Steroid elimination within 24 hours after orthotopic liver transplantation:effectiveness and tolerability
2012-06-11
Guangzhou,China
Introduction
As a cornerstone of immunosuppression in solid organ transplantation,corticosteroids have played an important role in the prevention and treatment of acute and chronic rejection after orthotopic liver transplantation (OLT).However,the introduction of steroids into organ transplantation has not yet been supported by prospective clinical trials.And the use of steroids is associated with a wide range of side-effects,such as osteoporosis,infections and de novo diabetes,[1-4]and so may be disadvantageous for patients' quality of life and long-term survival.
The application of novel powerful immunosuppressants makes it possible to minimize the use of steroids after OLT.[5-8]Currently,most immunosuppressive strategies applied in organ transplantation centers involve steroid withdrawal in 3 months or even later post-transplant.The well-known safety and efficiency of steroid withdrawl within 3 months encouraged our group to further minimize the use of steroids.This study aimed to evaluate the effectiveness and tolerability of an immunosuppressive regimen with steroid elimination within 24 hours after OLT.
Methods
This is a prospective study on consecutive adult Chinese patients who underwent de novo liver transplantation in the Organ Transplantation Center of the First Affiliated Hospital of Sun Yat-Sen University between January 2006 and June 2008.Two protocols of steroid use were carried out in our center,namely 24-hour elimination and 3-month withdrawal.The patients were randomized to receive one of the two protocols according to a random sequence generated by the Statistical Package for Social Sciences (SPSS,version 13.0,Chicago,IL).All patients were treated using the uniform protocol except for steroid use in our center.Informed consents were obtained from all patients enrolled in the study.
To avoid intrinsic bias,patients with any one of the following characteristics were excluded:(1)pre-transplant infections (except chronic HBV/HCV infection); (2) donor/recipient ABO blood group incompatibility; (3) pre-transplant diabetes,hypertension or hyperlipidemia; (4) hepatocellular carcinoma (HCC) lesions beyond the Milan criteria; (5)receiving marginal grafts,including grafts from donors with moderate to severe fatty liver,HBV infection,age >60 years or grafts with a cold ischemia time longer than 14 hours; (6) combined transplantation or retransplantation; and (7) partial liver transplantation,including living donor liver transplantation and split liver transplantation.
According to the exclusion criteria,76 recipients were enrolled,with 40 in the 3-month withdrawal group and 36 in the 24-hour elimination group.The basic characteristics of the recipients are summarized in Table 1.The preoperative characteristics were comparable between the two groups.
Immunosuppressive protocols
Steroids were eliminated or withdrawn in the groups.In the 3-month withdrawal group,500 mg methylprednisolone (MP) was used intraoperatively and on day 1,60 mg i.v.q6h on day 2,and tapered 40 mg every day from day 3 post-transplant.Intravenous use of MP was replaced by oral tablets on day 8,and tapered 8 mg every 3 days,then maintained at 4 mg and discontinued at three months.In the 24-hour elimination group,500 mg MP was used intraoperatively and on day 1 after the operation,then any form of steroids was discontinued unless for the treatment of acute rejection.
Follow-up
All patients received routine follow-up after discharge until December 2009.Patient survival,acute rejection episodes and steroid-resistant acute rejection,steroid-related complications (infections,nonhealing wound,new-onset diabetes,hypertension and hyperlipoidemia),and recurrence of HBV and HCC were compared between the two groups.
Since protocol biopsy is not performed routinely in our center,rejection in this study was diagnosed by pathological study of graft biopsy samples in clinically suspected patients with biochemical abnormalities.Each specimen was scored by two pathologists blinded to the immunosuppressant regimens according to the Banff scoring system.Patients with acute rejection were treated according to the following strategies.In those with mildly impaired liver function,immunosuppressants were strengthened by augmentation with basic immunosuppressive agents or combined use of MMF or sirolimus.A high-dose bolus of steroid was administered in patients who had no response to strengthened immunosuppressants or those who had a sharp increase in biochemical parameters.Anti-T3-receptor antibody or antithymocyte globulin treatment was given to those patients with steroid-resistant rejection.Steroid-resistant rejection was defined as acute rejection episodes with persistent elevation of bilirubin or transaminase levels above 1.5 times the upper limit of normal value and a repeated biopsy showing acute rejection after treatment with one or more courses of MP bolus.Infection was diagnosed according to clinical manifestations,laboratory study and pathogen culture.Diabetes was diagnosed when 2 consecutive fasting glucose levels were >7 mmol/L postoperatively.Hypertension was diagnosed as more than three random systolic pressures higher than 140 mmHg and/or diastolic pressure higher than 90 mmHg.Hyperlipidemia was diagnosed as a fasting total cholesterol level >5.72 mmol/L and or a fasting serum triglyceride level >1.7 mmol/L.
Statistical analysis
All analyses were performed using the Statistical Package for Social Sciences (SPSS,version 13.0,Chicago,IL).Continuous variables were tested for normal distribution and expressed as mean±standard deviation(SD) or median (range) as appropriate.Categorical variables were compared by the Pearson's product moment correlation coefficient and the chi-square test,and continuous variables were compared by Student's t test.Univariate survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test.For all analyses,P values <0.05 were considered statistically significant.
Results
Survival of patients
Three patients died in the peri-operative stage,two in the 3-month withdrawal group (one died of cerebrovascular accident on day 7 and the other of hepatic artery thrombosis on day 15).One patient in the 24-hour elimination group died from severe fungal infection on day 12.The peri-operative survival rates in the 3-month withdrawal and 24-hour elimination groups were 95% and 97.2%,respectively (P>0.05).
All recipients were followed up until December 2009.The mean follow-up period of the 3-month withdrawal group was 31.8±11.8 months,and in the 24-hour elimination group was 30.7±10.1 months.In the 3-month withdrawal group,besides the two who died in the early stage after operation,one died of biliary ischemia at 5 months and the other died of HCC recurrence at 36 months.In the 24-hour elimination group,except the patient who died in the early stage after operation,one died of biliary ischemia at 9 months.The survival curves are shown in Fig.,and the difference between the two groups was not significant (the log-rank test,χ2=0.461,P=0.497).
Acute rejection episodes
Nine (11.8%) of the 76 patients experienced acute rejection after the operation.At least one acute rejection episode occurred in 5 (12.5%) of the 40 patients of the 3-month withdrawal group with a mean Banff score of 6.1±0.7.And acute rejection occurred in 4 (11.1%) of the 36 patients of the 24-hour elimination group with a mean Banff score of 6.2±0.8.The incidence rate of acute rejection (χ2=0.035,P=0.852) and the mean Banff score (t=0.612,P=0.502) between the two groups were not different.Two episodes of rejection were reversed by strengthening the basic immunosuppressants,and seven episodes were cured by steroid bolus therapy.No acute rejection episode was found to be steroid-resistant.
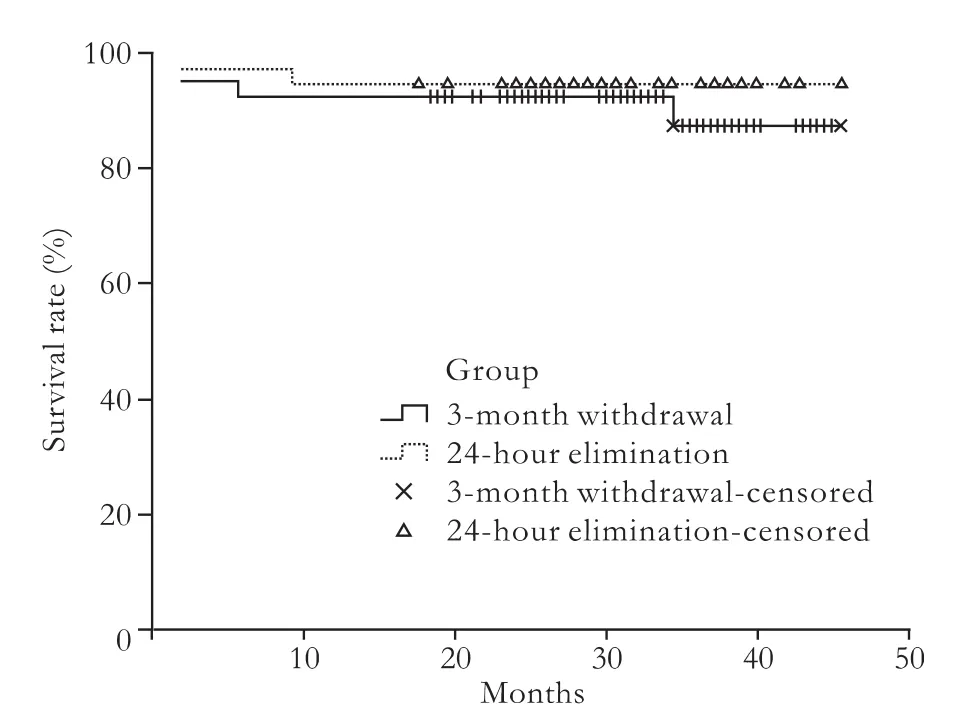
Fig.Survival curve by the Kaplan-Meier method.The difference between patients in the two groups was not significant (log-rank test,χ2=0.461,P=0.497).
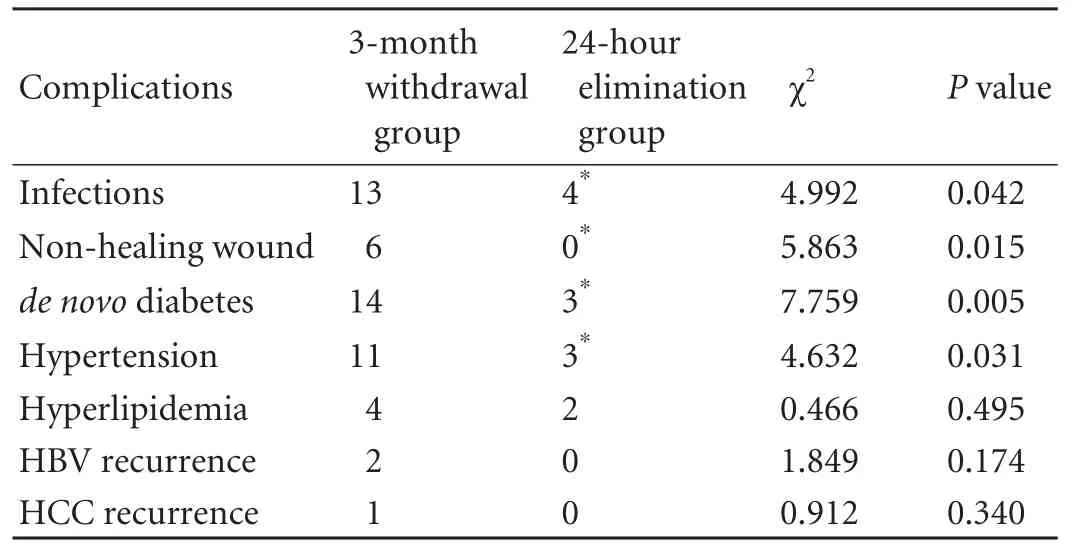
Table 2.Post-transplant complications
Post-transplant complications
Thirteen patients in the 3-month withdrawal group had at least one episode of infection after transplantation:bacterial infection in eight,fungal infection in two,co-existing bacterial and fungal infection in two,and CMV infection in one.Four patients in the 24-hour elimination group experienced infections after transplantation:bacterial infection in three and fungal infection in one.Six patients had a nonhealing wound in the 3-month withdrawal group:fat liquefaction of the incision infive and wound infection in one.No patients had a non-healing wound in the 24-hour elimination group.de novo diabetes developed in fourteen patients in the 3-month withdrawal group and three in the 24-hour elimination group.Eleven recipients in the 3-month withdrawal group and three in the 24-hour elimination group had hypertension.Hyperlipidemia appeared in four patients in the 3-month withdrawal group and two in the 24-hour elimination group.Two patients had HBV recurrence and one had HCC recurrence in the 3-month withdrawal group,whereas no patients had HBV or HCC recurrence in the 24-hour elimination group.Table 2 shows a detailed list of the post-transplant complications.
Discussion
Since the very beginning of solid organ transplant,steroids have been one of the primary agents for preventing and treating rejection.Meanwhile,longterm application of steroids leads to side-effects such as post-transplant diabetes mellitus,hypertension,hyperlipidemia,infectious diseases,osteoporosis,and growth retardation.[9]Minimization of steroids following solid organ transplantation has attracted great attention of researchers over the past decade.In the field of OLT,with the development of a wide variety of immunosuppressive drugs,physicians all over the world can control acute rejection very well,so this is no longer an important risk factor for long-term patient and graft survival.[10]A number of clinical trials on steroid reduction in OLT recipients have been conducted successfully.[5-8]Since most acute rejection episodes occur during the period from day 7 to day 60 posttransplant and about 60%of rejections occur within the first two weeks,current strategies for steroid withdrawal include 3-month and 2-week protocols.[10]Among these strategies,the common principle is to minimize the dosage and duration of steroid administration under the premise of an acceptable incidence rate of acute rejection.These studies have documented that steroid minimization decreases the incidence of steroid-related complications without increasing the risk of rejection,and thus is beneficial for the long-term patient outcome.[5-8,11,12]
Currently,the majority of liver transplantation centers adopt the immunosuppressive strategy with steroid withdrawal in three months or even later after the operation; this is the classical regimen yielding satisfactory immunosuppressant effects.However,this strategy might compromise the long-term patient and graft outcome because of the development of steroidrelated side-effects.Our previous study on a cohort of Chinese OLT recipients showed that steroid withdrawal within seven days after the operation is effective and safe in preventing acute rejection.[13]This success encouraged us to further investigate the safety and feasibility of an immunosuppressive regimen with steroid elimination within 24 hours after the operation.
IL-2 receptor antibody is mainly used for induction therapy to decrease the risk of acute rejection and to attenuate the renal impairment caused by early exposure to calcineurin inhibitors after OLT.[14-17]Tacrolimus is a potent and effective immunosuppressant and currently a basic element of immunosuppressant maintenance for liver transplant recipients.It guarantees good longterm patient and graft survival with a low incidence of graft rejection and side-effects.[10,18]Tacrolimus is well known for its steroid-sparing effect due to its powerful immunosuppressant capacity.An immunosuppressive strategy based on tacrolimus and IL-2 receptor antibody induction enables steroid elimination within 24 hours and even steroid-free treatment after the operation.[7,8,14,18]In the present study,steroid elimination within 24 hours after OLT did not increase the risk of rejection (a rejection rate of 11.1% in the 24-hour elimination group and 12.5% in the 3-month withdrawal group),indicating that both regimens offer a potent immunosuppressive effect.Since protocol biopsy is not routinely performed in our center,however,the possibility of low-grade rejection,early stages of vanishing bile duct syndrome andfibrosis could not be ruled out.Thus the pathologic changes in patients of the 24-hour elimination group may have been underestimated.Importantly,when steroids were eliminated within 24 hours,the incidence of steroidrelated side-effects such as infections,non-healing wound,de novo diabetes and hypertension were significantly decreased.The incidence of hyperlipidemia was also lower,but the difference between the two groups was not statistically significant,possibly because of the Chinese diet and a relatively short follow-up period.
In Western countries,the top-ranking liver transplant candidates are those with alcoholic cirrhosis,liver cirrhosis secondary to HCV infection and autoimmune hepatitis,while in the mainland of China,over 90% of liver transplant candidates are infected with HBV (about 40% have concurrent HCC).Studies have shown that the use of steroids over one year significantly increases the recurrence of primary diseases,such as HBV,HCV and HCC.[19-22]Therefore,there is no reason for us to use steroids,except for limiting ischemiareperfusion injury,which may be achieved by very shortterm use of steroids.In the current study,the recurrence rate of HBV and HCC was lower in the 24-hour elimination group than the 3-month withdrawal group,but the difference was not statistically significant.There are three possible reasons.First,all candidates with HCC in this study were within the Milan criteria,which could lead to a very low tumor recurrence rate.In addition,we routinely used low doses of hepatitis B immunoglobulin and lamivudine to prevent post-transplant HBV recurrence and this may explain its low recurrence rate in this study.Finally,our follow-up period may not have been long enough or the sample size may not have been large enough to detect a difference in disease recurrence between patients in the two groups.
Recipients with pretransplant diabetes and hypertension would gain the greatest benefit from a steroidfree immunosuppressive regimen; however,these patients were excluded from this study since the 24-hour elimination strategy was used in most of these patients for their safety.Therefore,we did not assess the advantage of the steroid avoidance regimen in this group of patients.On the other hand,with this strict patient enrollment criteria,we showed a clear benefit of virtual steroid avoidance in the prevention of post-OLT new onset diabetes and hypertension.Since diabetes itself has negative effects on patient survival,and hypertension is a well-known risk factor for cardiovascular accidents,[23,24]we anticipate that steroid elimination within 24 hours would likely prolong longterm survival although it was not shown in this study.
In conclusion,when using IL-2 receptor antibody induction and tacrolimus-based maintenance,steroids can be safely eliminated within 24 hours after operation,which significantly reduces steroid-related complications without increasing the risks of rejection.A prospective,randomized trial of the use of this immunosuppressant regimen is needed to further confirm these findings and the apparent beneficial effects on reducing the recurrence of HBV and HCC.
Contributors:HXS proposed the study.WLW and HXS wrote the first draft.GZY analyzed the data.All authors contributed to the design and interpretation of the study and to further drafts.HXS is the guarantor.
Funding:This study was supported by grants from the Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan of China (2008BAI60B02 and 2008BAI60B06) and the Science and Technology Planning Project of Guangdong Province (2008B030301308).
Ethical approval:The study protocol was approved by the ethical committee of the First Affiliated Hospital,Sun Yat-Sen University.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Compston JE.Osteoporosis after liver transplantation.Liver Transpl 2003;9:321-330.
2 Davidson J,Wilkinson A,Dantal J,Dotta F,Haller H,Hernández D,et al.New-onset diabetes after transplantation:2003 International consensus guidelines.Proceedings of an international expert panel meeting.Barcelona,Spain,19 February 2003.Transplantation 2003;75:SS3-24.
3 Stegall MD,Everson GT,Schroter G,Karrer F,Bilir B,Sternberg T,et al.Prednisone withdrawal late after adult liver transplantation reduces diabetes,hypertension,and hypercholesterolemia without causing graft loss.Hepatology 1997;25:173-177.
4 Stockmann M,Steinmüller T,Nolting S,Neuhaus P.Posttransplant diabetes mellitus after orthotopic liver transplantation.Transplant Proc 2002;34:1571-1572.
5 Greig P,Lilly L,Scudamore C,Erb S,Yoshida E,Kneteman N,et al.Early steroid withdrawal after liver transplantation:the Canadian tacrolimus versus microemulsion cyclosporin A trial:1-year follow-up.Liver Transpl 2003;9:587-595.
6 Pageaux GP,Calmus Y,Boillot O,Ducerf C,Vanlemmens C,Boudjema K,et al.Steroid withdrawal at day 14 after liver transplantation:a double-blind,placebo-controlled study.Liver Transpl 2004;10:1454-1460.
7 Washburn K,Speeg KV,Esterl R,Cigarroa F,Pollack M,Tourtellot C,et al.Steroid elimination 24 hours after liver transplantation using daclizumab,tacrolimus,and mycophenolate mofetil.Transplantation 2001;72:1675-1679.
8 Pirenne J,Aerts R,Koshiba T,Van Gelder F,Roskams T,Schetz M,et al.Steroid-free immunosuppression during and after liver transplantation--a 3-yr follow-up report.Clin Transplant 2003;17:177-182.
9 Guckelberger O,Mutzke F,Glanemann M,Neumann UP,Jonas S,Neuhaus R,et al.Validation of cardiovascular risk scores in a liver transplant population.Liver Transpl 2006;12:394-401.
10 Matinlauri IH,Nurminen MM,Höckerstedt KA,Isoniemi HM.Changes in liver graft rejections over time.Transplant Proc 2006;38:2663-2666.
11 Moench C,Barreiros AP,Schuchmann M,Bittinger F,Thiesen J,Hommel G,et al.Tacrolimus monotherapy without steroids after liver transplantation--a prospective randomized double-blinded placebo-controlled trial.Am J Transplant 2007;7:1616-1623.
12 Toyoki Y,Hakamada K,Narumi S,Totsuka E,Nara M,Ono H,et al.Primary immunosuppression regimen of rapid steroid withdrawal after living related liver transplantation:a singlecenter experience.Transplant Proc 2004;36:2279-2281.
13 Hu AB,He XS,Wu ZP,Zhu XF,Ma Y,Wang DP,et al.Evaluation of efficacy and safety on steroid withdraw at the seventh day after liver transplantation.Zhonghua Wai Ke Za Zhi 2008;46:1126-1128.
14 Langrehr JM,Glanemann M,Guckelberger O,Klupp J,Neumann U,Machens C,et al.A randomized,placebocontrolled trial with anti-interleukin-2 receptor antibody for immunosuppressive induction therapy after liver transplantation.Clin Transplant 1998;12:303-312.
15 Aw MM,Taylor RM,Verma A,Parke A,Baker AJ,Hadzic D,et al.Basiliximab (Simulect) for the treatment of steroidresistant rejection in pediatric liver transpland recipients:a preliminary experience.Transplantation 2003;75:796-799.
16 Lee KH,Da Costa M,Lim SG,Tan KC.Delayed tacrolimus is safe with basiliximab induction therapy.Liver Transpl 2002;8:732.
17 Neuhaus P,Clavien PA,Kittur D,Salizzoni M,Rimola A,Abeywickrama K,et al.Improved treatment response with basiliximab immunoprophylaxis after liver transplantation:results from a double-blind randomized placebo-controlled trial.Liver Transpl 2002;8:132-142.
18 Ringe B,Braun F,Schütz E,Füzesi L,Lorf T,Canelo R,et al.A novel management strategy of steroid-free immunosuppression after liver transplantation:efficacy and safety of tacrolimus and mycophenolate mofetil.Transplantation 2001;71:508-515.
19 Vivarelli M,Burra P,La Barba G,Canova D,Senzolo M,Cucchetti A,et al.In fluence of steroids on HCV recurrence after liver transplantation:A prospective study.J Hepatol 2007;47:793-798.
20 Filipponi F,Callea F,Salizzoni M,Grazi GL,Fassati LR,Rossi M,et al.Double-blind comparison of hepatitis C histological recurrence Rate in HCV+ Liver transplant recipients given basiliximab+steroids or basiliximab+placebo,in addition to cyclosporine and azathioprine.Transplantation 2004;78:1488-1495.
21 Marcos A,Eghtesad B,Fung JJ,Fontes P,Patel K,Devera M,et al.Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation:with particular reference to hepatitis C virus.Transplantation 2004;78:966-971.
22 Liu CL,Fan ST,Lo CM,Chan SC,Ng IO,Lai CL,et al.Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation:a protocol with early elimination of steroids and reduction of tacrolimus dosage.Liver Transpl 2004;10:728-733.
23 John PR,Thuluvath PJ.Outcome of liver transplantation in patients with diabetes mellitus:a case-control study.Hepatology 2001;34:889-895.
24 Desai S,Hong JC,Saab S.Cardiovascular risk factors following orthotopic liver transplantation:predisposing factors,incidence and management.Liver Int 2010;30:948-957.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Double inferior vena cava does not complicate para-aortic nodal dissection for the treatment of pancreatic carcinoma
- Cerebral protective effect of nicorandil premedication on patients undergoing liver transplantation
- Association of polymorphisms in non-classic MHC genes with susceptibility to autoimmune hepatitis
- Correlation of the occurrence of YMDD mutations with HBV genotypes,HBV-DNA levels,and HBeAg status in Chinese patients with chronic hepatitis B during lamivudine treatment
- Efficacy and factors in fluencing treatment with peginterferon alpha-2a and ribavirin in elderly patients with chronic hepatitis C
- Clinical outcome in patients with hilar malignant strictures type II Bismuth-Corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage
