两个由2-(4′-氯-苯甲酰基)苯甲酸构筑的铜、锌配合物的合成及晶体结构
2012-09-15李秀梅牛艳玲王志涛
李秀梅牛艳玲 王志涛
(通化师范学院化学系,通化 134002)
两个由2-(4′-氯-苯甲酰基)苯甲酸构筑的铜、锌配合物的合成及晶体结构
李秀梅*牛艳玲 王志涛
(通化师范学院化学系,通化 134002)
通过水热法合成了2个新的配合物Cu2(cbba)4(phen)2(1)和Zn(cbba)2(bipy)(2)(Hcbba=2-(4′-氯-苯甲酰基)苯甲酸,phen=1,10-邻菲罗啉,bipy=2,2′-联吡啶),并对其进行了元素分析、红外光谱、热重和X-射线单晶衍射测定。这2个配合物通过π-π相互作用形成了三维超分子网状结构。此外,还研究了配合物2的荧光性质。
水热合成;晶体结构;铜配合物;锌配合物
0 Introduction
Recently,the design and synthesis of coordination compounds have attracted much attention due to their diversity structures[1]and potential applications[2-7].A successfulstrategy for preparing coordination compoundsisthe assembly reaction between a transition metal ion and two types of organic ligands,one acting as a terminal ligand and the other as a chelting ligand.In this aspect,the bridging ligand 2,2′-bipyridine and 1,10-phenanthrolin has been widely used in the construction of metal-organic coordination polymers[8].In addition,as a rigid and versatile ligand,2-(4′-chlorine-benzoyl)benzoic acid (Hcbba)has been relatively less studied to constructcoordination compounds containing transition metals[9].
The hydrothermal technique is well suited to the preparation of crystals of synthetic minerals,new inorganic materials,and organometallic coordination polymers.Ofparticular interestto us is the construction of transition metal polymers with new structural features by utilizing hydrothermal synthesis.To extend our recent work[9],we report here the preparation and crystal structure of the title compounds Cu2(cbba)4(phen)2(1)and Zn(cbba)2(bipy)(2),which exhibit3D supramolecularnetwork through π-π stacking interactions.
1 Experimental
1.1 General procedures
All materials were commercially purchased and used without further purification.Infrared spectra(KBr pellets)were taken on a Perkin-Elmer 2400LS II spectrometer and elemental analyses for C,H and N were performed on a Perkin-Elmer 240C analyzer.The TG studies were performed on a Perkin-Elmer TGA7 analyzer.The fluorescent studies were carried out on a computer-controlled JY Fluoro-Max-3 spectrometer at room temperature.
1.2 Synthesis
Cu2(cbba)4(phen)2(1):A mixture of CuCl2·2H2O(0.2 mmol,0.034 g),Hcbba(0.2 mmol,0.052 g),phen(0.2 mmol,0.036 g)and H2O(18 mL)was sealed in a 30 mL Teflon-lined autoclave underautogenous pressure at 140℃for 7 d.After cooling to room temperature,blue block crystals were collected by filtration and washed with distilled water in 36%yield(based on Cu).Anal.Calcd.(%)for C80H48Cl4Cu2N4O12:C,62.96;H,3.17;N,3.67.Found(%):C,62.90;H,2.98;N,3.41.IR(KBr,cm-1):3423w,3088w,2361w,1663s,1642s,1612w,1587m,1519w,1482w,1429m,1385m,1 334m,1 270w,1 169w,1 142w,1 107w,1 088 m,1 014w,931s,845w,839w,781m,746s,723s,689 w,651w,528w,479w.
Zn(cbba)2(bipy)(2):A mixture of Zn(OAc)2·2H2O(0.2 mmol,0.044 g),Hcbba (0.4 mmol,0.10 g),bipy(0.2 mmol,0.032 g)and H2O(18 mL)was sealed in a 30 mL Teflon-lined autoclave underautogenous pressure at 150℃for seven days.After cooling to room temperature,colorless block crystals were collected by filtration and washed with distilled water in 42%yield (based on Zn).Anal.Calcd.(%)for C38H24Cl2N2O6Zn:C,61.60;H,3.26;N,3.78.Found(%):C,61.37;H,2.99;N,3.56.IR(KBr,cm-1):3423 w,3 079w,2 026w,1 670s,1 644w,1 634w,1 608w,1588m,1566w,1482w,1443w,1399m,1357m,1 415 w,1301w,1289w,1277w,1250w,1175w,1151w,1088 m,1 030w,1 013w,965w,932s,893w,861w,845w,837w,808w,786w,773m,746m,734w,716m,682w,653w,637w,574w,484w,456w.

Table 1 Selected bond lengths(nm)and bond angles(°)for compound 1 and 2
1.3 Structure determination
Single crystal diffraction data of 1 and 2 were respectively collected on a Bruker SMART APEXCCD diffractometer equipped with a graphite-monochromatic Mo Kα (λ=0.071 073 nm)radiation at room temperature.The structure was solved by direct methods with SHELXS-97 program[10]and refined by full-matrix least-squares techniques on F2with SHELXL-97[11].All non-hydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically.The selected bond parameters are given in Table 1.
Crystal data for 1:C80H48Cl4Cu2N4O12,triclinic,space group P1,Mr=1 526.10, a=1.0180 9(5),b=1.0889 1(5),c=1.689 60(8)nm, α=79.250 0(10), β=74.154 0(10),γ=70.473 0(10)°,V=1.688 89(14)nm3,Z=1,F(000)=778,μ(Mo Kα)=0.858 mm-1,Dc=1.500 g·cm-3,9 342 reflections measured,6 360 unique(Rint=0.022 8),5 007 observed reflections with I>2σ(I),R=0.045 5,wR=0.119 2,S=1.027.
Crystal data for 2:C38H24Cl2N2O6Zn,triclinic,space group P1,Mr=740.85,a=1.232 52(16),b=1.285 6(3),c=1.291 33(17)nm,α=109.295(3),β=115.476(2),γ=98.143(3)°,V=1.643 9(5)nm3,Z=2,F(000)=756,μ(Mo Kα)=0.961 mm-1,Dc=1.497 g·cm-3,8 994 reflections measured,6 360 unique (Rint=0.022 8),5 007 observed reflections with I>2σ(I),R=0.045 5,wR=0.119 2,S=1.027.
CCDC:859144,1;859145,2.
2 Results and discussion
2.1 IR spectrum
Complex 1:The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 587 cm-1(Ⅴ(OCO)assym)and 1 385 cm-1(Ⅴ(OCO)sym)[12],respectively.The ΔⅤ(Ⅴ(OCO)assym-Ⅴ(OCO)sym)are 202 cm-1(>200),showing the presence of monodentate linkage of carboxylates in the dianions.Thus the carboxylates coordinate to the metal as monodentate ligands via the carboxylate groups[13].
Complex 2:The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 670,1 588 cm-1(Ⅴ(OCO)assym)and 1 399 cm-1(Ⅴ(OCO)sym)[12],respectively.The ΔⅤ(Ⅴ(OCO)assym-Ⅴ(OCO)sym)are 271 cm-1(>200),189 cm-1(<200),showing the presence of both monodentate and bidentate linkages of carboxylates in the dianions.Thus,the carboxylates coordinate to the metal as monodentate and bidentate ligands via the carboxylate groups[13].
In addition,X-ray diffraction analysis further indicates the monodentate coordination manners of carboxylate groups in 1 and monodentate and bidentate coordination manners in 2.
2.2 Description of the structure
The molecular structure of complex 1 is shown in Fig.1.There are two same coordination centers,Cu(1)and Cu(1A),in the crystal with same coordination modes.The Cu(1)ion is five-coordinated by three carboxylate oxygen atoms(O(1),O(1A),O(4))from three different cbba ligands and two nitrogen atoms(N(1),N(2))from phen ligands,showing a distorted square-pyramidal geometry.Two carboxylate oxygen(O(1),O(4))and two nitrogen(N(1),N(2))atoms define an equatorial plane,while the axial coordination sites are occupied by one carboxylate oxygen atom(O(1A)).The bond distances of Cu-O in compound 1 fall in the 0.194 15(16)~0.239 98(16)nm range,and those of Cu-N in 0.200 23(19)~0.202 35(19)nm.
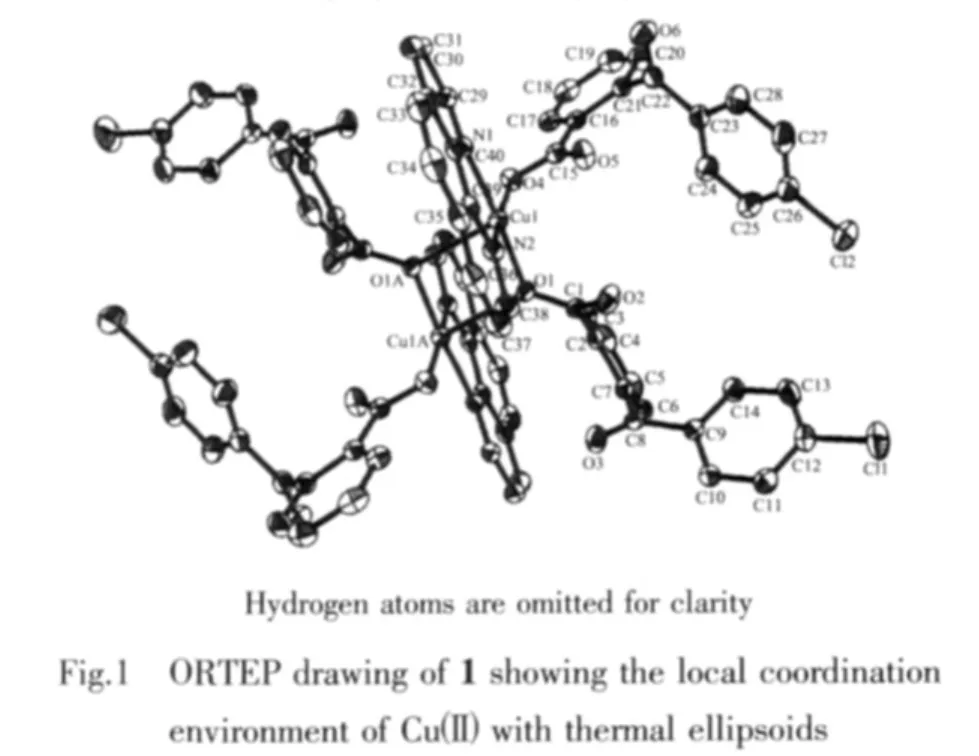
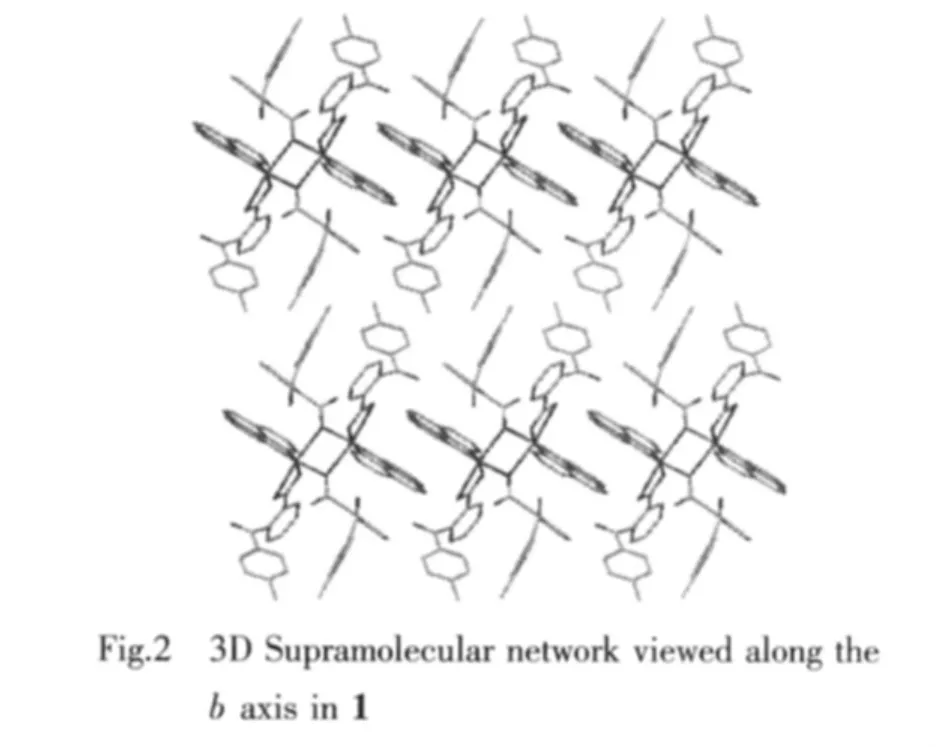
One coordination mode of the cbba ligand is present in the structure of compound 1,namely monodentate bridging mode.Based on this,two Cu(Ⅱ)ions are linked by cbba ligands to form dinuclear subunits.Moreover,there are many significant π-π interactions in the packing diagram between the neighboring aromatic cycles contained in phen and cbba ligands with the distances between ring centroids in the range of 0.347 93(15)~0.399 18(19)nm(Table 2),as a result,through π-π interactions,the networkstructures are further extended into a threedimensional supramolecular framework(Fig.2).
尽管当前河南生猪产品调运解禁,但压栏依然存在。养殖户压栏原因,一是由于活猪价格较低,养殖户有观望情绪,期待10月初生猪产品解禁和11月初活猪全面解禁后价格回升;二是存在一定程度销售困难,经纪人收购量减少;三是超过140千克的大猪省内屠宰场机器无法屠宰也无消费市场,经纪人拒收超大猪。
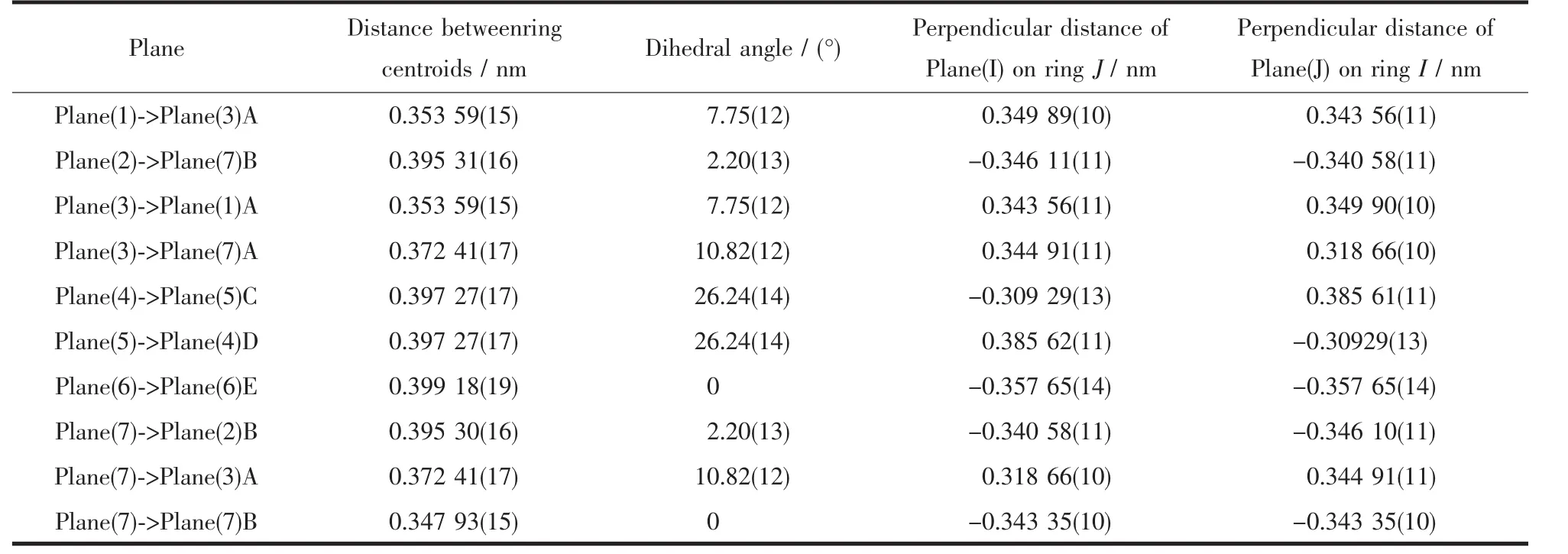
Table 2 Parameters between the planes for compound 1
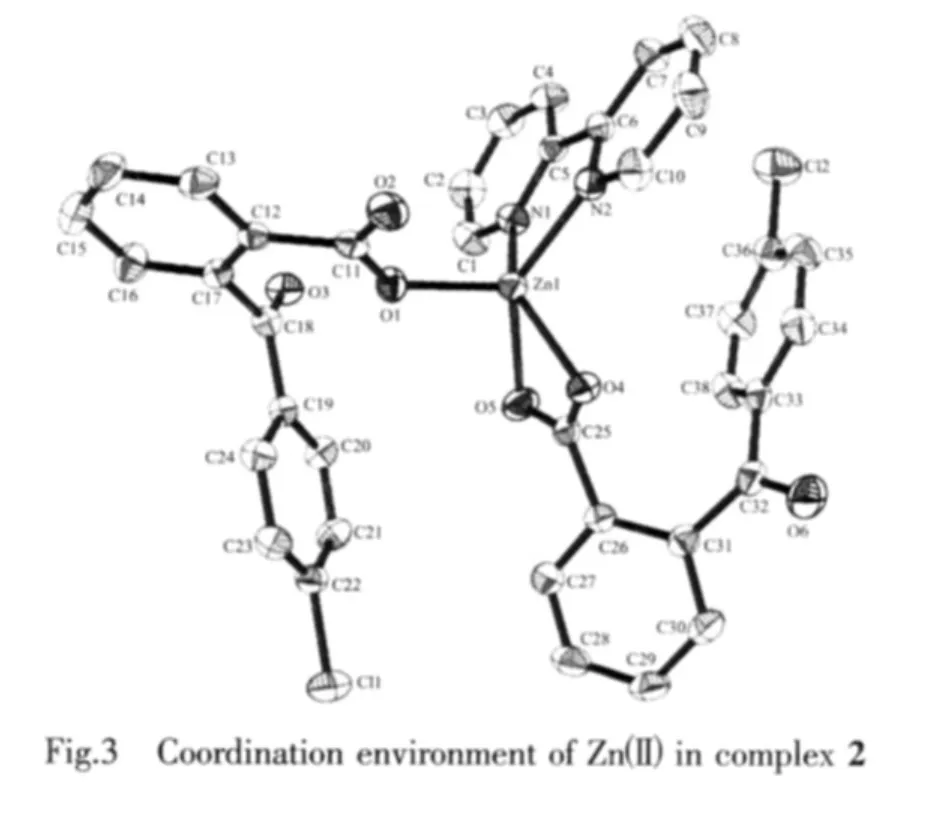
The coordination environment of Zn(Ⅱ)in omplex 2 is shown in Fig.3.There are one Zn(Ⅱ) ion,one bipy ligand and two cbba ligands in the asymmetric unit.The Zn(1)ion is five-coordinated by three carboxylate oxygen atoms(O(1),O(4),O(5))from two different cbba ligands and two nitrogen atoms(N(1),N(2))from bipy ligand,showing a distorted square-pyramidal geometry.The O(1)atoms locate at the apex site,two carboxylate oxygen atoms and two nitrogen atoms(O(4),O(5),N(1),N(2))define an equatorial plane.The bond distances of Zn-O in complex 2 fall in the range of 0.1913 9(19)~0.229 6(2)nm,and those of Zn-N in 0.206 3(2)~0.207 1(2)nm.
Two coordination mode of the cbba ligand is presentin the structure ofcomplex 2,namely monodentate and bidentate bridging mode.The bipy ligand also exhibits classic chelting mode.
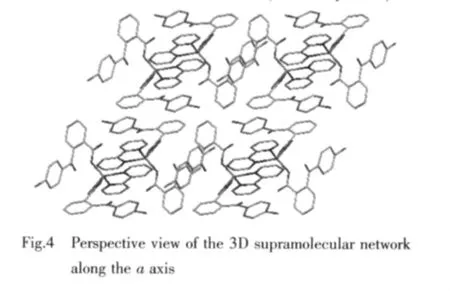
Moreover,there are π-π interactions in complex 2 between bipy ligands in the different moleculores and cbba ligands in the same moleculores(Fig.4).The centroid-to-centroid distances between adjacent aromatic rings are 0.377 7 nm for Zn1O4C25O5 and C33C34C35C36C37C38 and 0.399 2 nm for N1C1C2 C3C4C5 and N2C6C7C8C9C10 (1-x,1-y,1-z)aro-matic rings.The perpendicular distances are 0.358 8 nm for Zn1O4C25O5 and C33C34C35C36C37C38 and 0.3482nm forN1C1C2C3C4C5andN2C6C7C8 C9C10 (1-x,1-y,1-z)aromatic rings.Therefore,through π-π interactions,the complex is further extended into a three-dimensionalsupramolecular framework(Fig.4).
2.3 Thermal analysis
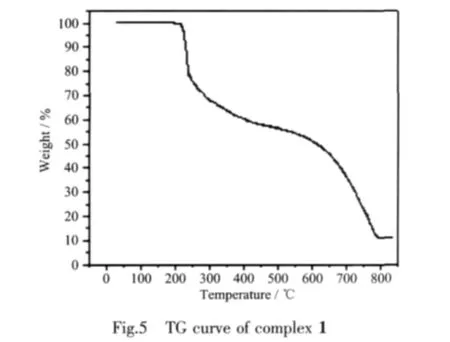
TG curve of 1 (Fig.5)show that the first weight loss of 25.1%from 215 to 251 ℃ corresponds to the removal of phen ligands(calcd.23.6%).Upon further heating,an obvious weight loss(66.2%)occurs in the temperature range of 251~780 ℃,corresponding to the release of cbba ligands(calcd.68.1%).After 780℃ no weight loss is observed,which means the complete decomposition of 1.The residual weight should be CuO.
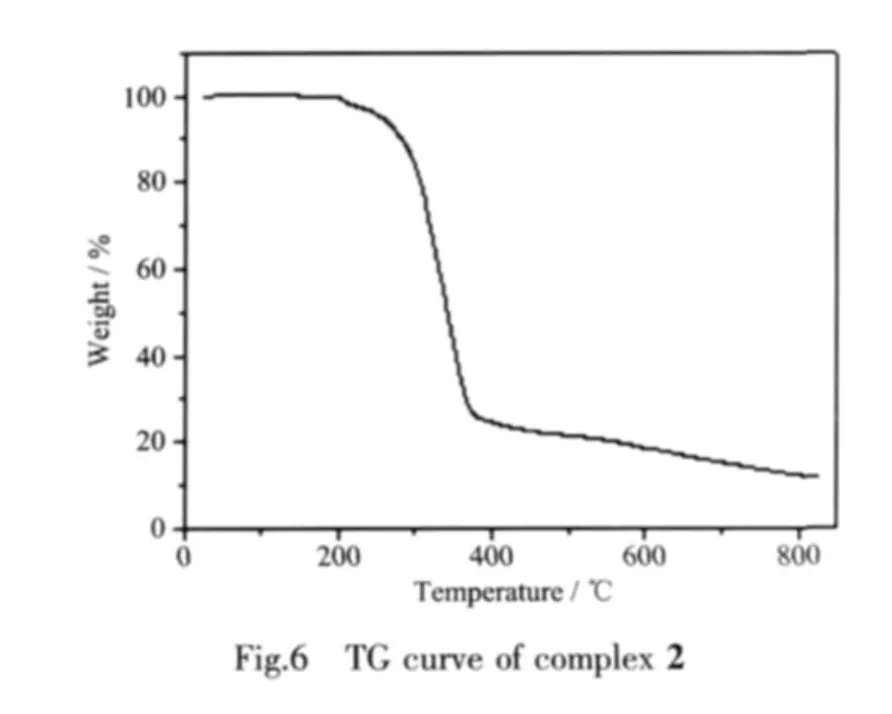
TG curve of 2 (Fig.6)show that the first weight loss of 71.3%from 152 to 368 ℃ corresponds to the removal of cbba ligands(calcd.70.1%).Upon further heating,an obvious weight loss(17.2%)occurs in the temperature range of 368~828 ℃,corresponding to the release of bipy ligand(calcd.21.1%).After 828 ℃no weight loss is observed,which means the complete decomposition of 2.The residual weight should be ZnO.
2.4 Luminescence property
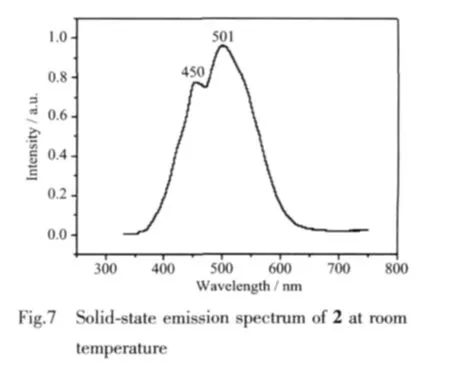
The solid-state photoluminescence spectra of 2,free Hcbba and bipy ligands were investigated at room temperature.Excited by 325 nm,coordination polymer 1 gives wide blue emission with the maximum peak at 501 nm plus shoulder peak at 450 nm (Fig.7).However,no obvious emission bands are observed for the free Hcbba and bipy ligand in the range of 400~800 nm under the same experimental conditions.The significant phenomenon of the fluorescenc emission of 2 here could be tentatively assigned to the ligand-tometal charge transfer(LMCT)[14].For possesses strong fluorescent intensity,it appears to be good candidates fornovelhybridinorganic-organicphotoactivematerials.
Reference:
[1](a)Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658(b)Eddaoudi M,Moler D B,Li L H,et al.Acc.Chem.Res.,2001,34:319-330
[2]Sun D F,Cao R,Liang Y C,et al.Dalton Trans.,2001:2335-2340
[3]Kobel W,Hanack M.Inorg.Chem.,1986,25:103-107
[4]Eddaoudi M,Kim J,Rosi N,et al.Science,2002,295:469-472
[5]Noro S I,Kitagawa S,Kondo M,et al.Angew.Chem.,Int.Ed.,2000,39:2081-2084
[6]Chen B L,Eddaoudi M,Hyde S T,et al.Science,2001,291:1021-1023
[7]Seo J S,Whang D,Lee H,et al.Nature,2000,404:982-986
[8](a)Li X M,Wang Q W,Liu B,et al.Chinese J.Struct.Chem.,2009,10:1257-1260(b)Li X M,Wang Q W,Cui Y C,et al.Chinese J.Struct.Chem.,2009,10:1317-1320(c)Li X M,Wang Q W,Liu B,et al.Chinese J.Inorg.Chem.,2011,6:1207-1211
[9](a)Wang Q W,Li X M,Meng Q L,et al.Chinese J.Struct.Chem.,2009,3:335-337(b)LI Xiu-Mei(李秀梅),WANG Qing-Wei(王庆伟),LIU Bo(刘博),et al.Chinese J.Inorg.Chem.(Wujie Huaxue Xuebao),2010,26(10):1904-1907(c)LI Xiu-Mei(李秀梅),NIU Yan-Ling(牛艳玲),WANG Zhi-Tao(王志涛).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(8):1659-1663(d)LI Xiu-Mei(李秀梅),NIU Yan-Ling(牛艳玲),WANG Zhi-T ao(王志涛).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(9):1837-1841(e)NIU Yan-Ling(牛艳玲),LI Xiu-Mei(李秀梅),WANG Li-Bin(王立斌),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(11):2298-2302
[10]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[11]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[12]Devereux M,Shea D O,Kellett A.et al.Inorg.Biochem.,2007,101:881-892
[13]Bellamy L J.The Infrared Spectra of Complex Molecules.New York:Wiley,1958.
[14]Fu Z Y,Wu X T,Dai J C,et al.Eur.J.Inorg.Chem.,2002,2730-2735
Synthesis and Crystal Structure of Two Complexes of Copper,Zinc Assembled by 2-(4′-Chlorine-benzoyl)-benzoic Acid
LI Xiu-Mei*NIU Yan-Ling WANG Zhi-Tao
(Department of Chemistry,Tonghua Teachers College,Tonghua,Jilin 134002,China)
Two complexes Cu2(cbba)4(phen)2(1)and Zn(cbba)2(bipy)(2)(Hcbba=2-(4′-chlorine-benzoyl)-benzoic acid,phen=1,10-phenanthroline,bipy=2,2′-bipyridine)have been hydrothermally synthesized and structurally characterized by elemental analysis,IR spectrum,TG and single-crystal X-ray diffraction.They are further extended into a three-dimensional supramolecular network structure through π-π interactions.Moreover,the luminescent property of complex 2 has been investigated in the solid state.CCDC:859144,1;859145,2.
hydrothermal synthesis;crystal structure;copper complex;zinc complex
O614.24+1
A
1001-4861(2012)08-1769-06
2011-12-21。收修改稿日期:2012-03-28。
吉林省教育厅科学技术研究(吉教科合字[2011]第459号)资助项目。
*通讯联系人。E-mail:lixm20032006@yahoo.com.cn
