Serum cytokine levels in chronic hepatitis B patients receiving peginterferon alpha-2a therapy
2012-06-11
Seoul,Korea
Introduction
Hepatitis B virus (HBV) is a common etiologic agent of chronic hepatitis.HBV is thought not to be cytolytic,but rather HBV infection induces a host immunoreaction against its viral products.In this process,cytokines are produced by various types of immune cells including lymphocytes,macrophages,natural killer cells and dendritic cells,and these cells play crucial roles in the course of chronic hepatitis B (CHB).[1,2]
In flammation in HBV-infected hepatocytes is caused by cytokines,such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α),[3]and these molecules also trigger elimination of the virus.Cytokines such as transforming growth factor-β (TGF-β) promotefibrosis of the liver,[4,5]and hepatocarcinogenesis is also associated with abnormal immune responses initiated by various types of cytokines including TGF-β and interleukin (IL)-10.[6-8]In a recent study,[9]IL-6 limited adaptive immune responses and protected HBV-infected hepatocytes from death.Another study[10]reported that increased IL-6 expression was associated with liver dysfunction after HBV infection.
Studies[11-13]also reported the relationship between the expression pattern of cytokines and the progression of CHB.TNF-α and IL-10 levels were found to be higher in the progress of the CHB phases.[11]TNF-α,IL-4,and TGF-β levels were also higher in CHB patients than in controls,whereas IFN-γ level was lower in patients than in controls in another study.[12]Hepatic flares in CHB patients were also associated with high serum levels of IFN-γ inducible chemokines such as chemokine (C-X-C motif) ligand (CXCL)-9 and CXCL-10.[13]However,roles of various cytokines in the progression of CHB are not completely understood,and the association of cytokines with treatment responses to drugs in CHB has rarely been reported.[14]
In the present study we analyzed serum levels of cytokines in CHB patients treated with peginterferon α-2a in their treatment phases and responses to the therapy.Epidermal growth factor (EGF),IL-1α,IL-1β,IL-2,IL-4,IL-6,IL-8,IL-10,INF-γ,monocyte chemotactic protein-1 (MCP1),TNF-α,and vascular endothelial growth factor (VEGF) were simultaneously assayed in specimens obtained from the CHB patients.Predictive values of the cytokines for the responses to peginterferon α-2a were also assessed.
Methods
Study subjects
Altogether 93 serum samples were prospectively collected with written informed consents from 20 CHB patients treated with peginterferon α-2a between January 2009 and December 2011.CHB diagnosis was based on positive serum HBsAg for more than 6 months and positive HBeAg with HBV DNA over 20 000 IU/mL or negative HBeAg with HBV DNA over 2000 IU/mL along with persistent elevation of alanine aminotransferase(ALT).[15]The patients showed serum HBV DNA levels greater than 20 000 IU/mL before initiation of the therapy,and were treated with 180 μg of peginterferon α-2a per week for 48 weeks.From each patient,one or more samples were drawn at the following time points:before peginterferon therapy,during peginterferon therapy (at 3rd and 6th months after initiation of the therapy),and at 6th and 12th months after the therapy.
The patients were classified as virologic responders(VRs) when serum HBV DNA levels at 6th month after initiation of the therapy were decreased to less than 2000 IU/mL,[15-18]and the other patients were classified as virologic non-responders (NRs).The VRs were subdivided into the sustained virologic responders(SVRs) when virologic breakthrough (increase in serum HBV DNA by >1 log10[10-fold] above nadir after achieving virologic response) was not observed until 12 months off-treatment.This study was approved by the Institutional Review Board of Severance Hospital and conducted according to the ethical principles of the Declaration of Helsinki.
Measurement of cytokine levels
All serum samples were separated within 2 hours after taking venous blood samples and stored at -70 ℃until use.A protein chip analyzer,Evidence Investigator and Cytokine Array I reagents (Randox,Antrim,UK)were used to measure 12 cytokines including IL-1α,IL-1β,IL-2,IL-4,IL-6,IL-8,IL-10,VEGF,INF-γ,TNF-α,MCP1 and EGF.The assay was carried out according to the manufacturer's instructions.Frozen serum specimens and the reagent carrier comprising 9 protein chips were left at room temperature for about 15 minutes when unpacked.Monoclonal antibodies for the 12 cytokines were attached on each protein chip surface.Assay diluent of 200 μL was placed into each well of the chips,and then 100 μL of serum was added.The chips were placed in a thermo shaker at 37 ℃,370 rpm for 1 hour.After washing the chips with washing fluid for six times,300 μL of conjugate fluid containing enzymelabeled antibody was added to each well.After another one-hour reaction in the same condition,the chips were washed again for six times.Substrate reagent containing luminol and peroxidase was added to each well of the chips and then after 2 minutes the carrier was inserted into the Evidence Investigator analyzer.A chargecoupled device camera detected the chip signals and saved images in the computer.The computer program then calculated cytokine levels from the chip signals using calibration curves for the respective cytokines.
Other assays
Serum HBV DNA concentrations were determined by Cobas AmpliPrep/Cobas TaqMan HBV test v2.0(Roche Molecular Systems,Inc.,Pleasanton,CA.,USA).Aspartate aminotransferase (AST) and ALT levels were measured by a Hitachi 7600 DDP modular chemistry analyzer (Hitachi High-Technologies Co.,Tokyo,Japan).Each assay was performed according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were made using Analyseit Method Evaluation Edition software version 2.26(Analyse-it Software Ltd.,City West Business Park,Leeds,UK).Continuous variables between the two groups were compared using the Mann-Whitney U test.Multiple comparisons of continuous variables including levels of cytokines among the patient subgroups and among the treatment phases were performed using the Kruskal-Wallis test with Bonferroni correction to compensate for alpha-statistical errors.Friedman's test was also carried out to compare cytokine levels among the paired group.Categorical variables between the two groups were analyzed for the difference in proportion by Fisher's exact test.Area under the receiver operating characteristic curves (AUROCs) of the cytokines and HBV DNA were calculated to assess their predictive values for virologic responses.P values less than 0.05 were considered as statistically significant.
Results
Characteristics of the CHB patients
Characteristics of the patients are summarized in Table 1.Age and male proportions were not different between the VRs (n=11) and NRs (n=9).Serum AST and ALT levels were not different between the VRs and NRs before peginterferon therapy,but they were higher in the NRs during and after the therapy.Serum HBV DNA levels before,during and after the therapy were lower in the VRs than in the NRs.Among the VRs,four (36.4%)demonstrated HBeAg seroconversion during or after the therapy,andfive (45.5%) showed recurrence of active chronic hepatitis at 6 to 12 months after the therapy,thus,six (54.5%) patients were classified into SVRs.
Serum levels of cytokines according to treatment phases and responses to the therapy
Serum levels of 12 cytokines in the phases of peginterferon therapy in the VR and NR groups are summarized in Table 2.The levels of all cytokines except for EGF,IFN-γ,IL-1α,IL-4,IL-6,IL-8,and MCP1 were not different among the groups according to the responses to the therapy.The levels of MCP1 were higher during the therapy than before the therapy at 3rd and 6th months in the VR group (P=0.0371 at 3rd month and P=0.0011 at 6th month) (Fig.1),whereas IL-4 levels were lower during the therapy than before the therapy at 3rd and 6th months in the same group (P=0.0037 at 3rd month and P=0.0133 at 6th month)(Fig.1).IL-6 levels were also higher during the therapy than before the therapy (P=0.0159 at 3rd month and P=0.0174 at 6th month).On the other hand,levels of those three cytokines were not significantly changed according to the phases of the therapy in the NR group.In addition,MCP1 levels were also higher in the VRs than in the NRs during and after the therapy (P<0.05),and EGF levels were decreased at 3rd month in the VR group during the therapy compared with those before the therapy (P=0.0080).
Correlation between the serum levels of HBV DNA and cytokines
Serum HBV DNA levels were weakly correlated with the serum levels of EGF (r=0.33,95% CI,0.13 to 0.50,P=0.0016),IL-4 (r=0.31; 95% CI,0.11 to 0.49; P=0.0025),IL-10 (r=0.31; 95% CI,0.11 to 0.49; P=0.0025),and IL-1β (r=0.28; 95% CI,0.08 to 0.46; P=0.0068) regardless of treatment phases and groups according to the treatment responses.Whereas,the serum levels of MCP1 (r=-0.49;95% CI,-0.64 to -0.32; P<0.0001),IL-6 (r=-0.39; 95% CI,-0.55 to -0.20; P=0.0002),and IFN-γ (r=-0.26; 95% CI,-0.44 to -0.06; P=0.0127) were inversely correlated with HBV DNA levels.
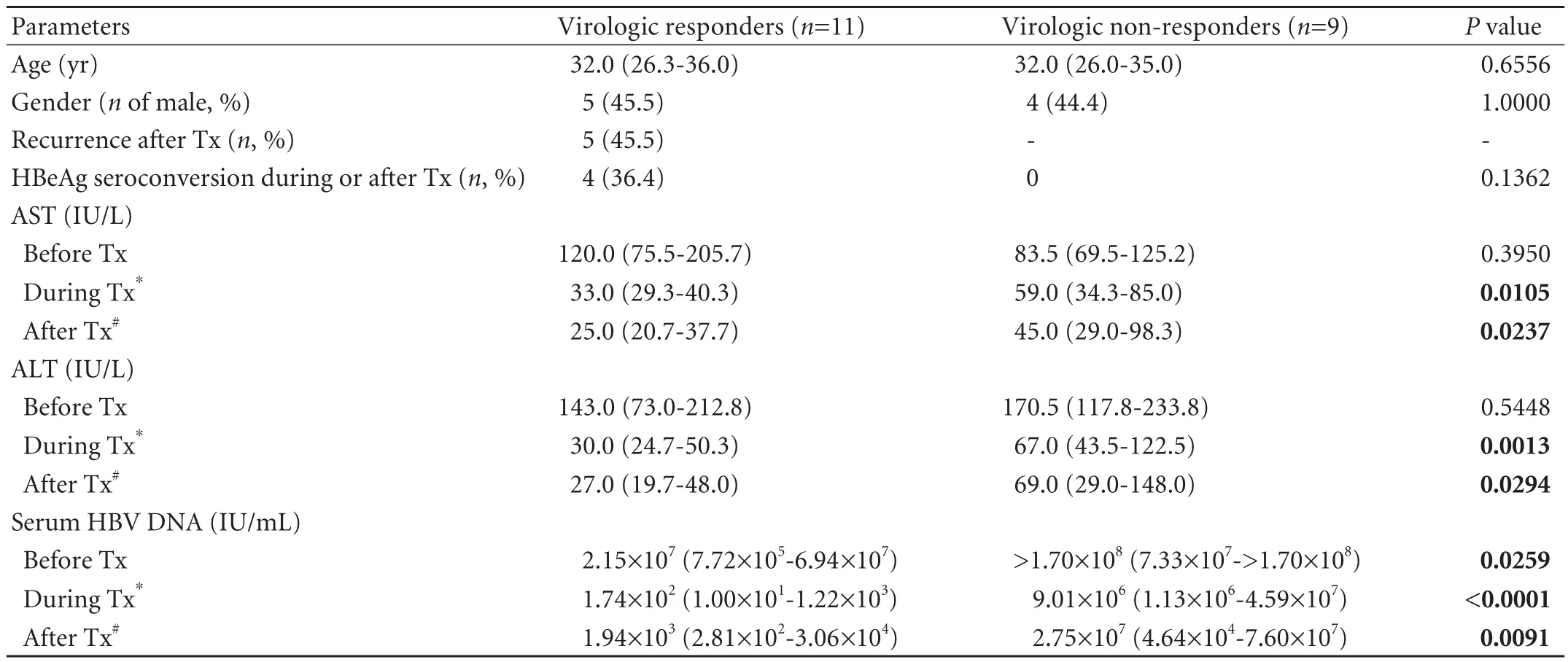
Table 1.Characteristics of patients with chronic hepatitis B
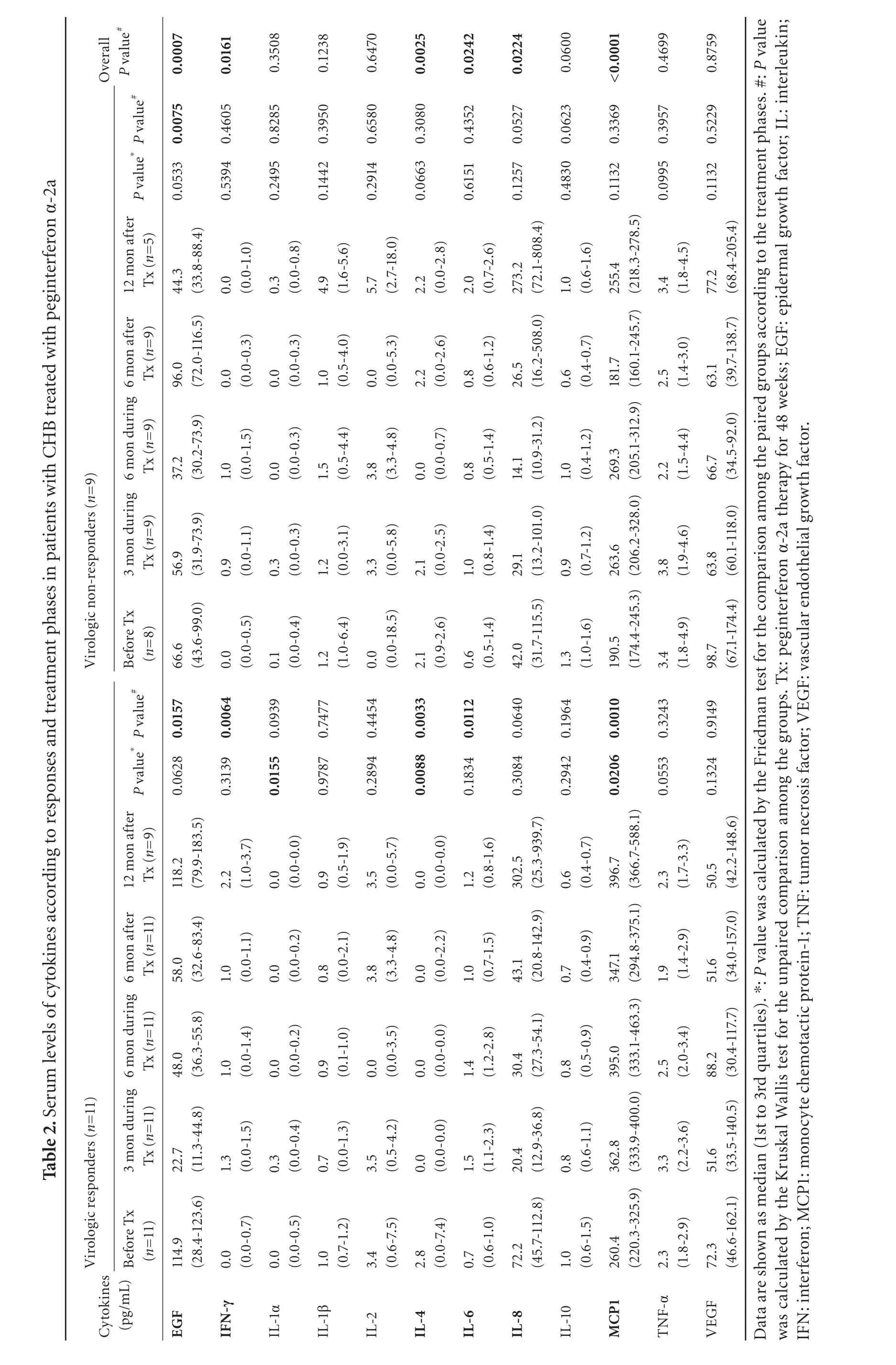
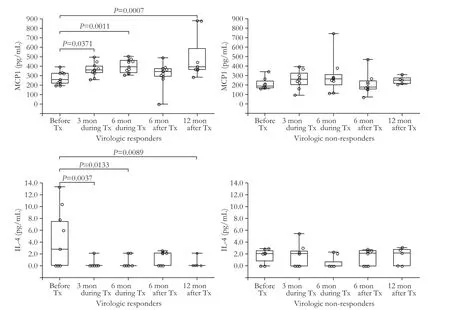
Fig.1.Serum levels of cytokines in CHB patients according to treatment phases and responses to peginterferon α-2a.Levels of MCP-1 were increased at 3rd and 6th months after treatment had been started,and at 12th month after treatment had beenfinished,while IL-4 levels were decreased during and after treatment in the same group.Levels of these two cytokines were not different according to the treatment phases in virologic non-responders.P values in thefigures were calculated by Mann-Whitney U test.The upper,lower ends of the boxes and box inner lines correspond to the upper and lower quartiles and median values,respectively.Whiskers denote extreme distribution values,and circles represent individual concentrations.
Predictive values of cytokines and HBV DNA
Diagnostic performances of HBV DNA and some cytokines are summarized in Table 3.The AUROC value of serum HBV DNA levels measured before peginterferon therapy was 0.81 (P=0.0017) in predicting VR (n=11) (Fig.2A),and the lower baseline HBV DNA level was related to the higher probability of VR.The sensitivity and specificity of baseline HBV DNA levels in predicting VR were 72.7% and 87.5%,respectively,when the cutoff DNA level was 7.33×107IU/mL.Meanwhile,the AUROC value of baseline MCP1 levels was 0.76(P=0.0230) in detecting VR (n=11),and the higher MCP1 level was related to VR.The sensitivity and specificity of the baseline MCP1 level in discriminating VRs from NRs were 81.8% and 75.0%,respectively,with the cutoff value of 213.9 pg/mL.The AUROC values for all other cytokines quantified in the samples before the therapy were not statistically significant in predicting VR.
In addition,the AUROC value of HBV DNA levels,which was measured at 3rd month during the treatment was 0.85 (P=0.0001) in predicting SVR (n=6) (Fig.2B),and the sensitivity and specificity of HBV DNA levels at that time point were 100.0% and 61.5%,respectively(cutoff,3.28×105IU/mL).The AUROC values of IL-6 and IL-8 determined at 3rd month during the treatment were 0.85 (P=0.0001) and 0.81 (P=0.0020) respectively in predicting SVR,and the higher IL-6 and IL-8 levels were associated with SVRs.The sensitivity and specificity of IL-6 were 100.0% and 69.2%,respectively,at the cutoff value of 1.05 pg/mL,and those of IL-8 were 76.9% and 83.3% (cutoff,18.55 pg/mL).
Similarly,the AUROC value of HBV DNA taken at 6th month during the treatment was 0.92 (P<0.0001)in predicting SVR,and the sensitivity and specificity of HBV DNA were 100.0% and 76.9% respectively,when the cutoff value was 1.18×103IU/mL.The AUROC value of IL-6 assessed at 6th month during the treatment was 0.78 (P=0.0058) in predicting SVR.The sensitivity and specificity of IL-6 were 100.0% and 69.2%,respectively when the cutoff value was 1.09 pg/mL.
In addition,changes in the serum levels of somecytokines during the treatment were also significantly associated with VR and SVR (Table 3).
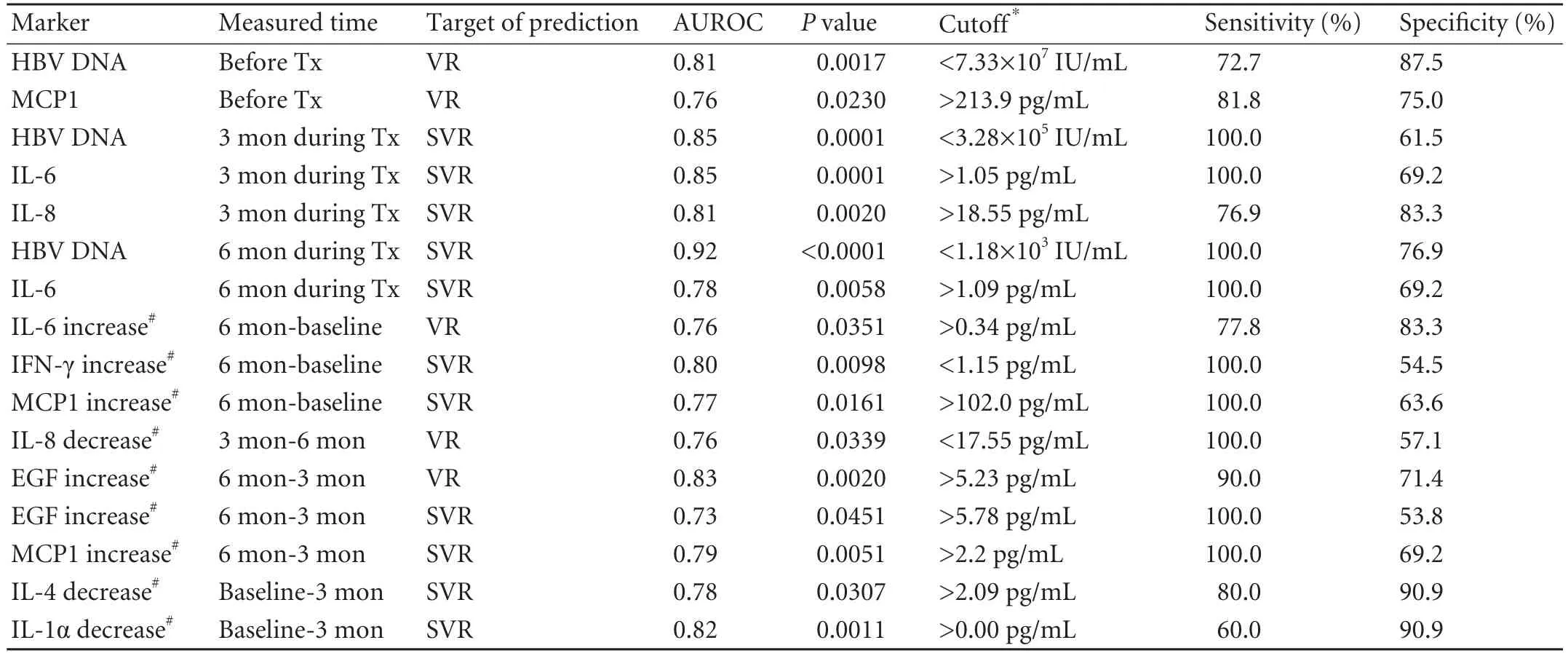
Table 3.Predictive values of HBV DNA and cytokines for virologic responses
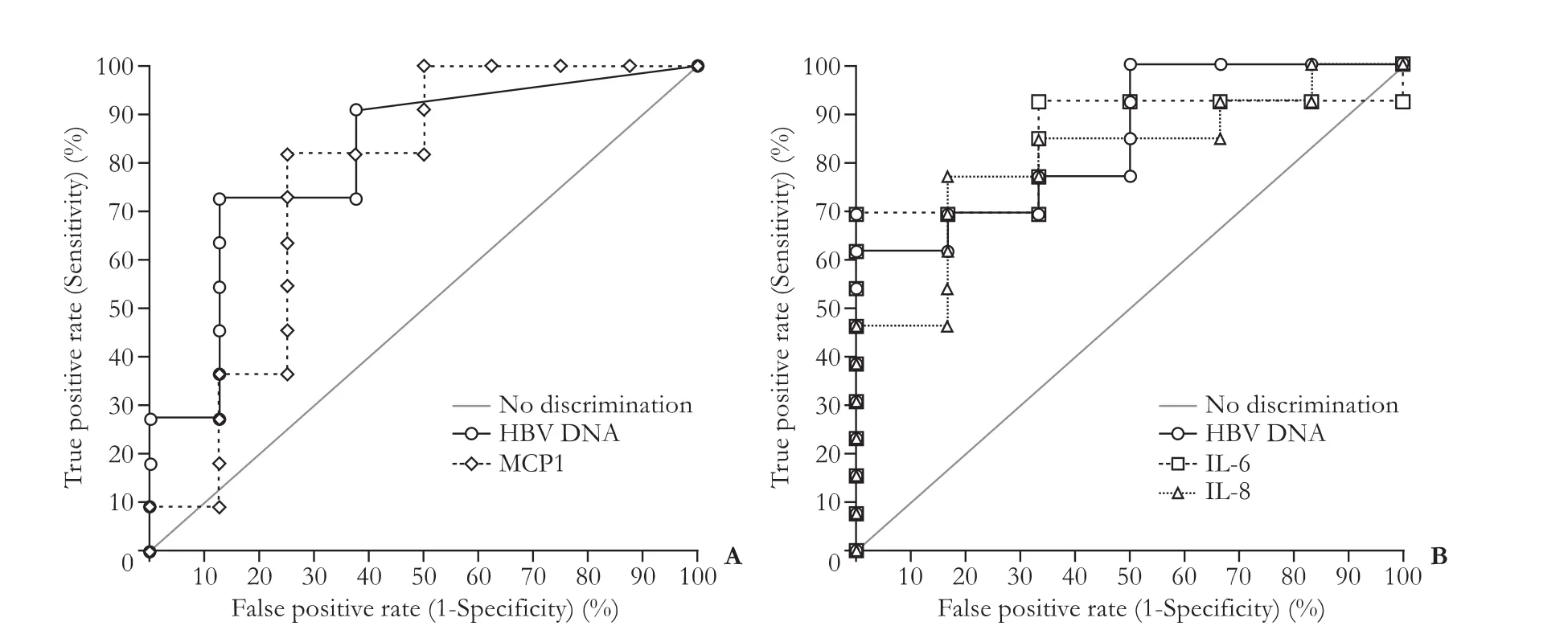
Fig.2.Receiver operating characteristic (ROC) curves of HBV DNA and cytokines for predicting virologic responses.A:AUROC of HBV DNA levels measured before treatment was 0.81 (P=0.0017) for the discrimination between VRs (n=11) and NRs (n=9) at 6th month during therapy,and that of MCP1 was 0.76 (P=0.0230).B:AUROC value of HBV DNA quantified at 3rd month during treatment was 0.85 (P=0.0001) in predicting SVR (n=6) until 12 months off-treatment,and that of IL-6 were 0.85 (P=0.0001).IL-8 level at 3rd month also demonstrated AUROC of 0.81 (P=0.0020) in predicting SVR.
Discussion
Liver damage after HBV infection is mediated by host immune responses.Acquired and innate immunity is thought to be related to the pathogenesis of CHB.In this study,we analyzed the serum levels of 12 cytokines in CHB patients to evaluate the patterns of changes in cytokines according to the treatment phases and responses to peginterferon α-2a,which is a widely used therapeutic agent for the treatment of CHB.In our study,the serum levels of MCP1,EGF,IFN-γ,IL-4,and IL-6 changed in the CHB patients according to the phases of peginterferon therapy.This would imply that the levels of those cytokines during each treatment phase may re flect the immunological status of patients undergoing peginterferon therapy.It is plausible that the host's immune responses can be in fluenced by the administration of peginterferon since the drug has a dual mode of action which acts not only as an antiviral agent but also as a regulator of the immune system.
In our study,MCP1 was significantly increased during the therapy (362.8 pg/mL at 3rd month and 395.0 pg/mL at 6th month) compared with that before therapy (260.4 pg/mL) in VRs but not in NRs.This would imply that serum MCP1 levels re flect treatment phase and response to the agent in CHB patients.A previous study[19]suggested that MCP1 plays an antiin flammatory role in T cell-mediated hepatitis by inhibiting T cell-derived IL-4 production through direct stimulation of its specific receptor chemokine(C-C motif) receptor 2 (CCR2).In our study,the serum levels of MCP1 were increased,but those of IL-4 were decreased during the treatment in the VR group.In addition,the serum MCP1 level was also increased during the treatment of patients with chronic hepatitis C by using IFN α-2b plus ribavirin,but in contrast to our study,the increase of MCP1 levels in non-responders was significant.[20]This discrepancy may be attributed to the difference of host immune responses to different viruses.MCP1,called as chemokine (C-C motif) ligand 2 (CCL2),small inducible cytokine A2 (SCYA2),and monocyte chemotactic and activating factor (MCAF),is known to act as a pro-in flammatory molecule,which plays a key role in the recruitment of monocytes to the site of infection.In a previous study,[21]the 2518G>A polymorphism of the MCP1 gene was reported to be associated with whether HBV infected individuals recovered spontaneously or became a chronic HBV carrier.Plasma MCP1 levels were also higher in patients with chronic hepatitis caused by HBV or HCV infection(median 111.4 pg/mL) than in healthy controls (median 49.7 pg/mL).[22]However,there are no reports on the role of MCP1 in monitoring CHB patients on peginterferon therapy.The results of our study provide a clue for the use of MCP1 as a biomarker in monitoring the efficacy of peginterferon therapy in CHB patients.Because MCP1 levels before treatment in our study showed an AUROC value of 0.76 in predicting virologic responses at 6th month during the treatment.
IL-6 is an endogenous pyrogen which causes fever as an in flammatory response in the body and acts as pro-in flammatory and anti-in flammatory cytokines.Its level was elevated during the therapy in the VR group in our study.IL-6 was reported to suppress HBV replication by preventing the formation of genomecontaining nucleocapsids,which is similar to the effect of interferons.[23]In contrast,low serum IL-6 level was reported as an early predictor for acute exacerbation of CHB.[24]Therefore,IL-6 also could be a marker for responses to peginterferon therapy.In our study IL-6 levels measured at 3rd and 6th months during the treatment were helpful in predicting SVR during the 12-month off-treatment.In addition,IFN-γ as one of the well-known cytokines has anti-viral effects,and the increase of IFN-γ level during the therapy in our study seems to be reasonable.
EGF was decreased during the early phase of peginterferon therapy compared to that before the therapy in our VR group.The finding indicates that the early decrease of serum EGF level during the therapy is a favorable prognostic factor for the peginterferon treatment.A study[25]reported that the EGF-dependent upregulation of integrin beta-1 chains and increased adhesion to extracellular matrix were additional cytoprotective mechanisms of HBV infected hepatocytes.This study suggested that EGF can interfere with CD95-mediated apoptosis and the action of cytotoxic T-cells through multiple mechanisms in human hepatocytes.However,EGF levels were increased in the later phase of peginterferon therapy (at 6th month during treatment)compared with those in the early phase,and this increase was also related to VRs in our patients.Thus,the dynamics of cytokines in CHB seems to be complex and needs further investigation.
Cytokines are key molecules in the complex signaling network of cell-mediated immunity.Analyses of changes in cytokine expression patterns during the treatment help to understand the pathogenesis of CHB and predict treatment responses.However,a small number of subjects were enrolled in this study,and thus the statistical power was not adequate for assessment of the usefulness of cytokines in monitoring CHB patients,and the levels of some cytokines were changed or unchanged according to statistical analysis.Also,VRs to the treatment of CHB patients can be in fluenced by various factors including viral genotypes as well as host's genetic factors such as HLA types and polymorphisms of many genes.Our data show changes in the levels of particular cytokines according to treatment phases.Further large-scale studies to control various confounding factors are necessary to prove the usefulness of cytokine levels as predictive factors in CHB patients treated with peginterferon or other agents.
We simultaneously evaluated 12 cytokines in the sera from CHB patients in order to detect their expression patterns according to treatment phases and responses to therapy.The serum levels of cytokines,particularly MCP1,EGF,IL-4,IL-6,and IL-8 re flected the pathological differences of the treatment phases.They could serve as indices for monitoring responses to peginterferon treatment in CHB patients.
Contributors:KHS proposed and supervised this study,and also revised thefinal manuscript.PY performed the statistical analysis and wrote the first draft.PJY and HKH provided the specimens and clinical data of the patients.All authors contributed to the design and interpretation of the study and to further drafts.KHS is the guarantor.
Funding:This study was supported by a faculty research grant of Yonsei University College of Medicine for 2006 (6-2006-0080).
Ethical approval:The study was approved by the Institutional Review Board of Severance Hospital (# 4-2009-0243).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Zhang JY,Zou ZS,Huang A,Zhang Z,Fu JL,Xu XS,et al.Hyper-activated pro-in flammatory CD16 monocytes correlate with the severity of liver injury andfibrosis in patients with chronic hepatitis B.PLoS One 2011;6:e17484.
2 Zhang Z,Zhang S,Zou Z,Shi J,Zhao J,Fan R,et al.Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients.Hepatology 2011;53:73-85.
3 Ito H,Ando K,Ishikawa T,Saito K,Takemura M,Imawari M,et al.Role of TNF-alpha produced by nonantigen-specific cells in a fulminant hepatitis mouse model.J Immunol 2009;182:391-397.
4 Wells RG.The role of matrix stiffness in hepatic stellate cell activation and liverfibrosis.J Clin Gastroenterol 2005;39:S158-161.
5 Bataller R,Brenner DA.Liverfibrosis.J Clin Invest 2005;115:209-218.
6 Rossmanith W,Schulte-Hermann R.Biology of transforming growth factor beta in hepatocarcinogenesis.Microsc Res Tech 2001;52:430-436.
7 Okumoto K,Hattori E,Tamura K,Kiso S,Watanabe H,Saito K,et al.Possible contribution of circulating transforming growth factor-beta1 to immunity and prognosis in unresectable hepatocellular carcinoma.Liver Int 2004;24:21-28.
8 Lee WC,Chiang YJ,Wang HC,Wang MR,Lia SR,Chen MF.Functional impairment of dendritic cells caused by murine hepatocellular carcinoma.J Clin Immunol 2004;24:145-154.
9 Hosel M,Quasdorff M,Wiegmann K,Webb D,Zedler U,Broxtermann M,et al.Not interferon,but interleukin-6 controls early gene expression in hepatitis B virus infection.Hepatology 2009;50:1773-1782.
10 Kao JT,Lai HC,Tsai SM,Lin PC,Chuang PH,Yu CJ,et al.Rather than interleukin-27,interleukin-6 expresses positive correlation with liver severity in naive hepatitis B infection patients.Liver Int 2012;32:928-936.
11 Ayada M,Ishikawa T,Okumura A,Tanabe J,Ito H,Ohashi T,et al.Alteration of serum cytokine balances among different phases of chronic hepatitis B virus infection.Hepatol Res 2006;34:214-221.
12 Akpolat N,Yahsi S,Godekmerdan A,Demirbag K,Yalniz M.Relationship between serum cytokine levels and histopathological changes of liver in patients with hepatitis B.World J Gastroenterol 2005;11:3260-3263.
13 Tan AT,Koh S,Goh W,Zhe HY,Gehring AJ,Lim SG,et al.A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B.J Hepatol 2010;52:330-339.
14 Park Y,Han KH,Kim HS.Serum cytokine levels in patients with chronic hepatitis B according to lamivudine therapy.J Clin Lab Anal 2011;25:414-421.
15 Lok AS,McMahon BJ.Chronic hepatitis B:update 2009.Hepatology 2009;50:661-662.
16 Lai CL,Gane E,Liaw YF,Hsu CW,Thongsawat S,Wang Y,et al.Telbivudine versus lamivudine in patients with chronic hepatitis B.N Engl J Med 2007;357:2576-2588.
17 Yuen MF,Sablon E,Hui CK,Yuan HJ,Decraemer H,Lai CL.Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy.Hepatology 2001;34:785-791.
18 Fukai K,Zhang KY,Imazeki F,Kurihara T,Mikata R,Yokosuka O.Association between lamivudine sensitivity and the number of substitutions in the reverse transcriptase region of the hepatitis B virus polymerase.J Viral Hepat 2007;14:661-666.
19 Ajuebor MN,Hogaboam CM,Le T,Swain MG.C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse.J Immunol 2003;170:5252-5259.
20 Panasiuk A,Prokopowicz D,Panasiuk B.Monocyte chemotactic protein-1 and soluble adhesion molecules as possible prognostic markers of the efficacy of antiviral treatment in chronic hepatitis C.World J Gastroenterol 2004;10:3639-3642.
21 Park BL,Kim YJ,Cheong HS,Kim LH,Choi YH,Lee HS,et al.Association of common promoter polymorphisms of MCP1 with hepatitis B virus clearance.Exp Mol Med 2006;38:694-702.
22 Marsillach J,Bertran N,Camps J,Ferre N,Riu F,Tous M,et al.The role of circulating monocyte chemoattractant protein-1 as a marker of hepatic in flammation in patients with chronic liver disease.Clin Biochem 2005;38:1138-1140.
23 Kuo TM,Hu CP,Chen YL,Hong MH,Jeng KS,Liang CC,et al.HBV replication is significantly reduced by IL-6.J Biomed Sci 2009;16:41.
24 Pan CJ,Wu HL,Kuo SF,Kao JH,Tseng TC,Liu CH,et al.Serum interleukin 6 level correlates with outcomes of acute exacerbation of chronic hepatitis B.Hepatol Int 2011;6:591-597.
25 Barreiros AP,Sprinzl M,Rosset S,Hohler T,Otto G,Theobald M,et al.EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes.Int J Cancer 2009;124:120-129.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Disease spectrum and use of cholecystolithotomy in gallstone ileus
- Xanthogranulomatous cholecystitis mimicking gallbladder cancer and causing obstructive cholestasis
- Liver transplantation in Crigler-Najjar syndrome type I disease
- High-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients awaiting liver transplantation
- Laparoscopic distal pancreatectomy with or without splenectomy:spleen-preservation does not increase morbidity
- Expression of HBx protein in hepatitis B virusinfected intrahepatic cholangiocarcinoma
