由α-萘乙酸构筑的一维铜(Ⅱ)配位聚合物的合成、结构和性质研究
2012-09-15尹福军赵宏许兴友杨绪杰
尹福军赵 宏许兴友杨绪杰
(1南京理工大学化工学院,南京 210094)
(2淮海工学院,江苏省海洋资源开发研究院,连云港 222005)
(3淮阴工学院,淮安 223003)
由α-萘乙酸构筑的一维铜(Ⅱ)配位聚合物的合成、结构和性质研究
尹福军1,2赵 宏2许兴友*,1,3杨绪杰1
(1南京理工大学化工学院,南京 210094)
(2淮海工学院,江苏省海洋资源开发研究院,连云港 222005)
(3淮阴工学院,淮安 223003)
合成了一维聚合物[Cu(NAA)2]n(HNAA=α-萘乙酸),采用单晶X-射线、FTIR和元素分析对生成的晶体进行了结构表征。该聚合物属于正交晶系,Pbcn 空间群,a=3.077 2(3)nm,b=1.272 56(11)nm,c=1.023 51(9)nm,V=4.008 0(6)nm3,Z=8。Cu(Ⅱ)的配位几何构型是五配位的扭曲四方锥,配体的羧基采用μ2-η1∶η1和μ3-η2∶η1两种不同的桥联配位模式,连结Cu(Ⅱ)离子形成1D链,1D链又被C-H-π作用力进一步连结形成2D层和3D固态超分子结构。磁性研究表明:在该聚合物中,相邻铜离子间存在反铁磁偶合作用。还对其热稳定性进行了研究。
α-萘乙酸;Cu(Ⅱ)聚合物;磁性;热重
0 Introduction
The design and construction of metal-organic coordination polymers(MOCPs)have attracted considerable attention due to their interesting structural chemistry and potential applications in gas storage,separation,catalysis,magnetism,luminescence,drugdelivery[1-6],and nonlinear optics(NLO),and optical limiting capability[7-8].The structures of coordination polymers rely on several factors,but to select suitable bi-or multi-dentate bridging ligands is no doubt the key factor because it has an obvious influence on the topologies of coordination polymers.The behaviors of carboxylate ligands have been adopted to construct various structural coordination frameworks,because they can exhibit a variety of bonding modes to metals,including(i)terminal monodentate,(ii)chelating to a single metal,(iii)bridging bidentate to two metals in a syn-syn-,syn-anti-,an anti-anti-fashion,and (iv)bridging tridentate to two metal centers[9-10].These interesting findings have prompted us to seek this kind of carboxylate ligands to synthesize desired complexes.Thus,we chose carboxylic acid ligand,αnaphthylacetic acid (HNAA)with a bulky aromatic group,because it may introduce additional π-π and C-H…π interactions in stabling the structures of metal-organic frameworks.By doing so,we hope to obtain interesting coordination polymers.Among our attempts,a new polymer,namely[Cu(NAA)2]n(1),was obtained as crystals suitable for single-crystal X-ray analysis.The magnetic and thermal properties of the polymer 1 were investigated as well.
1 Experimental
1.1 Instruments and materials
All chemicals were of reagent grade obtained from commercial sources and used without further purification.IR Spectrum was recorded on a Nicolet NEXUS 470-FTIR spectrophotometer as KBr pellets in the 400~4 000 cm-1region.Elemental analyses(C,H)were carried out on a FLASH EA1112 Elemental Analyzer.TG-DSC measurements were performed by heating the sample from 20 to 800℃at a rate of 10℃·min-1on a NETZSCH STA 409PC differential thermalanalyzer.Variable temperature magnetic susceptibility data were obtained on polycrystalline Samples from 2 to 300 K in a magnetic field of 2 kOe,using a Quantum Design MPMS-XL7 SQUID magnetometer.All magnetic data have been corrected for diamagnetism by using Pascals constants[11].
1.2 Synthesis of the title complex
0.093 g (0.5 mmol)HNAA and 0.05 g(0.25 mmol)copper(Ⅱ)acetate was dissolved in 20 mL deionized water,and the pH value of the solution was adjusted to about 8 with sodium hydroxide solution(0.1 mol·L-1).The mixture was heated to reflux for 1 h in a water bath and then cooled to room temperature with green precipitate.The precipitation was redissolved in 30 mL tetrahydrofuran and left to stand at room temperature for several days,green block crystals of 1 were obtained.Yield:79% (based on Cu).Crystals of 1 are stable in the air.Anal.Calcd.for C24H18CuO4(%):C:66.35,H:4.14.Found(%):C:66.04,H:4.30.IR (cm-1,KBr):3 442(s),3 045(w),2 921(w),1592(s),1511(m),1409(s),1299(w),1260(m),1 047(w),1 017(w),781(s),719(m),647(m),580(w),547(m).
1.3 Crystal structure determination
A green block single crystal of the title complex(0.10 mm ×0.10 mm ×0.10 mm)was selected and mounted on a glass fiber.All measurements were made on a Bruker Smart 1000 diffractometer with a graphite-monochromated Mo Kα radiation(λ=0.071 073 nm).All data were collected at 298(2)K using the ω-2θ scan mode and corrected for Lorenz-polarization effects.A total of 19 795 reflections in the range of 2.55°to 25.02° (-31≤h≤36,-13≤k≤15,-12≤l≤11)and 3 535 unique ones (Rint=0.090 9)were collected.The empirical absorption corrections by SADABS were carried out.
The structure was solved by direct methods and expanded by Fourier technique.The non-hydrogen atoms were refined with anisotropic thermal parameters.The maximum peak in the final difference Fourier map is 471 e·nm-3and the minimum-301 e·nm-3.In the final circle of refinement the largest parameter shift(Δ/σ)maxis 0.000.All calculations were performed with SHELX-97 crystallographic software package[12].The crystal data and refinement details for the compound 1 are listed in Table 1,and the selected bond lengths (nm)and bond angles(°)are given in Table 2.
CCDC:835977.

Table 1 Crystallographic data for complex 1
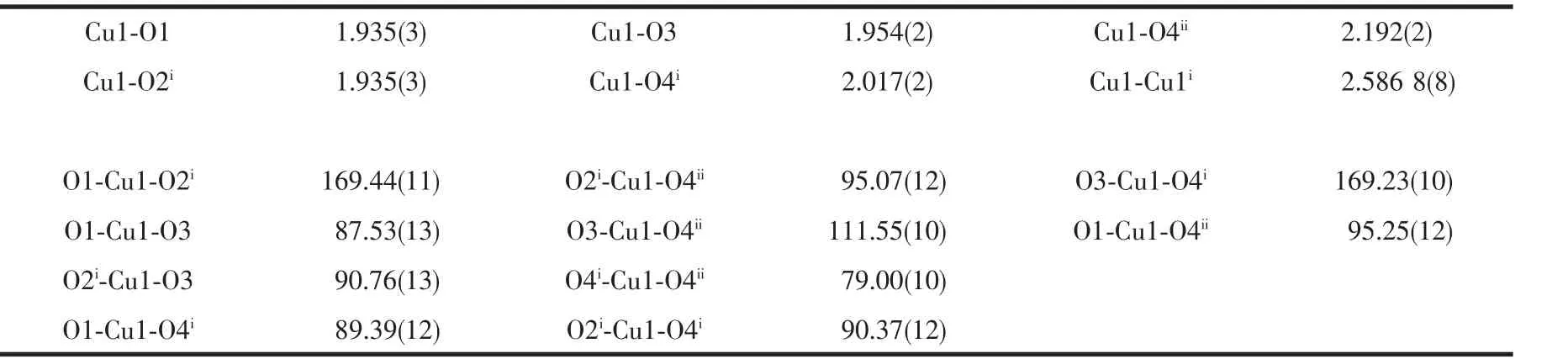
Table 2 Selected bond lengths(nm)and bond angle(°)of complex 1
2 Results and discussion
2.1 Crystal structure of 1
The asymmetric unit of 1 and the coordinated environment of copper(Ⅱ) ion showed in Fig.1.The crystal data and structure refinement for the title complex listed in Table 1.The asymmetric unit of the title polymeric compound (Fig.1)contains one Cu (Ⅱ)cation and two NAA-anion ligands.The Cu(Ⅱ) cation is coordinated by four O atoms (O1,O3,O2i,O4i,symmetry code:i-x+1,-y+1,-z+1)from four NAA-anions in the basal plane and the apical position is occupied by O4ii(symmetry code:iix,-y+1,z+1/2)from a fifth NAA-anion to complete the slightly distorted square-pyramidal coordination geometry.Cu1 atom is displaced by 0.018 09(4)nm from this plane in the direction of the apical O4iiatom.The corresponding equatorial Cu-O bonds are in the range 0.193 5(3)to 0.201 7(2)nm.The apical Cu1-O4iibond is 0.219 2(2)nm,it is longer than other Cu-O bonds in the basal plane,showing the typical Jahn-Teller distortion.The distance of Cu-Cu is 0.258 68(8)nm,which is shorter than the separation reported,indicating a Cu-Cu interaction in this complex[13].The max bond angles around central Cu1 ion are 169.44(11)°and 169.23(10)°,others range from 87.53(13)°to 95.25(12)°close to 90°,the distorted index τCu1=0.004(4)[14].The selected bond lengths and bond angles are listed in Table 2.
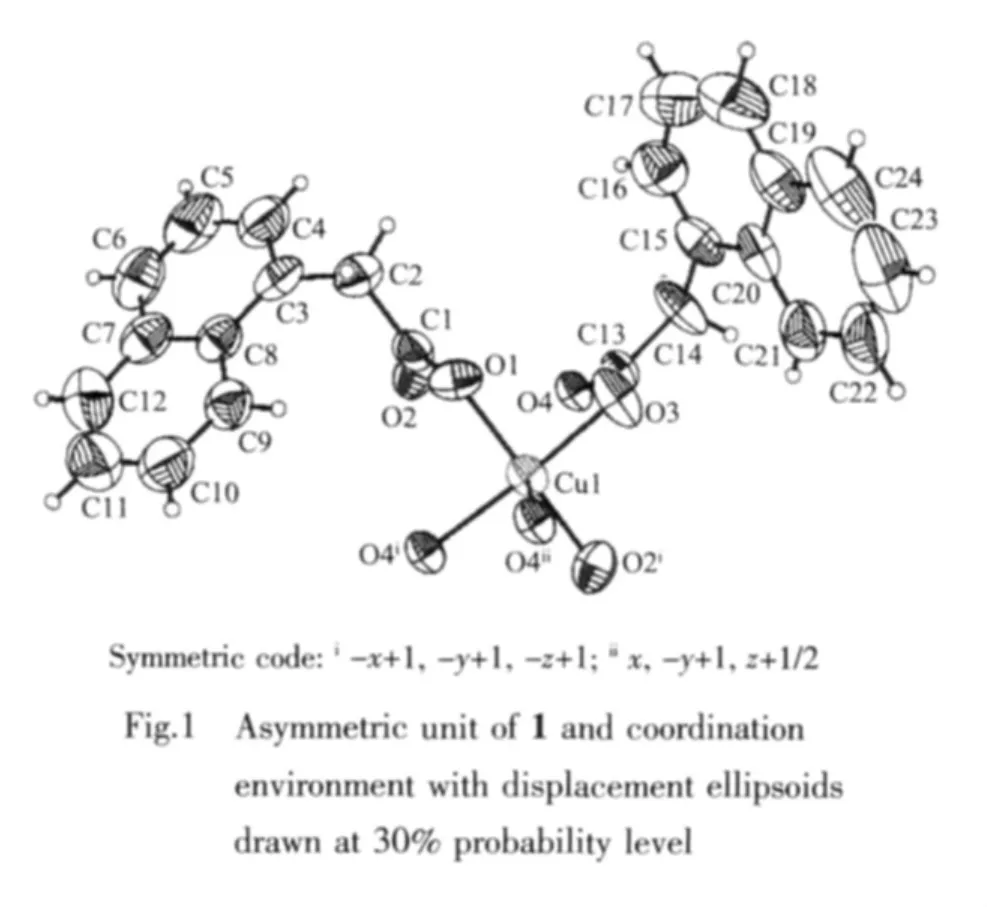
In NAA-anions,carboxylate groups take twodifferent coordination modes.One carboxylate group takes a μ2-η1∶η1chelating coordinate mode with Cu(Ⅱ)ion,the other one take a μ3-η2∶η1bridging coordination mode,which connects the adjacent Cu(Ⅱ)ion to form a infinite extension zigzag polymeric chain(Fig.2).
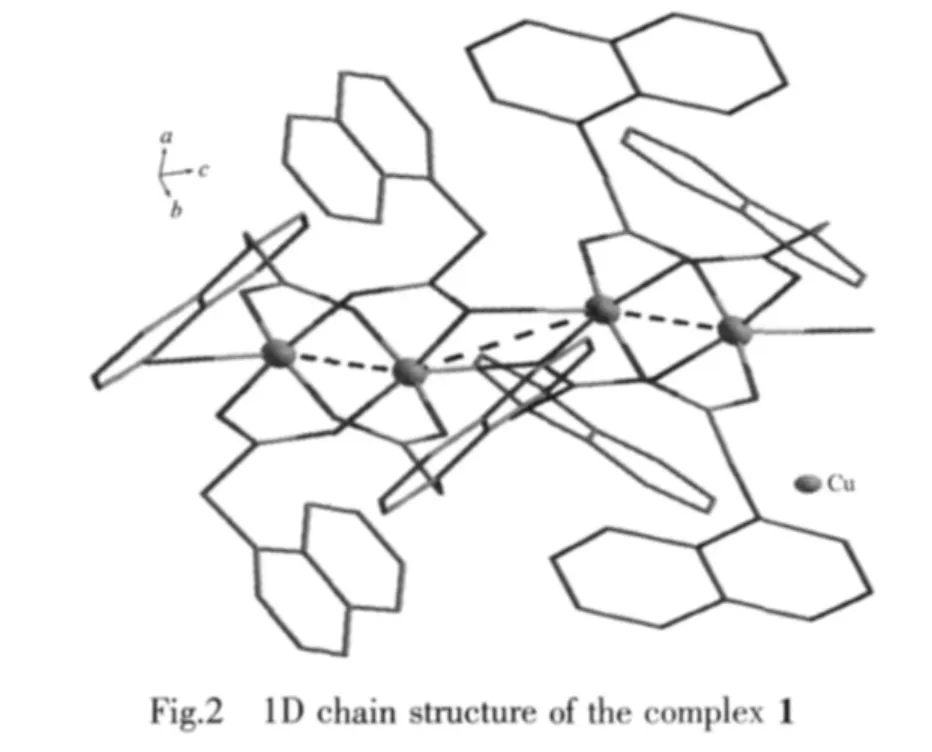
There are C-H-π interactions between naphthyl rings from neighbor chains since the separation of centroid-to-C is 0.354 3 nm.These one-dimensional chains are first extended into a 2D layer through this interaction.And then 2D layers further are linked together to give rise to 3D solid-state structure by CH-π with a distance of 0.441 0 nm[15].

2.2 Magnetic properties
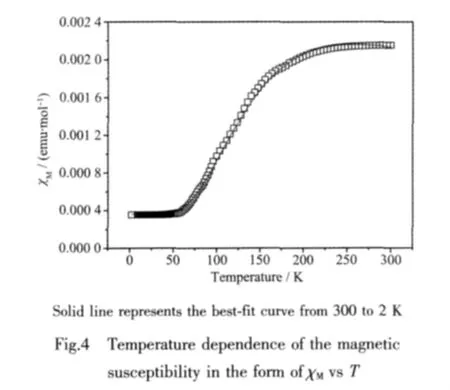
Magnetic susceptibility measurements of complex 1 were made in the temperature range 2~300 K.Fig.4 gives the plots of χMversus temperature (T).The χMvalue decreases as the temperature is lowered,which indicates the presence of antiferromagnetic interactions between the Cu(Ⅱ) centers bridged by the carboxylate groups.Although the expression for a simple dinuclear structure also reproduces correctly thetemperaturedependenceofχMofthechain compound,a better fit is obtained by using the expression developed by Hatfield and co-workers[16]for alternating chain compounds,which gives g=2.08,2J=-254 cm-1,an alternating factor α=0.016 and p=1.23%(the TIP was here fixed to 3.12×10-4cm3·mol-1.These results are in agreement with the many reports of coupling of Cuions through a tetracarboxylate bridge[17].In the case of the tetranuclear and chain compounds,the additional bridge corresponds to the apical coordination sites of the Cuions,where the spin density is expected to be negligible in any case,since the structure indicates that the magnetic orbital is d.On the contrary,the carboxylate bridges are coordinated in equatorial positions,in a syn-syn fashion,yielding a strong overlap and therefore a strong antiferromagnetic coupling.
2.3 Thermogravimetric analysis(TGA)
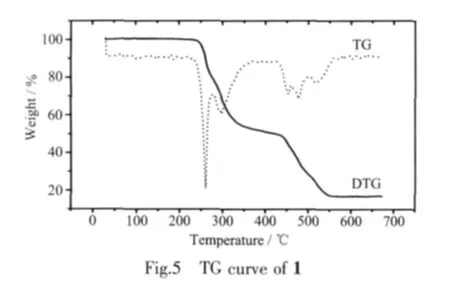
From the thermal analysis curve(TG/DTG)of the title complex 1 (Fig.5),we can see that there are two weight loss steps in the temperature range of 25~ca.570℃,corresponding to the decomposition of the organic ligand NAA.It firstly loses weight from room temperature to ca.470 ℃ (obsd.57.25%,calcd.59.07%),corresponding to the loss of two naphthalene molecules.The residue is copper acetate.The second weight loss of 28.96%from 470 to 560 ℃ results from the release of CO2and CH4molecules,corresponding to the decomposition of the copper acetate(calcd.27.72%).The residue is black CuO.
[1]Culp J T,Goodman A L,Chirdon D,et al.J.Phys.Chem.C,2010,114(5):2184-2191
[2]Cordovilla C,Coco S,Espinet P,et al.J.Am.Chem.Soc.,2010,132(4):1424-1431
[3]Ranford J D,Vittal J J,Wu D.Angew.Chem.Int.Ed.,1999,38:3498-3501
[4]Sharma C V K,Rogers R D.Chem.Commun.,1999,1:83-84
[5]Dietzel P D C,Morita Y,Blom R.et al.Angew.Chem.Int.Ed.,2005,44:1483-1492
[6]Li Y,Zheng F K,Liu X,et al.Inorg.Chem.,2006,45:6308-6316
[7]LI Jing(李静),JI Chang-Chun(季长春),WANG Zuo-Wei(王作为),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25:2083-2089
[8]ZHU Ying-Gui(朱英贵),JU Xue-Hai(居学海),SONG Ying-Lin(宋瑛林),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(12):2029-2034
[9]Zhou X P,Xu Z T,Zeller M,et al,Chem.Commun.,2009:5439-5441
[10]Abuhmaiera R,Lan Y,Ako A M.et al.CrystEngComm,2009:1089-1096
[11]Carlin R L.Magnetochemistry.Berlin Heidelberg:Springer-Verlag Publisher,1986:3
[12]Sheldrick G M.SHELX-97,Program for the Solution and Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[13]Wei H,Wu G,Liu Z F.Chinese J.Struct.Chem.,2011,30:1257-1264
[14]Liu Y F,Xu X Y,Xia H T.Synth.React.Inorg.Met-Org.Chem.,2009,39:400-405
[15]Wu G,Wang X F,Guo L,et al.J.Chem.Crystallogr.,2011,41:1071-1076
[16]James W H,Wayne E M,Robert R W.et al.Inorg.Chem.,1981,20:1033-1037
[17]Chiari B,Piovesana O,Tarantelli T,et al.Inorg.Chem.,1993,32:4834-4838
Synthesis,Structure and Properties of a 1D Copper(Ⅱ)Coordination Polymer Constructed by α-Naphthylacetic Acid
YIN Fu-Jun1,2ZHAO Hong2XU Xing-You*,1,3YANG Xu-Jie1
(1School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China)
(2Jiangsu Marine Resources Development Research Institute of Huaihai Institute of Technology,Lianyungang,Jiangsu 222005,China)
(3Huaiyin Insititute of Technology,Huai′an,Jiangsu 223003,China)
A copper(Ⅱ) coordination polymer,[Cu(NAA)2]n(HNAA=α-naphthylacetic acid)was synthesized and structurally characterized by means of X-ray single-crystal diffraction,IR spectrum and elemental analysis.It crystallizes in orthorhombic space group Pbcn with a=3.077 2(3),b=1.272 56(11),c=1.023 51(9)nm,V=4.008 0(6)nm3,Z=8.The coordination geometry of Cu(Ⅱ)is a distorted square-pyramid.Carboxylate groups take two kinds of bridging coordination modes: μ2-η1∶η1and μ3-η2∶η1,resulting in infinite zigzag 1D chains.These 1D chains are connected together to form layer and 3D stacking framework by C-H-π interactions between naphthyl rings.Magnetic measurement of the polymer shows that a strong antiferromagnetic coupling can be observed.The thermal stable property of the polymer was also investigated.CCDC:835977.
α-naphthylacetic acid;copper(Ⅱ) complex;synthesis;magnetic;thermal properties
O614.121
A
1001-4861(2012)08-1700-05
2011-11-18。收修改稿日期:2012-05-28。
江苏省海洋资源开发研究院自然科学基金(No.JSIMR11B03)资助项目。
*通讯联系人。E-mail:yfj1999@126.com
