in Fig. 5. Electrochemical corrosion kinetic parameters obtained by extrapolation of Tafel lines are given in Table 2. Both cathodic and anodic current-potential curves are nearly parallel to Tafel lines, and the values of anodic slope (βa) are modified slightly and those of cathodic slope (βc) changes slightly as temperature increases. The phenomenon indicates that higher temperature does not change the reactions controlling the process on cathode and anode.
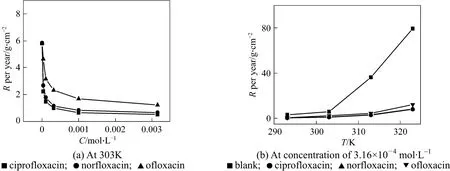
Figure 2 Corrosion rate of mild steel with inhibitors in 1 mol·L−1 HCl
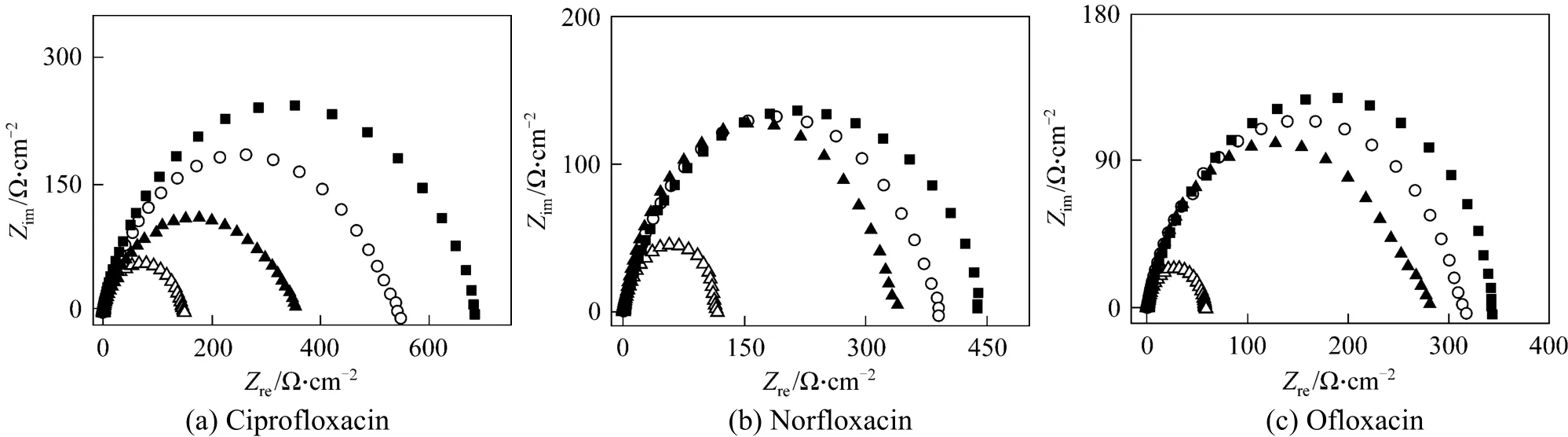
Figure 3 Nyquist diagram for mild steel in 1 mol·L−1 HCl and 3.16×10−4 mol·L−1 inhibitor at different temperatures■ 293 K; ○ 303 K; ▲ 313 K; △ 323 K
In Fig. 5 and Table 2, current density values of cathode (ik) and anode (ia), and the total current density Icorrincrease obviously with temperature. Although the anodic iron dissolution and the cathodic proton-discharge reaction are accelerated greatly at higher temperature, the inhibitory effect still exists,reflected by IE data. The values of Edesfor the three inhibitors decrease when T increases, since the inhibitor desorption is accelerated because of the thermal motion and metal dissolution. Thus, higher temperature reduces the inhibitory effect. Table 2 shows that the inhibitory ability from polarization experiment is also in the order of ciprofloxacin>norfloxacin>ofloxacin.
3.4 X-ray photoelectron spectroscopy measurement
X-ray photoelectron spectroscopy was used to characterize the steel sample immersed in 1 mol·L−1HCl with 3.16×10−4mol·L−1inhibitor for 3 h to validate the effective adsorption of the inhibitor on the metal surface, and the XPS spectra are shown in Fig. 6.XPS core-level spectra of C1s, O1s and N1s and their fitting curves for the inhibitors are shown in Figs. 7-9,and the peak area and area percentage are given in Table 3. The structure and the front orbital density distribution of inhibitors [26] are shown in Fig. 10 in order to analyze XPS measurement.
C1s core-level spectra for ciprofloxacin, norflox-acin and ofloxacin are shown in Fig. 7. All C1s spectra have two peaks. At 285.5 eV (ciprofloxacin), 285.3 eV(norfloxacin), and 285.4 eV (ofloxacin), the peaks belong to C atoms of C C bond and pyrazine circle,which are more positive than those at 284.6 eV (C C)and 285.2 eV (C N ) [35], of similar structure without interaction. In Fig. 10 C C bond and C atoms of pyrazine circle show stronger energy of the highest occupied molecular orbital (HOMO) distribution, so that they supply electrons to Fe atoms more easily and their binding energy shifts positively. The peaks at 289.1 eV (ciprofloxacin), 288.9 eV (norfloxacin) and 289.0 eV(ofloxacin) correspond to C atoms of C O ,
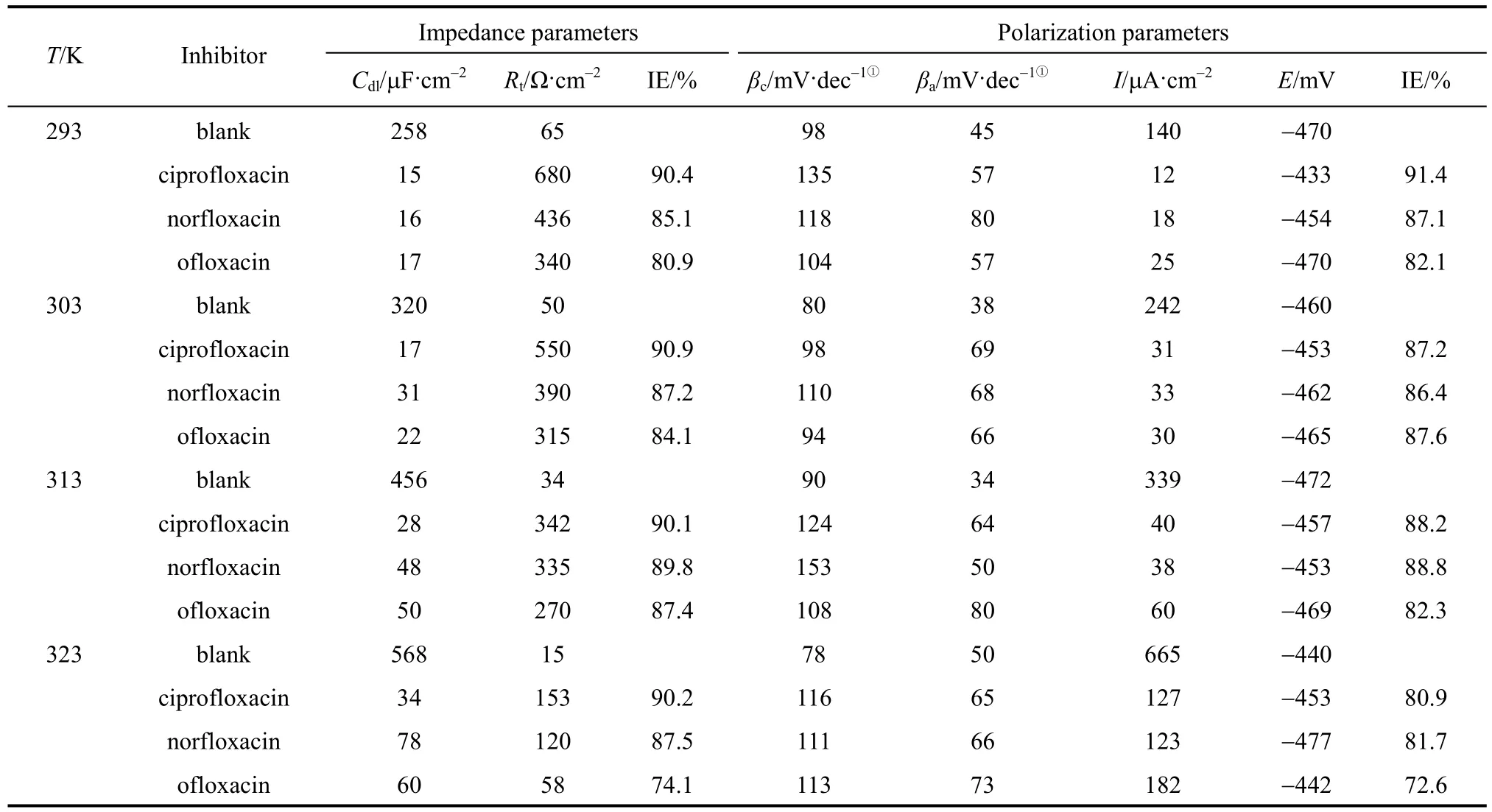
Table 2 Electrochemical parameters for mild steel in 1 mol·L−1 HCl with inhibitors
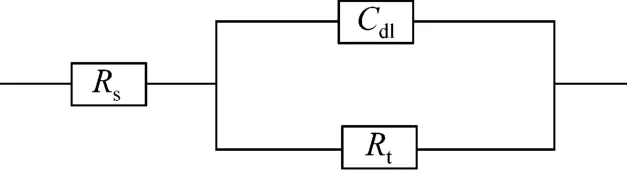
Figure 4 Equivalent circuit model for impedance analysis

Figure 5 Polarization curves for mild steel in 1 mol·L−1 HCl with inhibitor of 3.16×10−4 mol·L−1 1—293 K; 2—303 K; 3—313 K; 4—323 K
OC OH, which are more negative than the one at 290.8eV [35] of similar structure without interaction.The lowest unoccupied molecular orbital (LUMO) on C O, OC OH shows the stronger distribution, so these parts may accept the feedback electrons from Fe atoms and their binding energy shifts negatively.
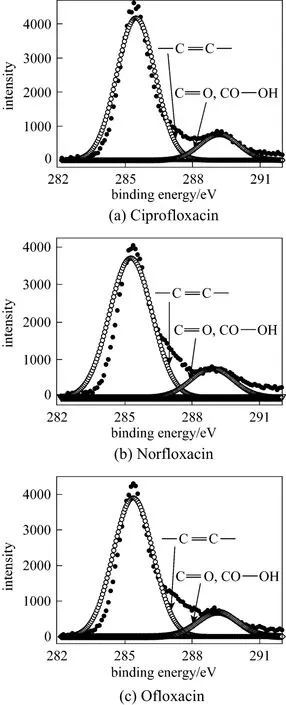
Figure 7 XPS core-level spectra of C1s for steel surface exposed to 3.16×10-4 mol·L-1 inhibitor+1 mol·L-1 HCl solution for 3 h at 303 K
Figure 10 and Table 3 show that C atoms of C C bond and pyrazine circle contribute considerably to the adsorption of inhibitor, more than C O, OC OH.The peak area percentage of C atoms of C C bond and pyrazine circle reaches 84% (ciprofloxacin), 82%(norfloxacin), and 84% (ofloxacin), which are almost five times those of C O, OC OH, since C atom content in C C bond and pyrazine circle is higher than that of C O, OC OH. In Table 3, the peak area of C follows the sequence of ciprofloxacin>norfloxacin>ofloxacin, and it is the sequence of the interaction ability.

Figure 6 XPS spectra of mild steel surface
N1s core-level spectra for ciprofloxacin, norfloxacin and ofloxacin are shown in Fig. 8. For ciprofloxacin, the peaks at 399.1, 399.2 and 399.8 eV correspond to N1s spectra of NH , N , and the peak at 403.1 eV is generated by protonized N+.For norfloxacin, the peaks at 399.0, 399.1 and 399.6 eV are generated by NH , N , and the peak at 403.0 eV is generated by protonized N+. For ofloxacin, the peaks at 399.0 and 400.6 eV are generated by NH , N , and that at 403.3eV is generated by protonized N+. The binding energy of the similar structure without interaction of NH ,N is 398.8 eV [36], which is more negative than those of inhibitors with interaction. In Fig. 10, N of NH , N shows stronger HOMO distribution intensity, offering electrons more easily to interact with Fe atoms, which is the reason for binding energy of N1s to shift positively. Moreover, N atoms of the inhibitors are protonized easily in the acidic solution,and can offer the lone pair electrons to H+and H2O in the protonized process, resulting in negative shift of N atom. However, protonized N+are only part of all N atoms, so the integral peak area is less than that of NH , N . From Table 3, the peak area of N element follows the sequence of ciprofloxacin>norfloxacin>ofloxacin, which shows the total interaction ability with Fe atoms.
Figure 9 shows O1s core-level spectra for ciprofloxacin, norfloxacin and ofloxacin. O1s spectra at 528.7 eV (ciprofloxacin), 528.5 eV (norfloxacin), and 528.7 eV (ofloxacin) are generated by O2−. The peaks at 530.4 eV (ciprofloxacin) and 531.2 eV (norfloxacin)are generated by O atoms of C O, OC OH, and the peak at 530.7 eV (ofloxacin) corresponds to O of ofloxacin, which shift negatively from 533.6 eV [35],the binding energy of similar structure (C O,OC OH) without interaction.
The peak of O2−is resulted from the oxidation in atmosphere during the period after the sample is removed from the solution and before it is tested, which may be several days, so that the integral peak area is larger. In Table 3, the peak area of O is in the order of ciprofloxacin>norfloxacin>ofloxacin, which represents the total interaction ability of O element with Fe atoms.

Figure 8 XPS core-level spectra of N1s for steel surface exposed to 3.16×10-4 mol·L-1 inhibitor+1 mol·L-1 HCl solution for 3 h at 303 K
From Table 3, we can deduce that the effective peak area of O element>that of C element>that of N element. Less O element content leads to stronger XPS test result, which may be resulted from O element of FeOOH from the metal oxidation. N element content is the least, and so does its effective peak area,but its ability to offer the electron should not be neglected, as reported by other researches [36-40].
4 CONCLUSIONS
Ciprofloxacin, norfloxacin and ofloxacin are effective inhibitors for mild steel in 1 mol·L−1HCl at different temperatures, and the inhibitory ability is as the sequence of ofloxacin<norfloxacin<ciprofloxacin at same temperature.
Mass loss experiment shows that corrosion rate R increases with temperature, but the inhibition efficiency IE does not decrease.
EIS displays a single capacitive loop, Rtdecreases and Cdlincreases when T increases. Potentiodynamic polarization curves indicate that the inhibitors suppress both anodic and cathodic processes, and the inhibition mechanism does not change at higher temperature, but the total corrosion current density Icorrand Edesdecrease.
XPS results show that C, N and O element aredetected. The corresponding peak shifts positively or negatively since the functional group of the inhibitors offers or accepts the electrons according with quantum chemical analysis.
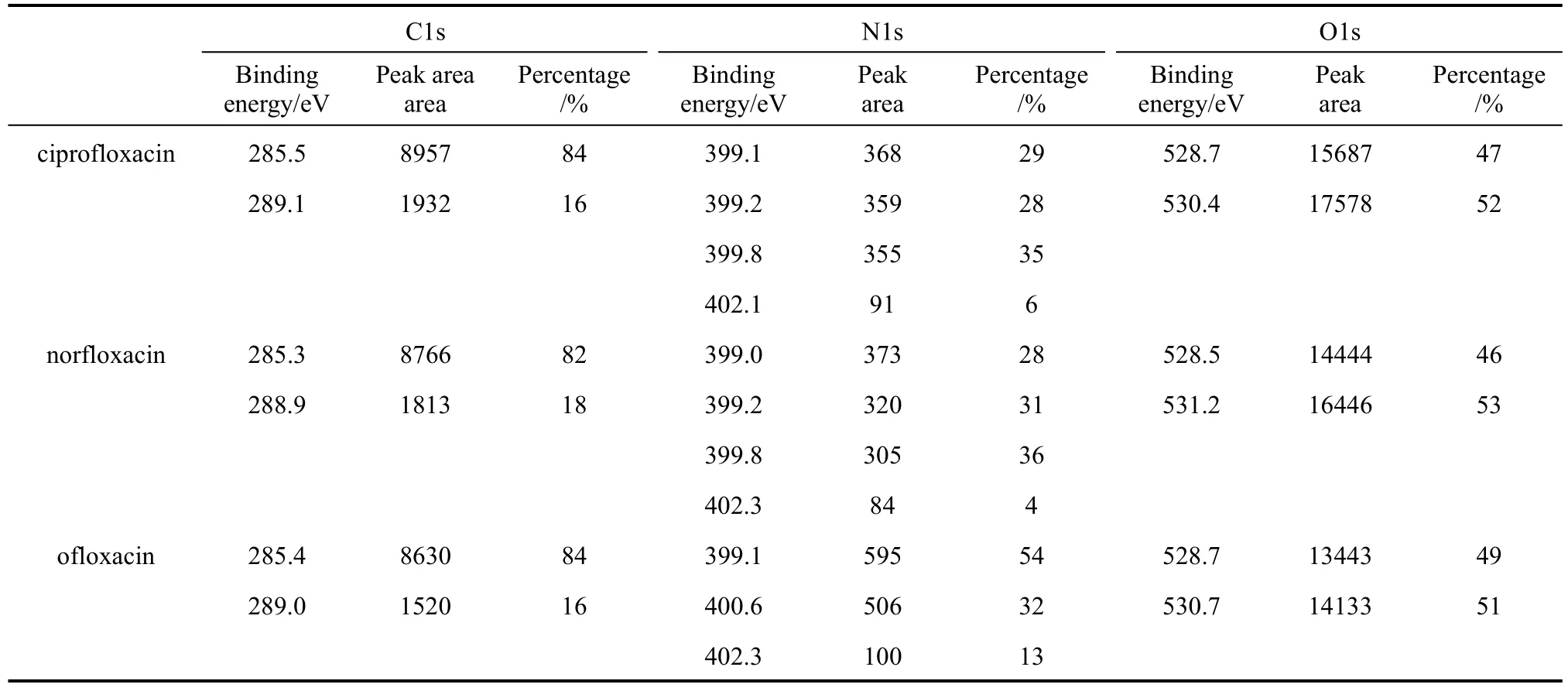
Table 3 Binding energy and peak area of compounds from C1s, N1s, and O1s spectra

Figure 10 The optimized geometry for inhibitors and the frontier molecule orbital density distribution
NOMENCLATURE
C concentration, mol·L−1
Cdlconstant phase elements modeling capacitance, F·cm−2
E corrosion potential, V
Edesdesorption potential
EHOMOenergy of the highest occupied molecular orbital, eV
ELUMOenergy of the lowest unoccupied molecular orbital, eV
f frequency, Hz
h the Planck constant
Icorrtotal corrosion current density, A·cm−2
IE inhibitory efficiency, %
iaanodic current density, A·cm−2
ikcathodic current density, A·cm−2
Rselectrolyte resistance, Ω·cm−2
Rtcharge transfer resistance, Ω·cm−2
v the optical frequency
Zimthe imaginary part of ac impedance
Zrethe real pant of ac impedance
βaanodic slope, mV·dec−1
βccathodic slope, mV·dec−1
1 Sanyal, B., “Organic compounds as corrosion inhibitors in different environments—A review”, Prog. Org. Coat., 9, 165-236 (1981).
2 Nathan, C.C., Corrosion Inhibitors, NACE, Houston (1973).
3 Ranney, M.W., Inhibitors—Manufacture and Technology, Noyes Data Corp, New Jersey (1976).
4 Putilova, N., Balezin, S.A., Barannik, V.P., Metallic Corrosion Inhibitors, Pergamon Press, London (1966).
5 Xiao, M.T., Huang, Y.Y., Meng, C., Guo, Y.H., “Kinetics of asymmetric reduction of phenylglyoxylic acid to R-(-)-mandelic acid by Saccharomyces cerevisiae FD11b”, Chin. J. Chem. Eng., 14 (1),73-80 (2006).
6 Eldredge, G.G., Warner, J.C., The Corrosion Hand Book, Wiley, New York, 905 (1948).
7 Solmaz, R., Kardas, G., Yazici, B., Erbil, M., “Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1,3,4-thiadiazole on mild steel in hydrochloric acid media”, Colloid. Surf. Physicochem. Eng. Aspect., 312, 7-17 (2008).
8 Negm, N.A., Zaki, M.F., “Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4N HCl”, Colloid Surface Physicochem Eng Aspect, 322, 97-102(2008).
9 Machnikova1, E., Whitmire, K.H., Hackerman, N., “Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives”,Electrochimica Acta, 53, 6024-6032 (2008).
10 Avci, G., “Corrosion inhibition of indole-3-acetic acid on mild steel in 0.5M HCl”, Colloid. Surf. Physicochem. Eng. Aspect., 317,730-736 (2008).
11 Satpati, A.K., Ravindran, P.V., “Electrochemical study of the inhibition of corrosion of stainless steel by 1,2,3-benzotriazole in acidic media”, Mater. Chem. Phys., 109, 352-359 (2008).
12 Lebrini, M., Traisnel, M., Lagrene’e, M., Mernari, B., Bentiss, F.I.,“Inhibitive properties, adsorption and a theoretical study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid”, Corros. Sci., 50, 473-479 (2008).
13 Solmaz, R., Kardas, G., Culha, M., Yazici, B., Erbil, M., “Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media”, Electrochimica Acta, 53 , 5941-5952 (2008).
14 Ramesh, S.V., Adhikari, A.V., “Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl) quinolin-4-yl]thio}propanohydrazide as an effective inhibitor of mild steel corrosion in HCl solution”, Corros. Sci.,50, 55-61 (2008).
15 Achary, G., Sachin, H.P., Naik, Y.A., Venkatesha, T.V., “The corrosion inhibition of mild steel by 3-formyl-8-hydroxy quinoline in hydrochloric acid medium”, Mater. Chem. Phys., 107, 44-50 (2008).
16 Ergun, U., Yüzer, D., Emregül, K.C., “The inhibitory effect of bis-2,6-(3,5-dimethylpyrazolyl) pyridine on the corrosion behaviour of mild steel in HCl solution”, Mater. Chem. Phys., 109, 492-499(2008).
17 Li, Y., Zhao, P., Hou, B.R., “Berberine as a natural source inhibitor for mild steel in 1 M H2SO4”, Appl. Surf. Sci., 252 (5), 1245-1253(2005).
18 Matamala ,G., Smeltzerand, W., Droguett, G., “Comparison of steel anticorrosive protection formulated with natural tannins extracted from acacia and from pine bark”, Corros. Sci., 42 (8), 1351-1362 (2000).
19 Lin, X.Z., Gong, M., Wu, J.P., “Study about use of abstracted oil from plant scrapas corrosion inhibitor for hydrochloric acid cleaning”, Corro. Sci. Prot. Tech., 18 (3), 222-224 (2006). (in Chinese)
20 Arenas, A., Bethencourt, M., Botana, F.J., Damborenea, de. J., Marcos, M., “Inhibition of 5083 aluminium alloy and galvanised steel by lanthanide salts”, Corros. Sci., 43 (1), 157-170 (2001).
21 Aballe, A., Bethencourt, M., Botana, F.J., Marcos, M.J., “CeCl3and LaCl3binary solutions as environment-friendly corrosion inhibitors of AA5083 Al-Mg alloy in NaCl solutions”, Alloys. Comp., 323/324,855-858 (2001). (in Chinese)
22 El-Sawy, S.M., Abu-Ayana, Y.M., Abdel-Mohdy, F.A., “Some chitin/chitosan derivatives for corrosion protection and waste water treatments”, Anticorros. Meth. Mat., 48 (4), 227-235 (2001). (in Chinese)
23 Feng, Y.F., Li, K.X., Xie, F., Sun, C.H., “HPLC determination of active metabolite of prulifloxacin: NM394 in human plasma with fluorescence detection”, Chin. J. Pharm. Ana., 26 (6), 755-757 (2006). (in Chinese)
24 Shen, J.Z., Li, J.S., Liang, B., Zhong, Q.F., Xiao, X.L., Liu, J.F.,“Study on the reproductive teratogenic toxicity of sarafloxacin to wistar rats”, Acta. Veter. Zoo. Sinica, 32 (4), 375-378 (2001). (in Chinese)
25 Lebrini, M., Lagrenée, M., Vezin, H., Gengembre, L., Bentiss, F.,“Electrochemical and quantum chemical studies of new thiadiazole derivatives adsorption on mild steel in normal hydrochloric acid medium”, Corros. Sci., 47 (2), 485-505 (2005).
26 Pang, X.H., Guo, W.J., Li, W.H., Xie, J.D., Hou, B.R., “Electrochemical, quantum chemical and SEM investigation of the inhibiting effect and mechanism of ciprofloxacin, norfloxacin and ofloxacin on the corrosion for mild steel in hydrochloric acid”, Science in China Series B: Chemistry, 51 (10), 928-936 (2008).
27 Khaled, K.F., “Experimental and theoretical study for corrosion inhibition of mild steel in hydrochloric acid solution by some new hydrazine carbodithioic acid derivatives”, Appl. Surf. Sci., 252 (12),4120-4128 (2006).
28 Wang, D.X., Li, S.Y., Ying, Y., Wang, M.G., Xiao, H.M., Chen, Z.X.,“Theoretical and experimental studies of structure and inhibition efficiency of imidazoline derivatives”, Corros. Sci., 41, 1911-1919 (1999).
29 Chebabe, D., Chikh, Z.A., Dermaj, A., Rhattas, K., Jazouli, T.,Hajjaji, N., Mdari, F.E., Srhiri, A., “Synthesis of bolaamphiphile surfactants and their inhibitive effect on carbon steel corrosion in hydrochloric acid medium”, Corros. Sci., 46, 2701-2713 (2004).
30 Bentiss, F., Traisnel, M., Chaibi, N., Mernari, B., Vezin, H., Lagrenée, M., “2,5-Bis(n-methoxyphenyl)-1,3,4-oxadiazoles used as corrosion inhibitors in acidic media, correlation between inhibition efficiency and chemical structure”, Corros. Sci., 44, 2271-2289 (2002).
31 Tebbji, K., Hammouti, B., Oudda, H., Ramdani, A., Benkadour, M.,“The inhibitive effect of bipyrazolic derivatives on the corrosion of steel in hydrochloric acid solution”, Appl. Surf. Sci., 252, 1378-1385(2005).
32 Hosseini, M., Mertens, S.F.L., Ghorbani, M., Arshadi, M.R., “Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media”, Mater. Chem. Phys., 78 (3), 800-808 (2003).
33 Cruz, J., Martínez, R., Genesca, J., Garc, E., “Experimental and theoretical study of 1-(2-ethylamino)-2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media”, J. Electroanal.Chem., 566 (1), 111-121 (2004).
34 McCafferty, E., Hackerman, N., “Double layer capacitance of iron and corrosion iInhibition with polymethylene diamines”, J. Electrochem. Soc., 119 (2), 146-154 (1972).
35 Liu, S.H., Wang, D.H., Pan, C.H., X-ray Photoelectron Spectroscopy Analysis, Science Press, Beijing (1988). (in Chinese)
36 Olivares, O., Likhanova, N.V., Gómez, B., Navarrete, J.,Llanos-Serrano, M.E., Arce, E., Hallen, J.M., “Electrochemical and XPS studies of decylamides of a-amino acids adsorption on carbon steel in acidic environment”, Appl. Surf. Sci., 252, 2894-2909 (2006).
37 Bentiss, F., Traisnel, M., Gengembre, L., Lagrenee, M., “Inhibition of acidic corrosion of mild steel by 3,5-diphenyl-4H-1,2,4-triazole”,Appl. Surf. Sci., 161, 194-202 (2000).
38 Bommersbach, P., Alemany-Dumont, C., Millet, J.P., Normand, B.,“Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods”, Electrochim Acta., 51,1076-1084 (2005).
39 Pang, X.H., Hou, B.R., Li, W.H., Liu, F.Q., Yu, Z.G., “2,3,5-Triphenyl-2H-tetrazolium chloride and 2,4,6-tri(2-pyridyl)-striazine on the corrosion of mild steel in HCl”, Chin. J. Chem. Eng., 15, 909-915 (2007).
40 Yang, K., Chu, G., Shao, L., Xiang, Y., Zhang, L., Chen, J.F., “Micromixing efficiency of viscous media in micro-channel reactor”,Chin. J. Chem. Eng., 17, 546-551 (2009).
2009-06-05, accepted 2009-11-02.
* Supported by the National Science & Technology Pillar Program (082603101c), China Postdoctoral Science Foundation(O92623101H), Shandong Postdoctoral Foundation (200902040), Open Project Program of Marine Corrosion and Protection Research Center of Institute of Oceanology, Chinese Academy of Science (200901005) and Doctor Foundation of University of Jinan (XBS0899).
** To whom correspondence should be addressed. E-mail: pxh791118@163.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
