Immunological tolerance of human hepatocyte xenograft induced by adenovirus vector-mediated CTLA4Ig gene transfer
2012-07-07YaoKaiChenXiaoCongLiuJunGangLiGuoDongLiuYanGuoLingChengandYuMingWang
Yao-Kai Chen, Xiao-Cong Liu, Jun-Gang Li, Guo-Dong Liu, Yan Guo, Ling Cheng and Yu-Ming Wang
Chongqing, China
Immunological tolerance of human hepatocyte xenograft induced by adenovirus vector-mediated CTLA4Ig gene transfer
Yao-Kai Chen, Xiao-Cong Liu, Jun-Gang Li, Guo-Dong Liu, Yan Guo, Ling Cheng and Yu-Ming Wang
Chongqing, China
BACKGROUND:Systemic administration of CTLA4Ig has been applied in inducing immunological tolerance of hepatocyte implants, but has potential for systemic immune inhibition. This study was designed to induce hepatocyte immunological tolerance by locally expressing CTLA4Ig in an attempt to improve the effectiveness of cell transplantation.
METHODS:A normal human liver cell line (L02) was transfected with adenovirus vector containing the CTLA4Ig gene (Ad-CTLA4Ig-EGFP)in vitro, and the expression of CTLA4Ig by transfected cells was assessed by fluorescent imaging and immunocytochemical staining. Transfected cells then were injected into the spleen of Sprague-Dawley rats, the survival of cells was determined by immunohistochemistry, and the immune status was examined through CD4+and CD69+T cellcounts and ELISA detection of IL-2 in peripheral blood.
RESULTS:L02 cells expressed CTLA4Ig in the cytoplasm for >4 weeks. Surviving L02 cells were observed in the experimental group at 3 and 4 weeks post-transplantation, while none was detected in the control group. Furthermore, the percentages of CD4+and CD4+CD69+T cells in the CTLA4-transfected group were 24.5% and 45.1%, markedly lower than those in the control group at 4 weeks post-transplantation (P<0.01). Furthermore, the IL-2 level was also lower in the CTLA4-transfected group than in the control group.
CONCLUSION:Adenovirus-mediated CTLA4Ig gene transfer into human hepatocytes has the potential to become an effective method of inducing immunological tolerance in hepatocyte transplantation.
(Hepatobiliary Pancreat Dis Int 2012;11:148-153)
CTLA4Ig; adenovirus vectors; hepatocyte transplantation; immune tolerance; graft rejection
Introduction
Hepatocyte transplantation has become a promising therapeutic approach to hepatic failure and metabolic liver diseases. Potential advantages of cell transplantation include a simpler, safer, less invasive and costly procedure, and more efficient use of donor organs compared to liver transplantation. Recently, several human hepatocyte cell lines which may be used as universal donors have been developed.[1]However, such cells still face immunological processes in transplantation. Cytotoxic T lymphocytes-associated with antigen 4-immunoglobulin (CTLA4Ig) could block the CD28/B7 co-stimulatory pathway, inhibit T cell activation, and thus induce immunological tolerance to a specific antigen.[2,3]There is a mass of data indicating that tolerance to a graft is induced by systemic administration of CTLA4Ig protein or transfection with the CTLA4Ig genein vivo.[4-7]Since both methods have the potential to extensively inhibit the immune system and cause adverse systemic effects (e.g., susceptibility to infections and malignancy),[8]we transferred the CTLA4Ig gene into L02 cells by adenovirus vector, in an attempt to establish a method by which hepatocytes express CTLA4Ig locally and temporarily to induce immunological tolerance.
Methods
Materials
The recombinant adenovirus vector Ad-CTLA4Ig-EGFP was constructed and provided by Chongqing Key Laboratory for Disease Proteomics, Institute of Burn Research, Southwest Hospital, the Third Military Medical University.
The target fragment CTLA4Ig-EGFP cDNA was inserted into cosmid pAxCAwt to construct the shuttle plasmid CTLA4Ig-EGFP-pAxCAwt. After amplification, the shuttle plasmid was co-transferred into 293 cells with adenovirus gene DNA-TPC, and through homologous recombination, the Ad-CTLA4Ig-EGFP was constructed successfully. Finally, Ad-CTLA4Ig-EGFP was expanded in the 293 cell line and concentrated with a condensing centrifuge tube.
The normal human liver cell line L02 was purchased from the cell bank of the Chinese Academy of Sciences, Shanghai. Adult male Sprague-Dawley rats weighing 200-250 g were used as recipients. All animals were purchased from the Experimental Animal Department of the Third Military Medical University, and were maintained under specific pathogen-free conditions. The protocols were approved by the Institutional Animal Care and Use Committee at the Third Military Medical University. Human CTLA4 ELISA and rat IL-2 ELISA kits were from Bender Medsystem (Austria). Goat anti-human albumin was from Bethyl Laboratories, Inc. (USA). Goat anti-rat CD69 and FITC-conjugated rabbit anti-rat CD4 were from Santa Cruz, Inc. (USA). Cy3-conjugated rabbit anti-goat IgG was from Boster Biological Technology, Ltd. (Wuhan, China).
Transduction of the CTLA4Ig gene into L02 cells
L02 cells were transfected by Ad-CTLA4Ig-EGFP at a MOI of about 600. Cell morphology and fluorescence were observed daily with a laser scanning confocal microscope (TCS-TIV, Leica, Germany). Goat antihuman CTLA4Ig polyclonal antibody was used to detect the expression of CTLA4Ig in L02 cells by immunocytochemical staining.
Cell transplantation
Intrasplenic injection is an effective method of hepatocyte transplantation, 55±7% of the implanted hepatocytes are transported to the recipient liver within 48 hours.[9]Twenty-four Sprague-Dawley rats were randomly and equally divided into 4 groups, group A: NaCl, group B: L02, group C: CTLA4-L02, and group D: EGFP-L02. All rats were anesthetized with 3% pentobarbital. A median abdominal incision was made to perform a 2/3 partial hepatectomy. Then the rats were given intrasplenic injections of NaCl (A), normal L02 cells (B), or CTLA4-transfected L02 cells (C). In group D, the implanted cells were transfected with adenovirus vector that only carried the reporter gene EGFP (Ad-EGFP). All implanted cells were suspended to 1×107in 100 µL sterile Dulbecco's Modified Eagle's Medium without serum, and injected into the tip of the spleen through 26-gauge needles. After suturation, the rats were fed in separate cages.
Immunohistochemistry
At 3 weeks post-operation, 3 mice from each group were sacrificed, and at week 4, the remaining 3 were sacrificed. Livers were harvested and paraffin sections were made for immunohistochemistry. Goat antihuman albumin was used as the first antibody to assess the survival of L02 cells, and color was displayed by 3, 3'-diaminobenzidine (DAB).
Flow cytometry
Peripheral blood was collected from the orbital venous plexus at week 4 post-transplantation, and erythrocytes were lysed by Tris-NH4Cl (pH 7.2). The residual cells were incubated successively with FITC-conjugated rabbit anti-rat CD4, goat anti-rat CD69 and cy3-conjugated rabbit anti-goat IgG for 30 minutes each. The percentages of CD4- and CD69-positive cells were determined by flow cytometry.
Enzyme linked immunosorbent assay (ELISA) test for IL-2 in serum
Peripheral blood was collected from the orbital venous plexus in coagulation tubes at week 4 posttransplantation. After centrifugation at 7500 rpm for 15 minutes, serum was collected for detection of rat IL-2 by ELISA.
Statistical analysis
All experiments were repeated twice. Results are expressed as mean±SD. Data were analyzed with Student'sttest or ANOVA as appropriate for the data set.P<0.05 was considered statistically significant.
Results
Expression of CTLA4Ig in L02in vitro
CTLA4Ig levels were measured by fluorescence microscopy at regular intervals after transfection with Ad-CTLA4Ig-EGFP. Green fluorescence was observedin the cytoplasm within 16 hours; the fluorescence intensity peaked at 48-72 hours and lasted for >4 weeks. No morphological changes were observed. Furthermore, immunocytochemical staining showed that CTLA4Ig was strongly expressed in the cytoplasm after transfection (Fig. 1).
Graft cell survival
Transfected L02 cells that expressed human albumin were detected in recipient livers in the CTLA4-L02 group at weeks 3 and 4 post-transplantation; they were mainly located around portal tracts. No L02 cells were observed in the other 3 groups (Fig. 2).
Effect of CTLA4Ig on T cell function after transplantation of xenogeneic hepatocytes

Fig. 1. CTLA4 expressed by L02 cells after transduction by Ad-CTLA4Ig-EGFP (original magnification ×400). (A) EGFP expressed in L02 at 48 hours post-transfection (laser scanning confocal microscopy). (B) L02 cells positive for CTLA4 immunocytochemical staining after transfection (inverted phase contrast microscopy). All experiments were repeated twice times with similar results.
CTLA4Ig functions as a negative regulator of T cell activation, and cells in the CD4+T subset are the main contributors to cell rejection. Therefore, we first tested the activated state of CD4+T cells in the transplanted rats by the expression of CD69, a marker of activated T cells. At week 4 post-transplantation, group C had a lower percentage of CD4+T cells in peripheral blood leucocytes but a higher percentage of CD69+T cells in CD4+T cells than groups B and D (Fig. 3).
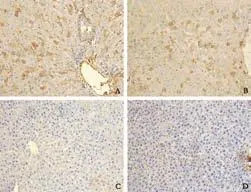
Fig. 2. Immunohistochemical staining of liver tissue from rats receiving transplantation with CTLA4-L02 cells, normal L02 cells and EGFP-L02 cells with human albumin. Immunohistochemical staining of liver tissue with human albumin-specific monoclonal antibody (original magnification ×200). (A) Group C (receiving CTLA4-L02 cells), 3 weeks after transplantation; and (B) Group C, 4 weeks after transplantation; clusters of transplanted L02 cells were positive for human albumin staining. (C) Group B (receiving normal L02 cells) and (D) Group D (receiving EGFP-L02 cells) were negative for human albumin immunocytochemical staining, 4 weeks after transplantation. All experiments were repeated twice with similar results.
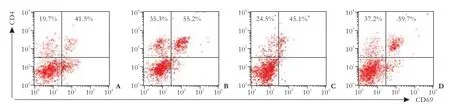
Fig. 3. Activation of CD4+T lymphocytes inhibited in rats receiving CTLA4-L02 cells compared to normal L02 cells and EGFP-L02 cells, 4 weeks after transplantation. Four weeks after transplantation, the percentage of CD4+T cells was 24.5% in the CTLA4-L02 group, but 35.3% in the L02 group (P=0.001), and 37.2% in the EGFP-L02 group(P<0.01); the percentage of CD4+CD69+T cells in CD4+T cells was 45.1% in the CTLA4-L02 group, but 55.2% in the L02 group (P=0.006) and 59.7% in the NaCl group (P=0.001). (A) Group A: rats receiving NaCl injection; (B) Group B: rats receiving normal L02 cells; (C) Group C: rats receiving CTLA4-L02 cells; (D) Group D: rats receiving EGFP-L02 cells. All experiments were repeated twice with similar results. *: P=0.001, vs group B; #: P=0.006, vs group B.
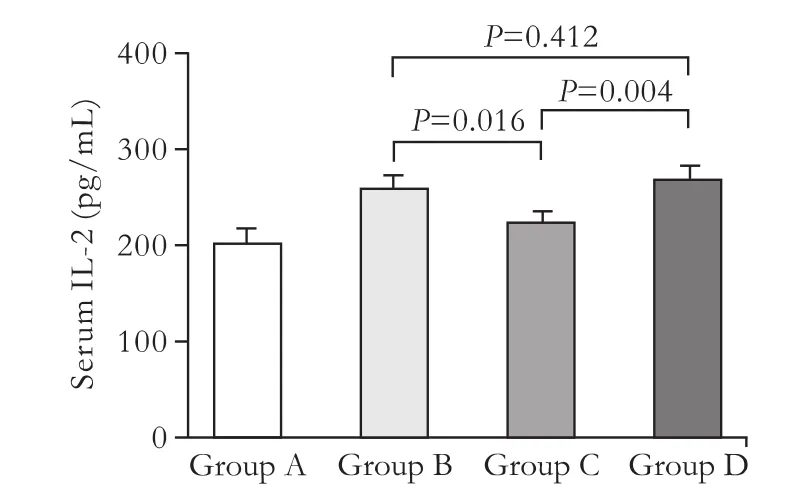
Fig. 4. The group receiving CTLA4-L02 cell transplants showed a lower level of IL-2 than that receiving normal L02 cells 4 weeks after transplantation determined by ELISA. Group A: rats receiving NaCl injection (200.9±17.0 pg/mL); group B: rats receiving normal L02 cells (259.5±14.7 pg/mL); group C: rats receiving CTLA4-L02 cells (223.9±11.3 pg/mL); group D: rats receiving EGFP-L02 cells (269.6±13.6 pg/mL) (mean±SD, n=3). One-way ANOVA was used to compare the three groups. All experiments were repeated twice with similar results.
T-cell mediated transplant rejection can be caused by the secretion of cytokines. We then asked whether CTLA4Ig altered cytokine released from antigen-specific T cells. Rats in group C had lower levels of IL-2 than those in groups B (P=0.016) and D (P=0.004) (Fig. 4), demonstrating that IL-2 release from T cells was partly inhibited by CTLA4Ig present in CTLA4-L02 cells.
Discussion
Hepatocyte transplantation has potential advantages over liver transplantation in its wide donor source, low cost and technical simplicity. What is more, the original liver remains after hepatocyte transplantation, which provides an opportunity for liver reconstruction. Therefore, hepatocyte transplantation may become a novel approach to treat liver diseases. However, immunological rejection remains the barrier to both hepatocyte and liver transplantation. Purified hepatocytes may become an ideal donor because they express little major histocompatibility complex-I and no major histocompatibility complex-II, which theoretically means no immunogenicity. However, Lunsford[10]pointed out that both CD4-dependent and CD8-dependent immunological rejections occur in hepatocyte transplantation. Therefore, immunosuppressive processes, especially suppressing T-cell-mediated rejection, are still essential in hepatocyte transplantation, which needs further study.[11]
The activation of CD4+T cells is considered as the key process in rejection. Generally, two basic signals are needed in the activation of CD4+T cells: the first is the combination of T cell receptor and major histocompatibility complex, which is antigenic specific; the second is the combination of co-stimulatory molecules on the surface of T cells and antigen presenting cells (APCs).[12]A lack of co-stimulatory signals causes T cells not to react to the specific antigen which is bound to T cell receptor.[13]Several co-stimulatory pathways have been discovered, such as B7/CD28, CD40/CD40L, CD2/ LFA3, CD95(Fas)/Fas L, VCAM1/VLA4, and ICAM1/ LFA1.[14-17]The B7/CD28 pathway is considered to be the most important one. The combination of B7 and CD28 mediates the generation of IL-2, which promotes the activation and proliferation of T cells. But there are several co-stimulatory molecules which provide negative regulation signals,[18]one of which is CTLA4. B7 is the common ligand of CTLA4 and CD28. The combination of CTLA4 and B7 provides negative regulation signals, which inhibit the activation and proliferation of T cells.[19]The affinity between CTLA4 and B7 is 10 times that between CD28 and B7,[20-22]probably because CTLA4 and B7 form a bivalent combination,[23]but CD28 forms only a monovalent combination.[24]The CTLA4 gene shows high homology between human and rat, and human CTLA4 binds to rat B7 and blocks the CD28/B7 co-stimulatory pathway.[25]
CTLA4Ig is a soluble protein, which is composed of the functional domain of CTLA4 and the constant region of IgG, and was first constructed by Linsley in 1991.[2]CTLA4Ig also binds to B7 to block the CD28/B7 co-stimulatory pathway. CTLA4Ig forms dimers and tetramers,[26]and the dimer has the higher activity.[27]The affinity between CTLA4Ig and B7 is 20 times that between CD28 and B7.[3]CTLA4Ig has been widely studied for the induction of transplant tolerance. Many reports have shown that graft tolerance is induced by systemic administration of CTLA4Ig protein or transduction with the CTLA4Ig genein vivo.[4-7]However, CTLA4Ig has the potential to extensively inhibit the immune system, because its mechanism is non-antigen-specific when it is administered continuously, systemically or directly into the recipient.[28]In view of this, localized CTLA4Ig gene expression was developed and successfully induced antigen-specific immune tolerance in liver, heart and islet cell transplantation.[7,29,30]Several kinds of vectors can be used to transiently express a gene, such as plasmid and retrovector, but such vectors have the risk of gene fusion, which may be harmful, and the CTLA4Ig gene expression often lasts for a long time in such vectors which may non-specifically inhibit the immune system. Compared to plasmid and retrovector, adenovector has the advantages of no risk of gene fusion, the efficiency of gene expression is higher, and gene expression is temporary. Therefore, in this study, we transferred the CTLA4Ig gene into L02 cells by adenovirus vector, in an attempt to establish a method by which hepatocytesexpress CTLA4Ig confined to the organ level to induce immunological tolerance and avoid systemic immunosuppression.
In our study, CTLA4Ig was successfully expressed in L02 cells mediated by adenovirus transfection. It was detected in the cytoplasm, and lasted for >4 weeks. L02 cells with normal ALB expression were detected in host rat livers in the CTLA4-transfected group, while none were observed in the control group at week 4 posttransplantation, which directly demonstrated that L02 cells expressing CTLA4Ig obtained immunological tolerance. The activation of CD4+T cells is considered the primary step in immune rejection. CD69 is a marker which only exists on the surface of activated T cells. Therefore, we analyzed the proportion of CD4+T cells and CD69+T cells in peripheral blood leucocytes. The results revealed that the activation intensity of the peripheral blood lymphocyte system in the CTLA4-transfected group was significantly weaker than in the control group. Furthermore, IL-2 is a key cytokine in T cell activation, and our study showed that the IL-2 level was significantly lower in the CTLA4-transfected group than the control group in rat serum at day 7 posttransplantation. All these results provide evidence that immune tolerance was induced by CTLA4Ig expressed in L02 cells.
In conclusion, adenovirus vector can successfully transfer the CTLA4Ig gene into the normal human liver cell line L02 which effectively induces immune tolerance to implants. This has the potential to become a feasible and effective method of inducing immunological tolerance in hepatocyte transplantation, and is likely to become a clinical tool for the treatment of liver diseases.
Contributors:CYK and LXC contributed equally to the manuscript. WYM designed the experiment; CYK and LXC accomplished the main experiments and wrote the first draft; LJG provided technical guidance in immunohistochemistry; LGD provided technical guidance in animal operation. All authors contributed to the design and interpretation of the study and to further drafts. WYM is the guarantor.
Funding:This study was supported by a grant from the National Basic Research of China (973 Program 2007CB512903).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Gupta S, Rajvanshi P, Sokhi RP, Vaidya S, Irani AN, Gorla GR. Position-specific gene expression in the liver lobule is directed by the microenvironment and not by the previous cell differentiation state. J Biol Chem 1999;274:2157-2165.
2 Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 1992; 257:792-795.
3 Gause WC, Halvorson MJ, Lu P, Greenwald R, Linsley P, Urban JF, et al. The function of costimulatory molecules and the development of IL-4-producing T cells. Immunol Today 1997;18:115-120.
4 Alegre ML, Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Curr Pharm Des 2006;12:149-160.
5 Glysing-Jensen T, Räisänen-Sokolowski A, Sayegh MH, Russell ME. Chronic blockade of CD28-B7-mediated T-cell costimulation by CTLA4Ig reduces intimal thickening in MHC classiand II incompatible mouse heart allografts. Transplantation 1997;64:1641-1645.
6 Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DM, Boon L, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol 2008;181:1034-1042.
7 Takekubo M, Tsuchida M, Haga M, Saitoh M, Hanawa H, Maruyama H, et al. Hydrodynamics-based delivery of plasmid DNA encoding CTLA4-Ig prolonged cardiac allograft survival in rats. J Gene Med 2008;10:290-297.
8 Hirakawa E, Yasunami Y, Nakano M, Shiiba M, Takehara M, Uede T, et al. Amelioration of hyperglycemia in streptozotocininduced diabetic mice with fetal pancreatic allografts: prevention of rejection by donor specific transfusion in conjunction with CTLA4Ig. Pancreas 2004;28:146-152.
9 Guha C, Deb NJ, Sappal BS, Ghosh SS, Roy-Chowdhury N, Roy-Chowdhury J. Amplification of engrafted hepatocytes by preparative manipulation of the host liver. Artif Organs 2001; 25:522-528.
10 Lunsford KE, Gao D, Eiring AM, Wang Y, Frankel WL, Bumgardner GL. Evidence for tissue-directed immune responses: analysis of CD4- and CD8-dependent alloimmunity. Transplantation 2004;78:1125-1133.
11 Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 1999;19: 39-48.
12 Guerder S, Flavell RA. Costimulation in tolerance and autoimmunity. Int Rev Immunol 1995;13:135-146.
13 Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996;14:233-258.
14 June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today 1990;11: 211-216.
15 Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995; 270:985-958.
16 Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998;338:1813-1821.
17 Reiser H, Stadecker MJ. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med 1996;335:1369-1377.
18 Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol 2002;2:116-126.
19 Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol 2003;24:314-319.
20 Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-SanchoR, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002;17:201-210.
21 van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 1997;185:393-403.
22 van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol 2003;21:659-684.
23 Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001;410:608-611.
24 Zhang X, Schwartz JC, Almo SC, Nathenson SG. Crystal structure of the receptor-binding domain of human B7-2: insights into organization and signaling. Proc Natl Acad Sci U S A 2003;100:2586-2591.
25 Harper K, Balzano C, Rouvier E, Mattéi MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol 1991;147:1037-1044.
26 Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, et al. Structure and dimerization of a soluble form of B7-1. Immunity 2000;12:51-60.
27 Cox GN, Pratt D, Smith D, McDermott MJ, Vanderslice RW. Refolding and characterization of recombinant human soluble CTLA-4 expressed in Escherichia coli. Protein Expr Purif 1999;17:26-32.
28 Wang Y, Ni Y, Wei H, Wang FC, Ge LP, Gao X. Stable skinspecific overexpression of human CTLA4-Ig in transgenic mice through seven generations. Acta Biochim Biophys Sin (Shanghai) 2006;38:171-178.
29 Lu S, Yu Y, Gao Y, Li GQ, Wang XH. Immunological inhibition of transplanted liver allografts by adeno-associated virus vector encoding CTLA4Ig in rats. Hepatobiliary Pancreat Dis Int 2008;7:258-263.
30 Potiron N, Chagneau C, Boeffard F, Soulillou JP, Anegon I, Le Mauff B. Adenovirus-mediated CTLA4Ig or CD40Ig gene transfer delays pancreatic islet rejection in a rat-to-mouse xenotransplantation model after systemic but not local expression. Cell Transplant 2005;14:263-275.
August 21, 2011
Accepted after revision January 27, 2012
Author Affiliations: Institute of Infectious Diseases, Southwest Hospital, Third Military Medical University, Chongqing 400038, China (Chen YK, Li JG, Liu GD, Guo Y, Chengland Wang YM), and Department of Digestive Diseases, General Hospital of Chengdu Military Command, Chengdu 610083, China (Liu XC)
Yu-Ming Wang, MD, Institute of Infectious Diseases, Southwest Hospital, Third Military Medical University, 29 Gaotanyan Main Street, Shapingba, Chongqing 400038, China (Tel: 86-23-68754141; Fax: 86-23-68754144; Email: wym417@163.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60140-4
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Steroid elimination within 24 hours after orthotopic liver transplantation: effectiveness and tolerability
- Management of hypersplenism in non-cirrhotic portal hypertension: a surgical series
- Feasibility of orthotopic fetal liver transplantation: an experimental study
- Percutaneous transhepatic portal catheterization guided by ultrasound technology for islet transplantation in rhesus monkey
- Pancreaticopleural fistula: etiology, treatment and long-term follow-up
