Feasibility of orthotopic fetal liver transplantation: an experimental study
2012-07-07XianPingZhouXuePingZhouWeiHuaPanWeiGongChengRenShiandZhiWeiQuan
Xian-Ping Zhou, Xue-Ping Zhou, Wei-Hua Pan, Wei Gong, Cheng-Ren Shi and Zhi-Wei Quan
Shanghai, China
Feasibility of orthotopic fetal liver transplantation: an experimental study
Xian-Ping Zhou, Xue-Ping Zhou, Wei-Hua Pan, Wei Gong, Cheng-Ren Shi and Zhi-Wei Quan
Shanghai, China
BACKGROUND:The use of livers from nonviable fetuses is particularly attractive for its potential to solve the current limitations of organ availability for the pediatric recipient. Therefore, it is essential to study the feasibility of orthotopic fetal liver transplantation.
METHOD:We measured the hepatic and extra-hepatic anatomical structures of fetal and neonatal lambs and established an orthotopic liver transplantation model of the fetal lamb.
RESULTS:Mean weight of the liver of fetal lambs at 142 to 145 days gestation was 34.75 g and the mean diameter of the portal vein was 3.03 mm, the supra-hepatic vena cava was 5.88 mm, and the infra-hepatic vena cava was 4.00 mm, which matched the corresponding sizes in neonatal lambs aged up to 2 weeks. Using standard surgical procedures we completed the vascular inosculation of fetal liver. However, all the newborn lamb recipients survived less than 24 hours.
CONCLUSIONS:Orthotopic transplantation of the fetal liver is anatomically and technically feasible. However, perioperative issues need to be resolved prior to clinical application.
(Hepatobiliary Pancreat Dis Int 2012;11:143-147)
surgical anatomy; operative surgery; survival time; experimental surgery
Introduction
Liver transplantation is still the only curative treatment for severe liver diseases, but it is limited by a serious shortage of donor organs for the pediatric recipient.[1]Furthermore, many patients are anatomically unsuitable for standard orthotopic transplantation. Thus, scientists around the world have actively searched for alternative innovative therapies over several decades, primarily in the form of artificial liver support devices and liver cell transplantation. Two major categories of liver support devices are currently under investigation. One is purely mechanical, and the other is an artificial biological device which uses liver cells to provide metabolic support as well as detoxification. Although liver support devices improve clinical and metabolic conditions, increased patient survival rates have not been gained due to the difficulty of removing toxins bound to large protein molecules.[2]Liver cell transplantation holds great promise as an alternative to whole organ transplantation.[3]However, the major limiting factor of mature liver cell transplantation is the shortage of good quality donor cells.[4,5]In addition, in the absence of a selective growth advantage, selective repopulation of the liver by transplanted cells does not occur.[6]It is worth mentioning that early fetal liver epithelial cells can repopulate the liver in a normal host environment.[7]The current research in this area focuses on the fetal liver cell in the first and second trimesters, but there is no report about potential applications for the third-trimester fetal liver in orthotopic liver transplantation.
The use of livers from nonviable fetuses sounds particularly attractive for its potential to solve the current limitations of organ availability and size discrepancy for the pediatric recipient. In theory, auxiliary liver transplantation has several advantages over orthotopic transplantation.[8]However, long-term complications linked to auxiliary liver transplantation may arise in the postoperative period, such as inadequate blood supply of the graft, oppression of theabdominal viscera, and the competition between native liver and graft,[9]which often require further surgical intervention. Therefore, it is necessary to study the feasibility of orthotopic transplantation of the fetal liver.
Methods
All experiments were carried out following the Chinese Institutional Ethical Committee guidelines for animal research. Ethical approval for this study was obtained from the Ethics Committee of Shanghai Jiaotong University School of Medicine. Four ewes of 125 to 145 days gestation (term 145 days) and 16 newborn lambs of 1-3 weeks (weighing 3.2-5.7 kg) were purchased from the Shanghai Academy of Agricultural Sciences.
Measurement of the liver
Under general anesthesia, abdominal exploration was performed on the fetal lambs. Cross-shaped incisions were made affording access to the hepatic vascular pedicles and macroscopic evaluation of intra-abdominal organs. Classic hepatectomy was performed.[10]Two transplantation surgeons performed the measurement of liver dimensions. The weight of the liver and the anatomy of the portal veins, umbilical vein, inferior vena and common bile duct were measured. The hepatic artery and its branches were determined. The inferior vena cava was dissected and released above its connection with the renal veins. The biliary ducts were cut from the upper duodenum.
Fetal liver transplantation with a venous-venous bypass
The newborn lambs were recipients of fetal liver. The surgical procedure was divided into four parts: harvesting, back-table, hepatectomy of the recipient and implantation. The donor operation, including the harvesting and preparation of the donor liver, was performed according to the modified procedure of hepatectomy.[11]
At the beginning of the operation, the jugular vein was exposed and freed from the neighboring tissues for the portal-jugular vein shunt. The hepatic hilar structures were dissected. The hepatic artery and bile duct were divided. Then the infra-hepatic vena cava was isolated to the emergence of the left renal vein. Next, the portal vein was cross-clamped at the bifurcation. After clamping the inferior vena cava, a temporary portal-jugular vein shunt was performed through a catheter. The liver graft was implanted after removal of the native liver. The inferior vena cava reconstruction was an end-to-end inosculation between the suprahepatic and infra-hepatic graft inferior vena cava and the corresponding recipient inferior vena cava segment. Then the portal-jugular vein shunt was taken down, and an end-to-end inosculation of the portal vein was performed between the umbilical vein of the graft and the recipient portal vein. Afterwards, the liver was refilled immediately after the last stitch of the portal vein inosculation. Subsequently, an arterial end-to-end inosculation was performed between the fetal aortic artery of the graft and the recipient proper hepatic artery at the level of artery bifurcation. In order to facilitate observation of biliary secretion, bile drainage was performed. The recipients were given fresh blood and crystal solution to maintain blood pressure.
Postoperative treatment
Postoperatively, intravenous injection of immunosuppressant (methylprednisolone 200 mg) was given after reopening of the portal vein. The animals were warmed, and all were given analgesic treatment. Antibiotics were not used in the postoperative period because the recipients had short survival times.
Results
Liver weight and anatomical dimensions of fetal and newborn lambs
To assess the anatomical feasibility of the fetus and newborn as liver donors, the weight and anatomical dimensions of the liver were measured. The mean weight of the liver of fetal lambs at 142 to 145 days gestation was 34.75 g, which met the conditions of a liver donor for newborn lambs up to 2 weeks old. The mean diameter of the portal vein was 3.03 mm, of the suprahepatic vena cava was 5.88 mm, and of the infra-hepatic vena cava was 4.00 mm (Table 1). All the anatomical data were similar to those of newborn lambs. Although there was no significant difference in the anatomy of the liver of fetal lambs between 125-135 and 142-145 days gestation (Table 2), the liver vessels of the fetal lamb at125-135 days gestation lacked toughness, and the vessels easily avulsed from the suture line during pruning of the liver. However, the liver vessels at 142-145 days gestation had acquired enough toughness and they were suitable for vascular inosculation.
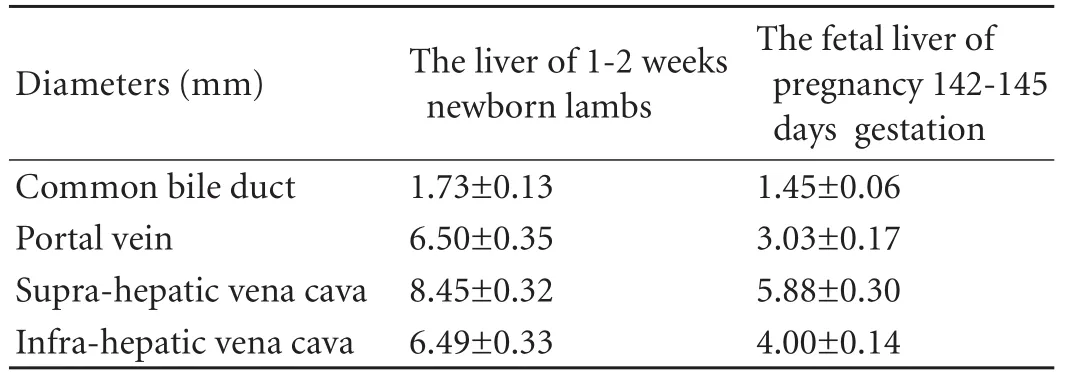
Table 1. Anatomical data of the fetal liver at 142-145 days gestation and in 1-2-week-old lambs

Table 2. Anatomical data of the fetal liver at 125-135 days and 142-145 days gestation
Model of orthotopic fetal liver transplantation
To study the technical feasibility of the fetus as a liver donor, we tried to establish a model of orthotopic transplantation of the fetal liver. Our initial attempts without venous-venous bypass were unsuccessful because of the severe congestion of the abdominal viscera. To overcome this obstacle, we attempted to establish a piggyback transplantation model, but it was unsuccessful. The newborn lamb has short hepatic veins and a relatively narrow inferior vena cava. When the vena cava was clamped above and below the hepatic veins, the blood flow from the inferior vena cava was almost completely blocked. Therefore, we decided to establish a model of orthotopic transplantation of the fetal liver with a portal-jugular vein shunt. A total of eight neonatal lambs underwent orthotopic liver transplantation. Because of the improvement of the test methods, the recipient blood pressure was stable during the anhepatic phase, without congestion of the abdominal viscera. Using standard vascular techniques, a complete fetal liver vascular inosculation was done successfully. When the blood flow to the liver was reestablished, the liver refilled well and there was no leakage of the vascular stomas. However, the newborn lamb recipients in all cases survived less than 24 hours.
Discussion

Fig. Blood pressures monitoring during perioperative period. Blood pressure of recipients was relatively stable before portal vein occlusion. The blood pressure significantly decreased after restoration of hepatic blood flow, and decreased gradually over time. 1: 20 minutes before portal vein occlusion; 2: 5 minutes after portal vein occlusion; 3: 20 minutes after portal vein occlusion; 4: portal vein reopening; 5: 10 minutes after reperfusion; 6: 30 minutes after reperfusion; 7: 60 minutes after reperfusion; 8: 120 minutes after reperfusion.
We analyzed the anatomical feasibility of the fetus as a liver donor based on the results of measurement of the liver of fetal lambs. The mean weigh of the liver at 142 to 145 days gestation was 34.75 g, and the anatomical data were also similar to those of newborn lambs of 1 to 2 weeks, which met the conditions of a liver donor for a newborn lamb up to 2 weeks old by orthotopic transplantation. Compared with the portal vein, the umbilical vein has a larger diameter and better tissue toughness. Moreover, the fetal liver receives 75% to 80% of blood flow from the umbilical vein and only about 20% from the portal vein.[12]Therefore, the best means of vessel reconstruction is an end-to-end inosculation between the umbilical vein of the graft and the recipient portal vein. The diameter of the hepatic artery of fetal lambs during late pregnancy was less than 1 mm, while the mean diameter of the abdominal aorta was 3.05 mm. Therefore, the use of the abdominal aorta of the graft rather than the hepatic artery for arterial inflow greatly simplified the procedure. The mean diameter of the common bile duct of lambs at 142 to 145 days gestation was 1.45 mm, which made it difficult to perform an endto-end bile duct inosculation. It is advisable that the gallbladder of the graft is inosculated to the jejunum loop of the recipient with mucosa-to-mucosa alignment. The mean diameters of the supra-hepatic vena cava and the infra-hepatic vena cava were similar to those of newborn lambs at 1 to 2 weeks, and the inferior vena cava reconstruction can be performed with an end-toend inosculation. Therefore, from the anatomical point of view, it is feasible for fetal lambs to serve as liver donors in orthotopic liver transplantation.
To investigate the technical feasibility of fetal lambs as liver donors, we developed the animal model of orthotopic fetal liver transplantation. Although there was discrepancy in vascular diameter, vessel reconstruction could still be successfully completedbecause we had chosen the reasonable method of vascular anastomosis mentioned above. In order to facilitate observation of bile secretion, biliary drainage was performed without bile duct anastomosis. In the transplantation model, the liver filled well and there was no leakage of the vascular stomas after completion of vascular inosculation. Therefore, orthotopic fetal liver transplantation is technically feasible.
It is noteworthy that the newborn lamb recipients in all cases survived less than 24 hours, therefore it is necessary to analyze the factors affecting recipient survival. We initially thought that blood group incompatibility led to the short survival of the recipients, because we had no way to identify the blood types of sheep. Hence we conducted 78 randomized crossmatching tests among 16 goats, but no agglutination occurred (data not shown). Therefore, we ruled out the possibility that blood group incompatibility led to a hemolytic reaction and hyperacute rejection. In addition, the neonatal immune system is immature and immune tolerance to the graft is easy to induce.[13]We inferred that rejection may not be the main cause of recipient death within 24 hours.
Hemodynamic stability is closely related to recipient survival. We monitored the changes of blood pressure during the perioperative period (Fig.). The blood pressure of the recipients was stable until restoration of hepatic blood flow. However, the blood pressure significantly decreased after restoration of hepatic blood flow, and it was not restored to the normal level although active steps were taken. Hemodynamic disorder has adverse effects on ischemia-reperfusion injury; in turn, hepatic reperfusion injury leads to hemodynamic deterioration. On one hand, metabolic acidosis has a detrimental effect on cardiac contractility,[14]and cardiac output is reduced. On the other hand, large amounts of oxygen free radicals are generated in the course of ischemia-reperfusion and they inhibit the production of coagulation factors,[15]which results in increased bleeding. In addition, a newborn lamb has a limited ability to compensate for body fluid changes. Therefore, hemodynamic instability was the main cause of early death in these newborn lamb recipients, and maintenance of hemodynamic stability may significantly improve recipient survival rate.
In the model of orthotopic liver transplantation, due to the venous-venous bypass, there was no congestion of the abdominal viscera during the anhepatic phase. However, the portal-jugular vein shunt also introduced a large number of toxic substances from the digestive tract directly into the systemic circulation without detoxification. The blood-brain barrier is not fully developed in newborns.[16]Thus, ammonia and other harmful substances may enter the brain and damage brain cells. This may affect many important functions that could cause even more problems, which are clearly detrimental to recipient survival.
In conclusion, orthotopic fetal liver transplantation is feasible from the anatomical and technical point of view. However, the recipients showed short survival times after transplantation. Among the factors affecting the survival time, hemodynamic instability was the main cause of early death in recipients in our experiment, and this needs to be resolved in follow-up studies.
Contributors:SCR and QZW proposed the study. ZXP and GW wrote the first draft. ZXP and PWH analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. QZW is the guarantor.
Funding:This work was supported by a grant from the Science and Technology Commission of Shanghai Municipality (07-JC14045).
Ethical approval:This study was obtained from the Ethics Committee of Shanghai Jiaotong University School of Medicine.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Nikeghbalian S, Nejatollahi SM, Salahi H, Bahador A, Dehghani SM, Kazemi K, et al. Experience of living donor liver transplantation in Iran: a single-center report. Transplant Proc 2009;41:2868-2871.
2 McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificial liver support. Semin Liver Dis 2008;28:210-217.
3 Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med 2009;266:339-357.
4 Lee SW, Wang X, Chowdhury NR, Roy-Chowdhury J. Hepatocyte transplantation: state of the art and strategies for overcoming existing hurdles. Ann Hepatol 2004;3:48-53.
5 Baccarani U, Adani GL, Sainz M, Donini A, Risaliti A, Bresadola F. Human hepatocyte transplantation for acute liver failure: state of the art and analysis of cell sources. Transplant Proc 2005;37:2702-2704.
6 Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol 2001;158:771-777.
7 Oertel M, Menthena A, Chen YQ, Shafritz DA. Comparison of hepatic properties and transplantation of Thy-1(+) and Thy-1(-) cells isolated from embryonic day 14 rat fetal liver. Hepatology 2007;46:1236-1245.
8 Belghiti J, Sommacale D, Dondéro F, Zinzindohoué F, Sauvanet A, Durand F. Auxiliary liver transplantation for acute liver failure. HPB (Oxford) 2004;6:83-87.
9 Schleimer K, Stippel DL, Kasper HU, Allwissner R, Yavuzyasar S, Hölscher AH, et al. Competition between native liver and graft in auxiliary liver transplantation in a rat model. Transplant Proc 2008;40:967-970.
10 Pararas N, Levi D, Selvaggi G, Moon J, Nishida S, Tryphonopoulos P, et al. Mass clamping of the hilum to facilitate difficult hepatectomy during liver transplantation: a single center 10 year experience. Ann Surg 2009;250:273-276.
11 Lladó L, Figueras J. Techniques of orthotopic liver transplantation. HPB (Oxford) 2004;6:69-75.
12 Kessler J, Rasmussen S, Kiserud T. The fetal portal vein: normal blood flow development during the second half of human pregnancy. Ultrasound Obstet Gynecol 2007;30:52-60.
13 West LJ. Developmental aspects of immunomodulation: exploiting the immature immune system for organ transplantation. Transpl Immunol 2002;9:149-153.
14 Alvis AG, Milesi V, Rebolledo A, Raingo J, Grassi de Gende AO. Influence of calcitonin gene-related peptide release on pH-induced mechanical depression in rat atria. Jpn Heart J 2001;42:507-517.
15 Zaets SB, Xu DZ, Lu Q, Feketova E, Berezina TL, Gruda M, et al. Recombinant factor XIII diminishes multiple organ dysfunction in rats caused by gut ischemia-reperfusion injury. Shock 2009;31:621-626.
16 Kimberlin DW, Shalabi M, Abzug MJ, Lang D, Jacobs RF, Storch G, et al. Safety of oseltamivir compared with the adamantanes in children less than 12 months of age. Pediatr Infect Dis J 2010;29:195-198.
Accepted after revision May 20, 2011
Wisdom comes alone through suffering.
—Aeschylus
February 19, 2011
Author Affiliations: Department of General Surgery (Zhou XP, Zhou XP, Gong W and Quan ZW) and Department of Pediatric Surgery (Pan WH and Shi CR), Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200092, China
Zhi-Wei Quan, MD, Department of General Surgery, Xinhua Hospital, 1665 Kongjiang Road, Shanghai 200092, China (Tel: 86-21-65790000ext7660; Fax: 86-21-65153984; Email: zhiweiquan@ yahoo.com.cn)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60139-8
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Steroid elimination within 24 hours after orthotopic liver transplantation: effectiveness and tolerability
- Management of hypersplenism in non-cirrhotic portal hypertension: a surgical series
- Immunological tolerance of human hepatocyte xenograft induced by adenovirus vector-mediated CTLA4Ig gene transfer
- Percutaneous transhepatic portal catheterization guided by ultrasound technology for islet transplantation in rhesus monkey
- Pancreaticopleural fistula: etiology, treatment and long-term follow-up
