分光光度滴定法测某些多齿配体与Ni(Ⅱ)配合物的稳定常数
2012-09-15HavaOzayAhmetUlgenYakupBaran
Hava OzayAhmet UlgenYakup Baran*,
(1Onsekiz Mart大学,文理学院,化学系,恰纳卡莱 17100,土耳其)
(2Erciyes大学,文理学院,化学系,开塞利,土耳其)
分光光度滴定法测某些多齿配体与Ni(Ⅱ)配合物的稳定常数
Hava Ozay1Ahmet Ulgen2Yakup Baran*,1
(1Onsekiz Mart大学,文理学院,化学系,恰纳卡莱 17100,土耳其)
(2Erciyes大学,文理学院,化学系,开塞利,土耳其)
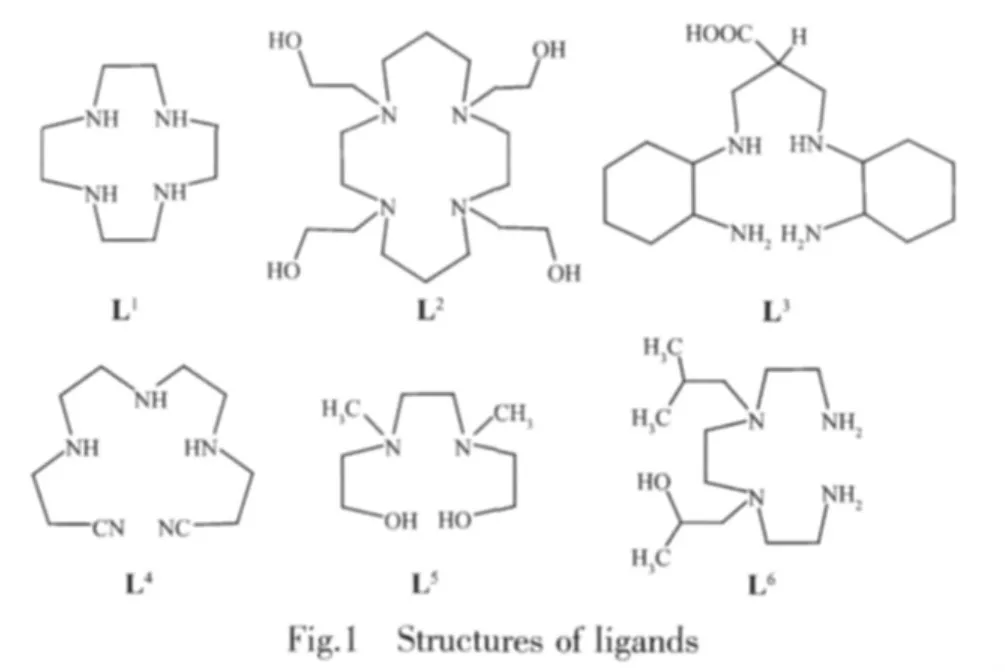
制备了多齿大环配体 1,4,7,10-四氮杂环十二烷(L1);1,4,8,11-四(2-羟乙基)-1,4,8,11-四氮杂环十四烷(L2)和无环多齿配体;3-(2-氨基环己氨基)-2-(2-氨基环己氨基甲基)丙酸(L3),4,7,10-十三烷二腈三氢氯化物(L4),2,2′-(1,2-二乙基-双((甲基二氮杂烷基)二乙醇(L5)and 1,1′-(1,2-二乙基-双((2-氨基乙基)二氮杂烷基))-2-二丙醇 (L6),并用 FTIR,NMR 和 MS 进行了表征,用配有二极管阵列检测器、蠕动泵和pH计的UV-VIS光度仪,经分光光度滴定法测定了它们与Ni(Ⅱ)的配合物的稳定常数。将稳定常数的数据与配体的开链和环状结构特性进行了关联讨论。还讨论了侧基对配合物稳定常数的影响。
配合物;多齿;分光光度滴定;Ni(Ⅱ)
0 Introduction
Forthe pastdecade,linearormacrocyclic polyamines have been studied extensively.They are an important class of compounds due to their role as polyprotic bases[1],biologically important compounds[2-3],sensors for the detection of metal ions and metal ion complexation[4-8].Transition metal complexes of multidentate ligands with N and O donors are used as model systems for many metalloenzymes[9-11],luminescencesensing,light-emitting devices,inter-metallic communication,catalysts,molecular electronics,chromotropic compounds,non-linear chromophores[12-16]and in coordination polymer chemistry[17].
Nickel has a very rich coordination chemistry[18].Nickel(Ⅱ)complexes are rich in color variation.They have coordination structures containing square-planar,tetrahedral,square-pyramidal,trigonal-bipyramidal and octahedral forms.Due to these properties of nickel(Ⅱ)complexes,a great number of studies relating to chromotropic metal complexes with applications as multi functional molecular devices have been carried out[19-20].In addition to these properties,metal ions may be part of the active sites of enzymes.There has been a great interest in the preparation of metal complexes which could mimic these metalloprotein′s active sites[21].
Potentiometric and spectrophotometric titration methods are generally used to investigate the equilibria in solutions to determine the acid-base constants[22-23].The potentiometric titration is used frequently due to the simplicity ofequipmentand minimaltime requirement[24].However,this method does not include all aspects of solution chemistry.In order to gain complete information about the species formed during titration,spectrophotometric titrations are usually carried out simultaneously[25].This technique shows how much equilibrium exists in the solution during the study and can be applied to structural analysis of compounds.A great number of studies have reported on the stability of nickel(Ⅱ)complexes with nitrogen and oxygen donor atoms.Basallote and co-workers reported equilibrium constants of mono-and bi-nuclear nickel complexes of the hexaazamacrocycle ligand.The equilibrium constants of complexes were obtained from potentiometric titration studies[26].Krot and co-workers investigated the stability constants of copper,nickel,silver and mercury complexes of a tetra amide ligand using the potentiometric titration method and determined that the nickel complexesarelessstablethantheircopperanalogues[27].
Here we report the synthesis of L1,L2,L3,L4,L5and L6and present the stability constants of nickel(Ⅱ)complexes obtained by spectrophotometric titration and subsequent global analysis of the data with Specfit/32 software package.
Fig.1 shows the structures of ligands.
1 Experimental
1.1 Chemicals and methods
All reagents were obtained commercially and used as received without further purification.Solvents were purified according to the standard methods prior to use.L1waspurchased from Sigma-Aldrich Chemical Company.Mass spectra were measured with a GC-MS,Thermo Finnigan Trace DSQ.NMR spectra were obtained with a Varian 300 MHzspectrometer.Spectrophotometric titration was measured with a UVVis,HP 8453 Diode Array Spectrophotometer.For the spectrophotometric titration acid or base solution was added to 1 cm quartz cell with a peristaltic pump(Cole Palmer,Masterflex)and the pH value of the solutions was measured with an Orion pH meter combined with a Metrohm semi-micro electrode.FTIR spectra were recorded with a Perkin Elmer BXII spectrometer.The molar magnetic susceptibilities of the complexes were measured on powdered sample at room temperature using a Sherwood Scientific Magnetic Susceptibility Balance.
1.2 Synthesis of ligands
1.2.1 Synthesis of 1,4,8,11-tetra(2-hydroxyethyl)-1,4,8,11-tetraazacyclo decane,L2·4H2O
Ethylene oxide(2.01 mL,40 mmol)was added to a solution of cyclam(1.38 g,4 mmol)in water(15 mL)at 0℃on a magnetic stirrer for six hours and warmed to room temperature.Solvent volume was reduced by a rotary evaporatorand the solution wasleftfor crystallization.Clear colorless crystals formed and werewashed with ice-cold water (2 mL)and dried under vacuum.Yield 1.1 g,63%.1H NMR (300 MHz,25 ℃,CDCl3,δ,J(Hz)):5.11(s),3.59(t,J=5.45,2H),2.27(s),2.49(s),2.43(t,J=6.12),2.13(m).13C NMR(300 MHz,25 ℃,CDCl3,δ):58.9,56.5,51.4,51.7,21.5,m/z:377(M+),FTIR(ATR,cm-1):Ⅴ(OH):3 349.
1.2.2 Synthesis of 3-(2-aminocyclohexylamino)-2-(2-aminocyclohexylaminomethyl)propionic acid,L3·4HCl·2H2O
L3was prepared by template synthesis from bis(cyclohexane-1,2-diamine)copper(Ⅱ),triethylamine,diethylmalonate and formaldehyde in methanol.The aqueous solution of copper(Ⅱ)perchlorate hexahydrate(11.28 g,30.00 mmol)was added to a solution of 1,2-diaminocyclohexane (6.85 g,60.00 mol)in deionized water (300 mL)on a magnetic stirrer.The reaction mixture was warmed to 50℃and was stirred at this temperature for 2 h.Then the solution was cooled to room temperature and Bis(cyclohexane-1,2-diamine)copper(Ⅱ) perchlorate was separated by filtering.
The solution ofbis(cyclohexane-1,2-diamine)copper(Ⅱ) perchlorate(6.00 g,12.00 mmol)in 250 mL methanol was heated to 50℃while stirring magnetically and to this solution,triethylamine (6 mL,43.05 mmol)and diethylmalonate(1.90 mL,12.00 mmol)were added.Then,a solution of formaldehyde(37%aqueous solution,3 mL)in methanol(50 mL)was added drop wise to the reaction mixture and the solution was stirred for 16 h at 50℃.The color of the reaction mixture converted to purple-red during to this time.This solution was diluted to 2 L with distilled water and then the solution was passed through a column (35×3.5 cm)of SP Sephadex C-25 resin (Na+form)and eluted with 0.2 mol·L-1NaClO4solution.After a while,two bands,one narrow and one broad,were observed.Both bands were collected and controlled.It was observed that a small amount of macrocyclic compound formed.The solvent of the acyclic compound was evaporated and dried.5 mL of triethylamine was diluted to 25 mL with deionized water and added to the solution of acyclic compound(3.5 g)in methanol(200 mL).The reaction mixture was stirred on a magnetic stirrer at 60℃for 12 h.Then the mixture was cooled to room temperature and diluted to 2 L with deionized water.The diluted solution was passed through a column (35×4 cm)of SP Sephadex C-25 resin (Na+form)and the column was elutedwith0.2mol·L-1NaClO4solution.Bands observed in the column were collected and the solution was concentrated to 300 mL by rotary evaporation.This solution and 3 mol·L-1HCl solution were simultaneously added over 2 hours drop wise from dropping funnels to Zn powder while stirring on a magnetic stirrer at room temperature.Then the solution was heated to 50 ℃ and stirred 30 min at this temperature.The solution was cooled to room temperature and was filtered on celite to remove Cu and residual Zn.The clear solution was diluted to 2 L with deionized water and the solution was passed through a column (35×3 cm)of Dowex 50 W×2 resin(H+form)and the column was eluted for a while with deionized water and afterwards with 1 mol·L-1HCl solution to remove Zn2+ions.Elution continued until no further Zn2+ions were present(checked by the addition of NaOH solution to eluent in order to observe Zn(OH)2).When the formation of jelly Zn(OH)2finished,the column was eluted with 3 mol·L-1HCl.After evaporation of the solvent by a rotary evaporator,the white colored crude product was obtained.Then the crude product was recrystallized in hot methanol and L3was obtained as a white powder(C16H32N4O2·4HCl·H2O),L3.Yield:2.1 g,52%.C16H35Cl4N4O2·4HCl·2H2O (Calcd.C,38.95;H,7.97;N,11.36)found%:C,38.79;H,7.81;N,11.44).1H NMR(300 MHz,D2O,δ,J(Hz)):1.28~2.11(m,16 H),2.13~2.46(m,6H),3.31~3.73(m,9H).13C NMR(300 MHz,D2O,δ):19.3,(2C);19.4(2C);22.8;23,1;26.1(2C);33.7;40.6;41.6;47.4 (2C);54.6;56.4;and 173.3.m/z:314(M+),FTIR(ATR,cm-1):Ⅴ(COOH):1 711 vs(br),2 017 m,1 612 s,1 514 s,Ⅴ(NH):3 369,3 152.
1.2.3 Synthesis of 4,7,10-triazatridecanedinitrile trihydrochloride,L4·3HCl
4,7,10-Triazatridecanedinitrile trihydrochloride was synthesized by condensation of diethylenetriamine(dien)and acrylonitrile according to the literature[27].Acrylonitrile(3.19 g,60.00 mmol)was added drop wise to a magnetically stirred dien solution (2.58 g,25 mmol),and the mixture was stirred for 20 h at roomtemperature.The crude product was purified as the trihydrochloride by recrystallization from methanol/water/HCl and L4·3HCl was obtained as a white powder.Yield:3.20 g,40%.C10H19N53HCl(Calc.C,37.69;H,6.96;N,21.98)found%:C,37.43;H,7.11;N,21.84).1H NMR(300 MHz,D2O,δ,J(Hz)):2.49(t,J=6.81,4H),2.55(t,J=5.97,4H),2.83(t,J=4.45,4H),2.89(t,J=5.77,4H).13C NMR(300 MHz,D2O,δ):120.2(2C);46.9(2C);46.5(2C);46.3(2C);17.9(2C);m/z:210(M+),FTIR(ATR,cm-1):Ⅴ(NH3+):2667,2435;Ⅴ(CN):2661.
1.2.4 Synthesis of 2,2′-(ethane-1,2-diyl)bis(methylazanediyl))diethanol,L5
Ethylene oxide (3.02 g,60.00 mmol)was added dropwise to a solution of N,N′-dimethylethylenediamine(2.00 g,22.68 mmol)in methanol(50 mL)at 0 C and the reaction mixture was stirred for 12 h.Then the reaction mixture was warmed to the room temperature and solvent volume was reduced by a rotary evaporator.The solution was left for crystallization and L5was obtained as a viscose oil.Yield:2.7 g,76%.C8H20N2O2(Calcd.C,38.88;H,8.16;N,11.33)found%:C,38.79;H,7.88;N,11.28).).1H NMR (300 MHz,D2O,δ,J(Hz)):3.83(t,J=6.0,4H),2.78(t,J=6.0,4H),2.53(t,J=6.0,4H),2.23(s,6H).13C NMR (300 MHz,D2O,δ):58.6(2C);58.1(2C);53.6(2C);41.7(2C);m/z:176.99(M+),FTIR(ATR,cm-1):Ⅴ(OH):3270 br.
1.2.5 Synthesis of 1,1′-(ethane-1,2-diylbis((2-aminoethyl)azanediyl))dipropan-2-ol,L6
Propylene oxide (2.32 g,40 mmol)was added dropwise to solution of triethylenetetraamine(2.92 g,20 mmol)in methanol(50 mL)at 0℃and the reaction mixture was stirred for 12 h.Then,the reaction mixture was warmed to room temperature and solvent volume was reduced by a rotary evaporator.The solution was left for crystallization and L5was obtained as a viscose oil.Yield:2.9 g,55%.C12H30N4O2(Calcd.C,54.93;H,11.52;N,21.35 found%:C,54.73;H,11.48;N,21.28).1H NMR(300 MHz,D2O,δ,J(Hz)):3.89(m,2H),3.28(t,J=6 Hz,4H),2.71(t,J=6,4H),2.49(t,J=6 Hz,4H),2.43(t,J=3,4H),1.22(d J=6,6H).13C NMR(300 MHz,D2O,δ):66.1(2C);61.1(2C);58.6(2C);53.7(2C);41.8(2C);21.5(2C)m/z:263.11(M+),FTIR(ATR,cm-1):Ⅴ(OH):3275 br,(NH):3357,3190.
1.3 Synthesis of the NiL1complex
A solution containing (1.13 mmol,0.36g)L1and 1.15 mmol,0.27g)NiCl26H2O in 80 mL argon saturated water was stirred and heated at 60℃for several hours.The green solution was then diluted to 500 mL with water,filtered and sorbed onto a column of SP Sephadex C25(Na+form)resin(20×5 cm).Upon elution 0.125 mol·L-1NaClO4,two bands were separated.A green band eluted with 0.2 mol·L-1NaClO4.This band was stable in acidic medium which was the initial indication of macrocyclic complex.The green band was reduced in volume by rotary evaporation and left to crystallize.The solid product was dried in vacuum desiccators.Anal.Calcd.for:[NiL1]Cl2·2H2O;C8H24Ni Cl2N4O2(%):C,28.44;H,7.16;N,16.58.Found(%)C,28.37;H,7.11;N,16.49.FTIR(cm-1,KBr):Ⅴ(N-H),3178,Ⅴ(Ni-N),543.
1.3.1 Synthesis of the NiL2complex
All the other complexes are prepared by the same method.Yield:68%,Anal.Calcd.for[NiL2]Cl2.H2O:C18H42NiCl2N4O5(%)C,41.25;H,8.08;N,10.69.Found(%):C,41.37;H,8.11;N,10.51.FTIR(cm-1,KBr):Ⅴ(NH),3166,Ⅴ(Ni-N),566.
1.3.2 Synthesis of the NiL3complex
Yield:73%,FTIR (KBr,cm-1):Anal.Calcd.for:[NiL3]Cl2·H2O;C16H36NiCl2N4O(%):C,44.68;H,8.44;N,13.03.Found(%)C,44.57;H,8.41;N,13.09.FTIR(cm-1,KBr):Ⅴ(N-H),3149,Ⅴ(Ni-N),577.
1.3.3 Synthesis of the NiL4complex
Yield:66%,Anal.Calcd.For%:[NiL4Cl]Cl·2H2O;C10H23NiCl2N5O2(%)C,32.04;H,6.18;N,18.64.Found(%):C,32.13;H,6.11;N,18.59.FTIR(cm-1,KBr):Ⅴ(NH),3182,Ⅴ(Ni-N),559.
1.3.4 Synthesis of the NiL5complex
Yield:75%,Anal.Calcd.For%:[NiL5]Cl2·H2O;C8H22NiCl2N2O3(%):C,29.67;H,6.85;N,8.65.Found(%)C,29.57;H,7.01;N,8.69.FTIR(cm-1,KBr):Ⅴ(NH),3176,Ⅴ(Ni-N),571.
1.3.5 Synthesis of the NiL6complex
Yield:58%,Anal.Calcd.For%:[NiL6]Cl2·H2O;C12H32NiCl2N4O3(%):C,35.15;H,7.87;N,13.66.Found(%):C,35.27;H,7.71;N,13.59.FTIR(cm-1,KBr):Ⅴ(NH),3174,Ⅴ(Ni-N),579.
1.4 Electronic spectra
The electronic spectra data for the Ni(Ⅱ)complexes are given in Table 1.There are four bands for the Ni(Ⅱ)complexes in UV-Vis spectrum.The low intensity bands around 560 and 950 nm could be assigned to dd,Laporte forbidden,spin allowed transitions of Ni(Ⅱ)ions.The medium intensity bands around 380 nm are due to metal-ligand charge transfer processes.
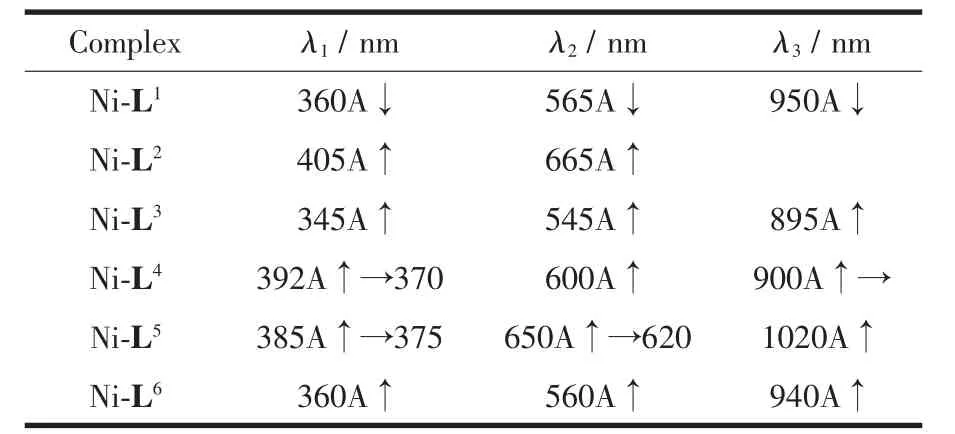
Table 1 Absorbance changes in Ni-L complexes during spectrophotometric titration
1.5 Magnetic measurements
The Ni(Ⅱ)complexes with the allligands are diamagnetic indicating the square planer structure of the complex.
1.6 Spectrophotometric titrations
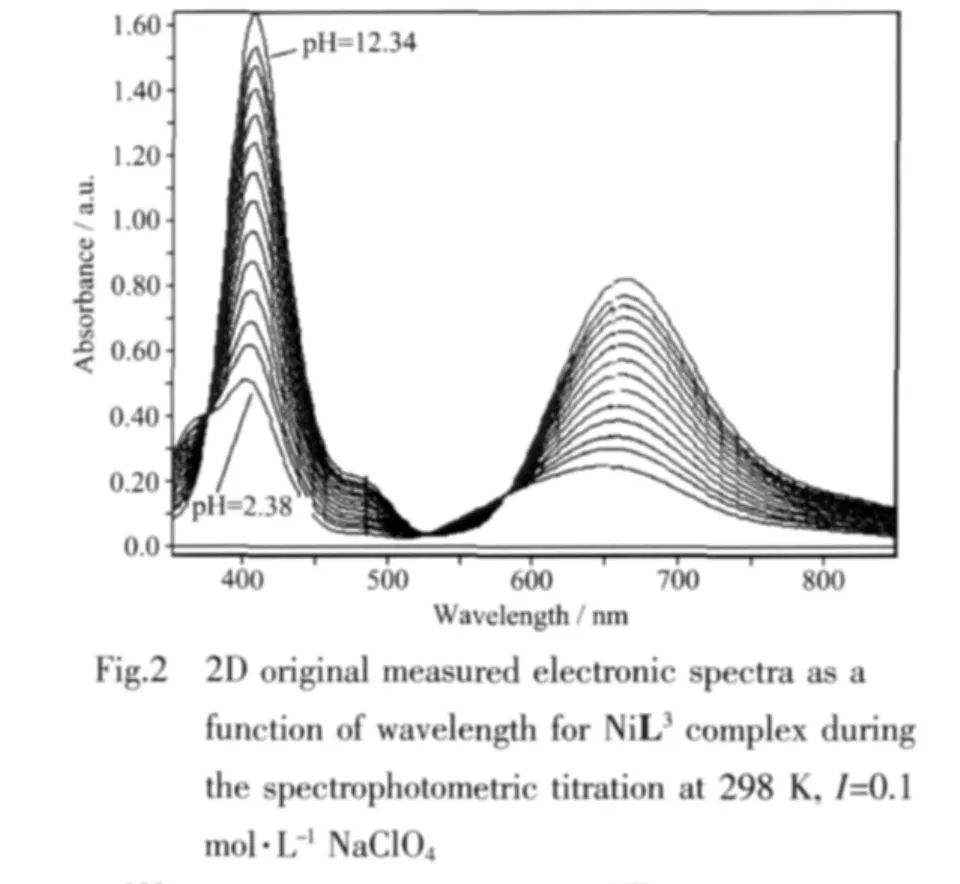
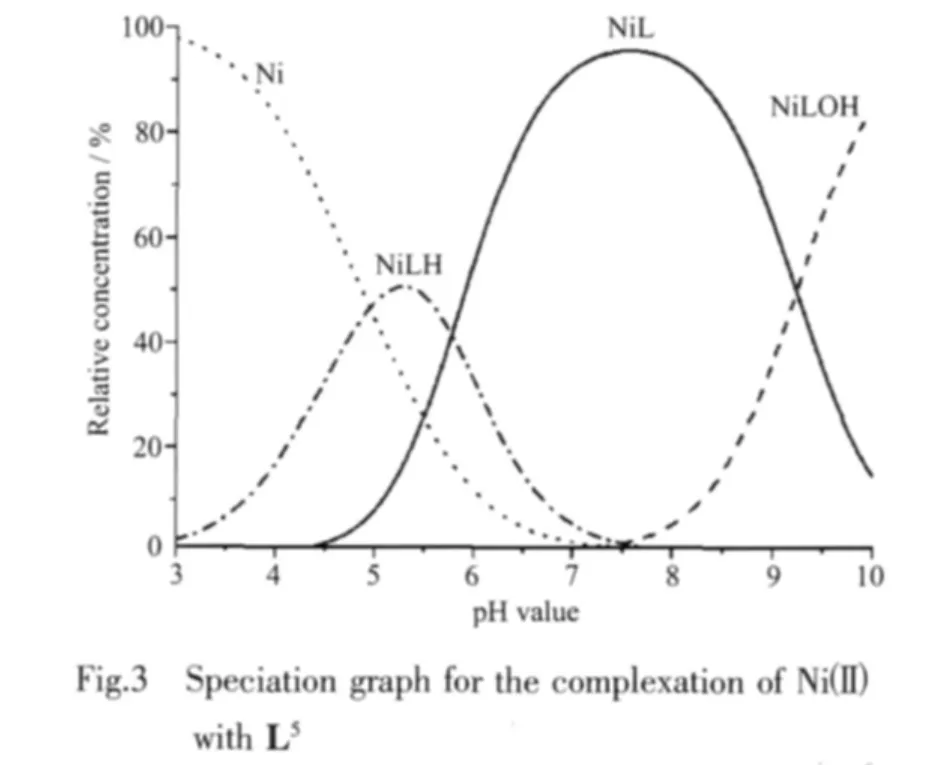
Stability constants of the complexes were measured with an automatic titration set up consisting of a computer interfaced to an Agilent HP 8453 Diode ArraySpectro-photometerwith astirrerundera thermostated cell holder,a peristaltic pump,Cole Palmer and an Orion pH meter combined with an Metrohm semi-micro electrode.The electrode was calibrated with pH value of 4.0 and 7.0 buffers for measurements in aqueous solutions.Argon-saturated solutions of the ligands(1.2 mmol)and the nickel(Ⅱ)(1.2 mmol)containing 0.1 mol·L-1NaClO4for the adjustment of ionic strength were titrated with base,0.1 mol·L-1NaOH,in 1 cm quartz cell and the cell compartment was thermostated to (25±0.1)℃ during titration.The cell was containing a pH electrode and a capillary tip from peristaltic pump.The UV-Vis spectrum was determined during the titration at 60 sec intervals over the wavelength range of 350~1 100 nm Fig.2 shows typical 2D absorption spectra of NiL3during spectrophotometric titration as a function of pH value.Fig.3 shows speciation graph for the complex formation of Ni(Ⅱ) with L5.The measurements were made over the pH value range of 2.0 to 11.0.Triplicate data analyses were performed for each complex.Data analysis was carried out using the nonlinear leastsquare fitting program Specfit/32.An initial guess for the equilibrium constants were entered and these values iteratively refined until the best fit was achieved.
2 Results and discussion
2.1 Stability of the complexes
All the macrocyclic and acyclic complexes are colored solid and stable at room temperature.They are soluble in water.Each Ni(Ⅱ) ion is coordinated to four nitrogen atoms in the NiL1,NiL2,NiL3and NiL6complexes.The Ni(Ⅱ) ion is incorporated into ligands to form square planer environment.The other ligands L4and L5which have N3and N2O2donor atoms form also square planer geometry.Magnetic measurements of thecomplexes support for the square planer geometry.All the complexes exhibit diamagnetism in solid state at room temperature.Pendant groups in L2,L4and L6ligands do not involve in coordination and stay as dangling group in the complexes.This is supported by FTIR study of the complexes and ligands.The O-H stretching vibration of the ligands does not change after complex formation.The trend in stability order for diamagnetic Ni(Ⅱ) complexes are observed that Ni(Ⅱ)ions prefer the smallest macrocycle L1.The same effect is found for the open-chain tetraazaamines L6~L3.The larger the cavity,the more their complexes are destabilized by the presence of six membered rings.Large differences in stability constants within the series are observed for the nickel(Ⅱ)complexes of macrocyclic and acyclic ligands.When NiL1stability is compared with open chain analogue NiL6,stability decreases from 21.61 to 17.93.As the number of chelate rings increase,stability of the complexes decrease for the Ni(Ⅱ)ions[28].When the intermediate in a five-membered chelate ring is compared with the six-membered chelate ring,it is observed that in the five-membered chelate ring,the free donor group will possess increased entropy.As a result,the small size chelate ring will show greatest entropy increase while the larger ring chelate will show a decrease in entropy.This effect may be observed when NiL1is compared with NiL2.NiL2has two six membered rings while NiL1has no six membered ring,as a result stability decrease from 21.61 to 19.52.The enhanced stability of the macrocyclic ligand over its acyclic analogue is explained by the macrocyclic effect.Solvation of the ligands is also important during complexation.Macrocycles are thought to be less solvated than their acyclic analogues which leads to an enhancement of the thermodynamic stability for cyclic ligands over their acyclic analogues.Pendant groups on the nitrogen atoms will cause a decrease in the basicity of the nitrogen donor atoms and as a result of this,the stability of the complexes will decrease.There is a wealth of stability constant data for the polyazamacrocycles with different metal ions[29-32].Macrocycles can be organized to select particular metal ions from solution and can be used in metal ion extractions.Selectivity of the macrocycles can be altered in different ways.By changing cavity size and adding pendant groups to the nitrogen atom in the ring,the selectivity of the macrocycles changes.
3 Conclusions
For the most of the complexation titrations,one protonated complex species is observed which can be assigned to protonation of the primary amine or one of the secondary amines in the macrocycle.It is expected that the stability and selectivity of the ligands with nickel(Ⅱ)can be classified according to the parameters mentioned.NiL1complex is the most stable.The macrocyclic effect,number of rings and ring size cause nickel(Ⅱ) to bind to L1selectively.The stability of the NiL2decreases when compared to the NiL1.The cavity size,change in basicity of the donor atoms and steric effect cause a decrease in the stability of the nickel(Ⅱ)complex with L2.L6and L5ligands are the acyclic analogue of L1and L2,respectively.The stabilities of NiL6and NiL5decrease as expected.The least stable complex is NiL5with an alkylated secondary amine and two oxygen donor atoms.The stability constant of NiL4is 12.31 with three donor nitrogens which do not saturate the coordination sphere of the nickel(Ⅱ).Tetradentate coordination has been established for nickel(Ⅱ) with all of the ligands studied.Similar complex formation constants are observed for macrocyclic NiL1and NiL2since nickel(Ⅱ)is bound to the macrocyclic plane in the same manner.Table 2 shows the stability constants of the complexes at 20℃,I=0.1 mol·L-1NaClO4.
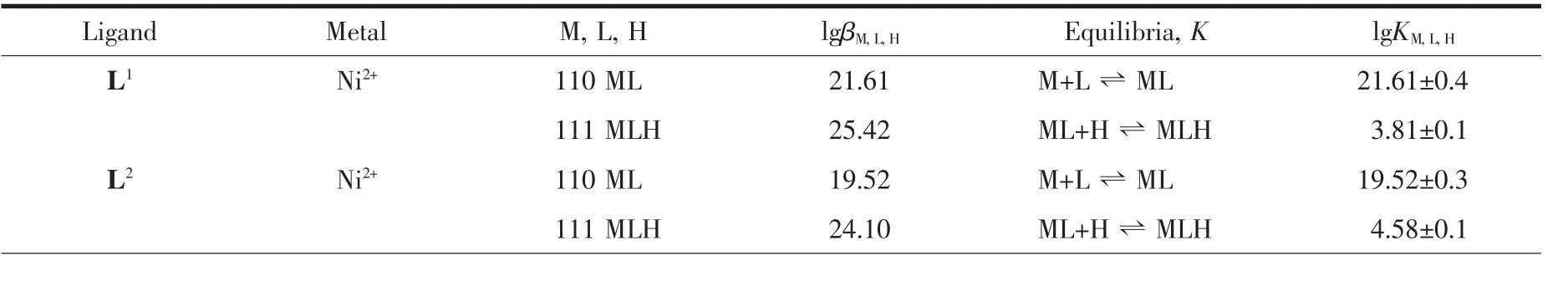
Table 2 Stability constants of the complexes at 20 ℃,I=0.1 mol·L-1NaClO4
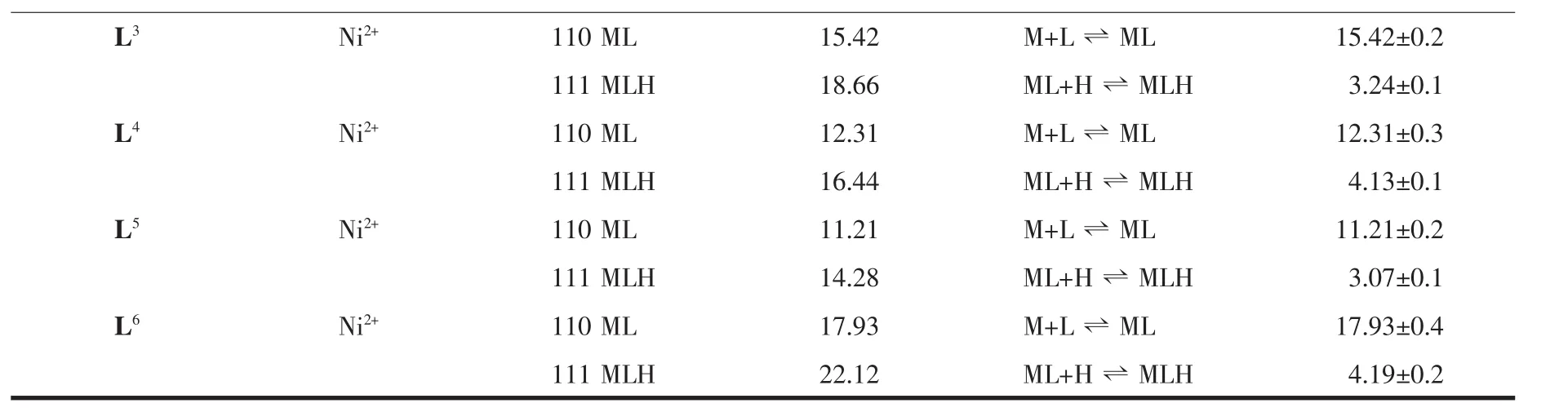
Continued Table 2
Acknowledgements:The authors thank the Scientific and Technological Research Council of Turkey (TUBITAK)for financial support(Project No.104T389).
[1]Cascio S,Robertis A D,Foti C.Fluid Phase Equilibr.,2000,170:167-181
[2]Silva J A,Felcman A L R,Lopes C C,et al.Inorg.Chim.Acta,2003,356:155-166
[3]Herve A C,Yaouanc J J,Toupet L,et al.J.Organomet.Chem.,2002,664:214-222
[4]Lai R A,Chakraborty M,Chanu O B,et al.J.Coord.Chem.,2010,63:1239-1251
[5]Ajibade A P,Zulu H N.J.Coord.Chem.,2010,63:3229-3239
[6]Ozay H,Baran Y.J.Coord.Chem.,2010,63:4299-4308
[7]Yamada Y,Takenoudhi S I,Okamoto K I,et al.J.Coord.Chem.,2010,63:996-1012
[8]Basallote M G,Domenech A,Verdejo B,et al.Inorg.Chim.Acta,2006,359:2004-2014
[9]Jubert C,Mohamadou A,Barbier J P,et al.Inorg.Chem.Commun.,2003,6:900-907
[10]Ambrosi G,Formica M,Pontellini R,et al.Inorg.Chim.Acta,2009,362:2667-2677
[11]Sarma M,Singh A,Mondal B,et al.Inorg.Chim.Acta,2010,363:63-70
[12]Shirase H,MiuraY,Fukuda Y.Monatsh Chem.,2009,140:807-814
[13]Shirase H,Mori Y,Uchiyama M,et al.Monatsh Chem.,2009,140:801-805
[14]Deplano P,Marchio L,Yagubski E B,et al.Monatsh Chem.,2009,140:775-781
[15]Amatore C,Jutand A,Rollin Y,et al.Monatsh Chem.,2000,131:1293-1304
[16]Panda G,Selim M,Mukherjea K K,et al.Monatsh Chem.,2009,140:281-286
[17]Kirillov A M,Kopylovich M N,Pombeiro A J L,et al.Angew Chem.Int.Ed.,2005,44:4345-4349
[18]Chattopadhyay T,Mukherjee M,Das D,et al.Inorg.Chem.,2010,49:3121-3125
[19]Murata F,Arakawa M,Fukuda Y,et al.Polyhedron,2007,26:1570-1578
[20]Koner S,Tsutake M,Banerjee S,et al.J.Mol.Struct.,2002,608:63-69
[21]Hubert S,Mohamadou A,Gerard C.Inorg.Chim.Acta,2007,360:1702-1710
[22]Ibanez G A,Escander G M.Polyhedron,1998,17:4433-4441
[23]Kadar M,Biro A,Huszthy P.Spectrochim Acta A,2005,62:1032-1038
[24]Dyson R M,Kaderli S,Zuberbühler A D,et al.Anal.Chim.Acta,1997,353:381-393
[25]Dyson R M,Lawrance G A,Maeder M,et al.Polyhedron,1999,18:3243-3251
[26]Basallote M G,Fernandez-Trujillo M J,Manez M A.Dalton Trans.,2002:3691-3695
[27]Krot K A,Namor A F D,Nolan K B,et al.Inorg.Chim.Acta,2005,358:3497-3505
[28]Polster J,Lachmann H.Spectrometric Titrations:Analysis of Chemical Equilibria,VCH,1989.
[29]Luckay R C,Hancock R D.Dalton Trans.,1991:1491-1494
[30]Martel A E,Smith R M.The Critical Stability Constants:Vol.1-6,N Y:Plenum Press,1974-1989.
[31]Bianchi A,Micheloni M,Paoletti P.Coord.Chem.Rev.,1991,110:17-113
[32]Izzat R M,Pawlak P,Breuning R L,et al.Chem.Rev.,1991,91:1721-1733
Stability Constants of Some Polydentate Ligands with Nickel(Ⅱ)by Spectrophotometric Titration
Hava Ozay1Ahmet Ulgen2Yakup Baran*,1
(1Onsekiz Mart University,Art and Science Faculty,Department of Chemistry,Canakkale 17100,Turkey)
(2Erciyes University,Art and Science Faculty,Department of Chemistry,Kayseri,Turkey)
The polydentate macrocyclic ligands,1,4,7,10-tetraazacyclododecane (L1),1,4,8,11-tetra(2-hydroxyethyl)-1,4,8,11-tetraazacyclotetradecane (L2);and acyclicpolydentate ligands;3-(2-aminocyclohexylamino)-2-(2-aminocyclohexylaminomethyl)propionic acid(L3),4,7,10-triazatridecane dinitrile trihydrochloride(L4),2,2′-(1,2-diyl)bis(methylazanediyl)diethanol(L5)and 1,1′-(ethane-1,2-diylbis((2-aminoethyl)azanediyl))dipropan-2-ol,(L6)were prepared and their structures were investigated by FTIR,NMR and MS.The stability constants of the nickel(Ⅱ)complexes with these ligands were determined by spectrophotometric titration using a diode array UV-VIS spectrophotometer equipped with peristaltic pump and pH meter.The values of the stability constants are discussed in terms of the open chain or cyclic nature of the ligands.The effect of pendant group on the stability of the complexes is discussed.
complex;polydentate;stability constants;spectrophotometric titration;nickel(Ⅱ)
O614.4;O614.81+3
A
1001-4861(2012)08-1680-07
2011-12-20。收修改稿日期(Date revised):2011-03-06。
The Scientific and Technological Research Council of Turkey(TUBITAK)(Project No.104T389)资助项目。
*通讯联系人(Corresponding Author)。E-mail:yakupbaran@yahoo.com
