Immobilization of Penicillin G Acylase on Magnetic Nanoparticles Modified by Ionic Liquids*
2012-03-22ZHOUHuacong周华从LIWei李伟SHOUQinghui寿庆辉GAOHongshuai高红帅XUPeng徐芃DENGFuli邓伏礼andLIUHuizhou刘会洲
ZHOU Huacong (周华从), LI Wei (李伟)**, SHOU Qinghui (寿庆辉), GAO Hongshuai(高红帅) XU Peng (徐芃), DENG Fuli (邓伏礼) and LIU Huizhou (刘会洲)**
1 State Key Laboratory of Biochemical Engineering, Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2 Graduate University of Chinese Academy of Sciences, Beijing 100049, China
1 INTRODUCTION
Penicillin G acylase (EC 3.5.1.11, PGA) is one of the important pharmaceutical enzymes during the production of β-lactam antibiotics. It can catalyze the hydrolysis of amidic bonds of penicillin G and cephalosporin G to produce 6-aminopenicillanic acid (6-APA)and 7-aminodeacetoxy-cephalosporanic acid (7-ADCA)[1]. However, difficulties in enzyme recycling limit application of native enzyme in industrial production.An efficient solution is to immobilize the enzyme on certain support materials [2]. Many organic or inorganic carriers have been used to immobilize PGA [3]. Although they have several advantages in immobilization of enzyme, the organic carriers have some inevitable disadvantages, such as the poor stability towards organic solvents, microbial attacks and disposal problems [4]. In contrast, many inorganic carriers are antimicrobial and stable towards organic solvents. Besides,they have high specific surface areas, large pore volume and strong physical strength. So more and more attention have been paid to the inorganic carriers [5, 6].
Among the inorganic supports, nano- or microparticles with magnetic properties have great potential applications because they can be separated rapidly and easily from reaction systems by applying an external magnetic field [7, 8]. Fe3O4nanoparticles exhibit superparamagnetic properties when their size is in a proper range [9]. Surface modification of magnetic nanoparticles (MNP) is a key procedure because it can make beneficial improvements in the surface properties and enzyme activity and/or stability. There have been many reports about the modification of inorganic supports. Shi et al. [10] introduced γ-aminopropyl groups on the silica surface through W/O microemulsion. After activated by glutaraldehyde, the materials were used as carrier for PGA immobilization. Composite microspheres containing hydroxyl groups were prepared by Wang et al [4]. Furthermore, other functional groups, such as epoxy-thiol group [11], were also used to modify the supports. Enzymes are immobilized on particle surfaces through reactions between these functional groups with certain groups on the surfaces of enzyme molecules. These reported approaches are efficient ways for immobilizing PGA but still have some disadvantages: (1) many steps are required to synthesize the composite materials, leading to long synthesis procedure and low final yield; (2) the use of many hazardous reagents could increase the environmental burden. Thus, it is necessary to find a new method for modifying carriers.
Ionic liquids (ILs) are a class of molten salts with no or very low volatile pressure, low melting point,thermal stability, excellent salvation properties, and structure-tunability [12], and have potential applications in catalysis, extraction, absorption and so on [13].It has been confirmed that ILs are suitable media for enzyme catalysis [14, 15]. These properties make ILs potential materials for enzyme immobilization. In previous work of our group, ionic liquids containing silane coupling group had been immobilized successfully onto MNPs, and this composite material applied in Candida rugosa lipase immobilization [16]. Herein,to further develop magnetic nanoparticles supported ionic liquids in enzyme immobilization, we prepared magnetic nanoparticles supported ionic liquids with a modified way and developed their application in PGA immobilization. ILs were loaded on the surface of magnetic silica nanoparticles (MSNP) through chemical bonds formed by reaction between ethoxy groups on ILs and silanol groups on MSNPs surface [17].PGA was immobilized onto the magnetic supportsviaphysical adsorption. The morphology and properties of the composite materials were characterized by the means of Fourier transform infrared spectroscopy(FTIR), Scanning electron microscopy (SEM), transmission electron microscopy (TEM), and vibrating sample magnetometer (VSM). The amount of loaded enzyme, activity and recovery performance of immobilized penicillin G acylase were also investigated.The results showed that MSNP was an effective material for immobilizing penicillin G acylase.
2 MATERIALS AND METHODS
2.1 Materials
Toluene (analytical grade) was dehydrated with 5A molecular sieve before use. All other reagents were analytical grade and used as received, including ferric chloride hexahydrate (FeCl3·H2O), ferrous chloride tetrahydrate (FeCl2·4H2O), ammonium hydroxide(NH3·H2O, 25% by mass), tetraethyl orthosilicate(TEOS),N-methylimidazole (purity>99%). Penicillin G potassium salt (purity>99%) was a gift from North China Pharmaceutical Group. Penicillin G acylase(774.03 U·ml-1, 23.1 mg protein·ml-1) was purchased from Haider Biochemical Corp., Zhejiang, China.
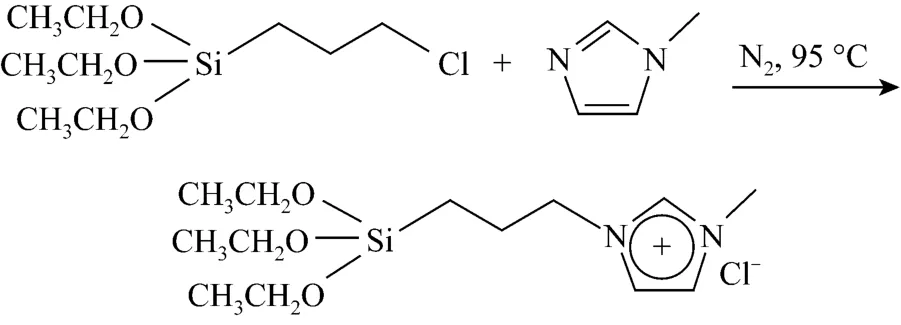
Scheme 1 Synthesis of functional ionic liquids
2.2 Synthesis of functional ionic liquids
Silane coupling group functionalized ionic liquids were synthesized according to the reported procedure[18]. The synthesis route was illustrated in Scheme 1.Equal molar ofN-methylimidazole and 3-chloropropyltriethoxy-silane were added into a flask and agitated at 95 °C under nitrogen for 24 h. After cooling down, an equal volume of ether was mixed and extracted for three times. The product (named [C1C(S)Im]Cl, where S denotes the silane substitute) was dried under vacuum at room temperature (yield: 94%).
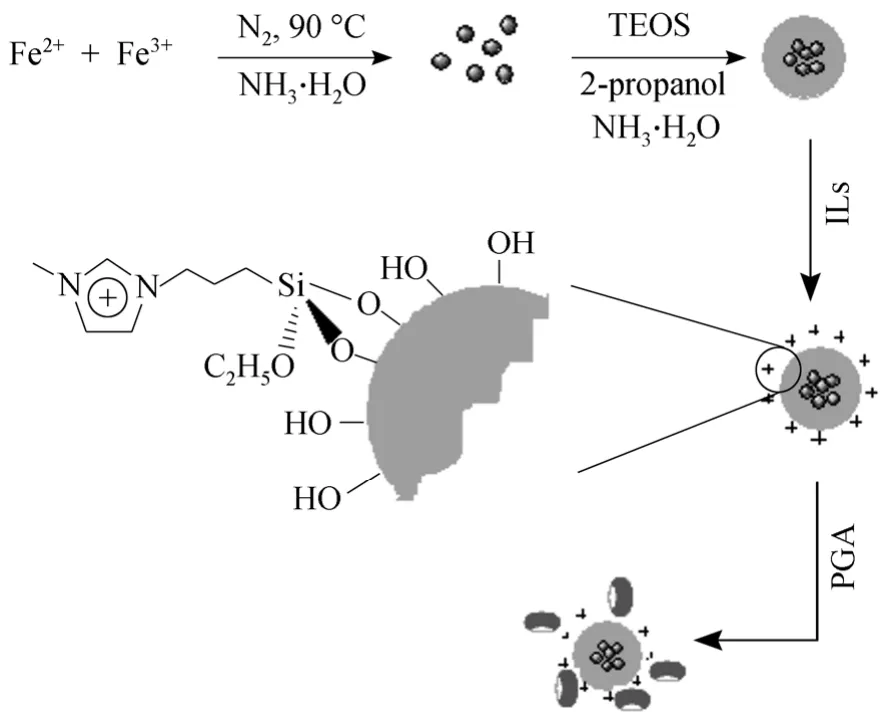
Scheme 2 Preparation of magnetic silica nanoparticles supported ionic liquids and immobilization of penicillin G acylasepenicillin G acylase; Fe3O4 particles; silica shell; IL,1-methyl-3-(tri-ethoxysilylpropyl)-imidazolium chloride
2.3 Preparation of magnetic silica nanoparticles
The whole process for support synthesis and PGA immobilization was illustrated in Scheme 2.Fe3O4was prepared first by modified co-precipitation method [17]. The deionized water was heated to a higher temperature (95 °C) than the reaction temperature (90 °C) for 30 min with nitrogen bubbling before ferric and ferrous salts were added for better removal of dissolved oxygen. After this, the system was cooled down to a lower temperature (90 °C) and 4.649 g(0.0172 mol) FeCl3·6H2O and 1.710 g (0.0086 mol)FeCl2·4H2O were dissolved under nitrogen. 10 ml 25% NH3·H2O was poured with vigorous stirring. After the color of bulk solution turned to black, the magnetic precipitates were separated and washed several times with deionized water until the pH value of eluent decreased to ~7. Then, silica shell was formed on the surface of Fe3O4through sol-gel method [17].2 g magnetic fluid containing 40 mg Fe3O4was diluted with 40 ml of water and 160 ml of 2-propanol. This mixture was dispersed under ultrasonification for 10 min and then 3 ml concentrated ammonium hydroxide was added under 30 °C in the presence of a constant nitrogen flux. 0.2 ml TEOS was added slowly with stirring. This suspension was stirred for 12 h to make the silica shell grow on the surface of nanoparticles.The obtained sample was washed with water for several times and dried under vacuum.
2.4 Immobilization of functional ionic liquids
0.1 g magnetic silica nanoparticles was dispersed in dry toluene by ultrasonification for 10 min [19]. About 10 g [C1C(S)Im]Cl was added and the mixture was stirred at 90 °C for 24 h. The product was isolated by an external magnet, washed with acetonitrile (100 ml)twice and methanol (100 ml) twice, and dried in vacuo.
2.5 Characterization of magnetic MNPs and MNPILs
The size and morphology of MNPs and MNPs-IL were observed by scanning electron microscopy (SEM,Japan, JSM-6700 F) and transmission electron microscopy (TEM, Japan, JEM 2010). Magnetization curves of samples were recorded with a vibrating sample magnetometer (VSM, Model 4 HF VSM, ADE Technologies, USA). Fourier transform infrared spectroscopy (FTIR, Bruker, Vecter 22) was used to record functional groups.
2.6 PGA immobilization and activity assay
The procedure for PGA immobilization was as follows: 0.05 g MNP-IL (dry mass) and different concentrations of PGA solution in 0.1 mol·L-1phosphate buffer (pH 9.0) were mixed and shaken at 37 °C, 180 r·min-1for 2 h. After that, the particles were isolated with magnet and washed for three times with 0.1 mol·L-1phosphate buffer (pH 9.0). Protein concentrations before and after the immobilization were measured by Bradford’s dye binding assay [20]. The immobilized protein amounts were calculated by mass balance.
The activities ImPGA were detected by the hydrolysis reaction of penicillin G potassium salt (PGP,Scheme 3). One unit of enzyme activity was defined as the amount of enzyme required to produce 1 μmol 6-APA per minute with 2% penicillin G potassium salt as substrate in 0.1 mol·L-1, pH 9.0 phosphate buffer at 37 °C, 180 r·min-1. Typical procedure was as follows:15 ml 2% PGP solution in 0.1 mol·L-1phosphate buffer under pH 9.0 was prewarmed at 37 °C for 30 min. Then certain amount of ImPGA was added and the system was shaken at 180 r·min-1. After reaction for 5 min, 10 ml sample was extracted and mixed vigorously with 5 ml anhydrous alcohol to stop the reaction. The enzyme activity was measured by the production of 6-aminopenicillanic acid (6-APA), one of the hydrolysis products, by the PDAB method [21].The experimental procedure was as follows: 3 ml 2 mol·L-1acetic acid buffer (pH 2.5) and 1 ml 0.5% ofp-dimethylaminobenzaldehyde (PDAB) in methanol were mixed and then 1 ml sample containing 6-APA was added. The mixture was allowed to react for exact 3 min at room temperature and the optical density value of reaction solution was determined at 415 nm.
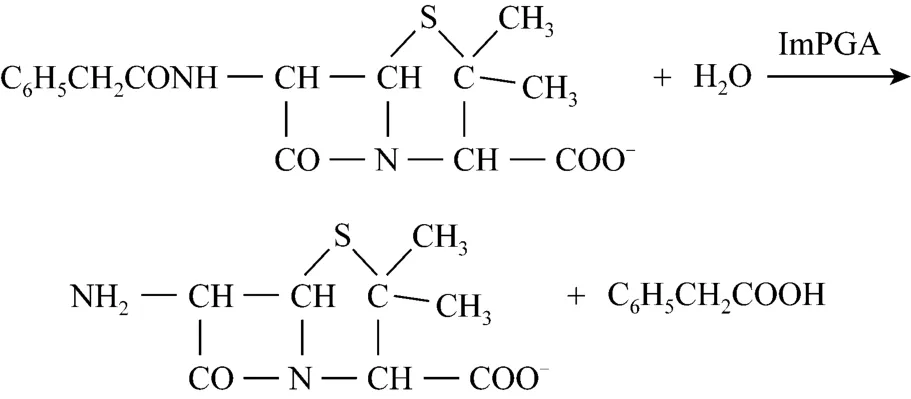
Scheme 3 Hydrolysis of penicillin G potassium salts under ImPGA
3 RESULTS AND DISCUSSION
3.1 Synthesis of magnetic nanoparticles supported ionic liquids
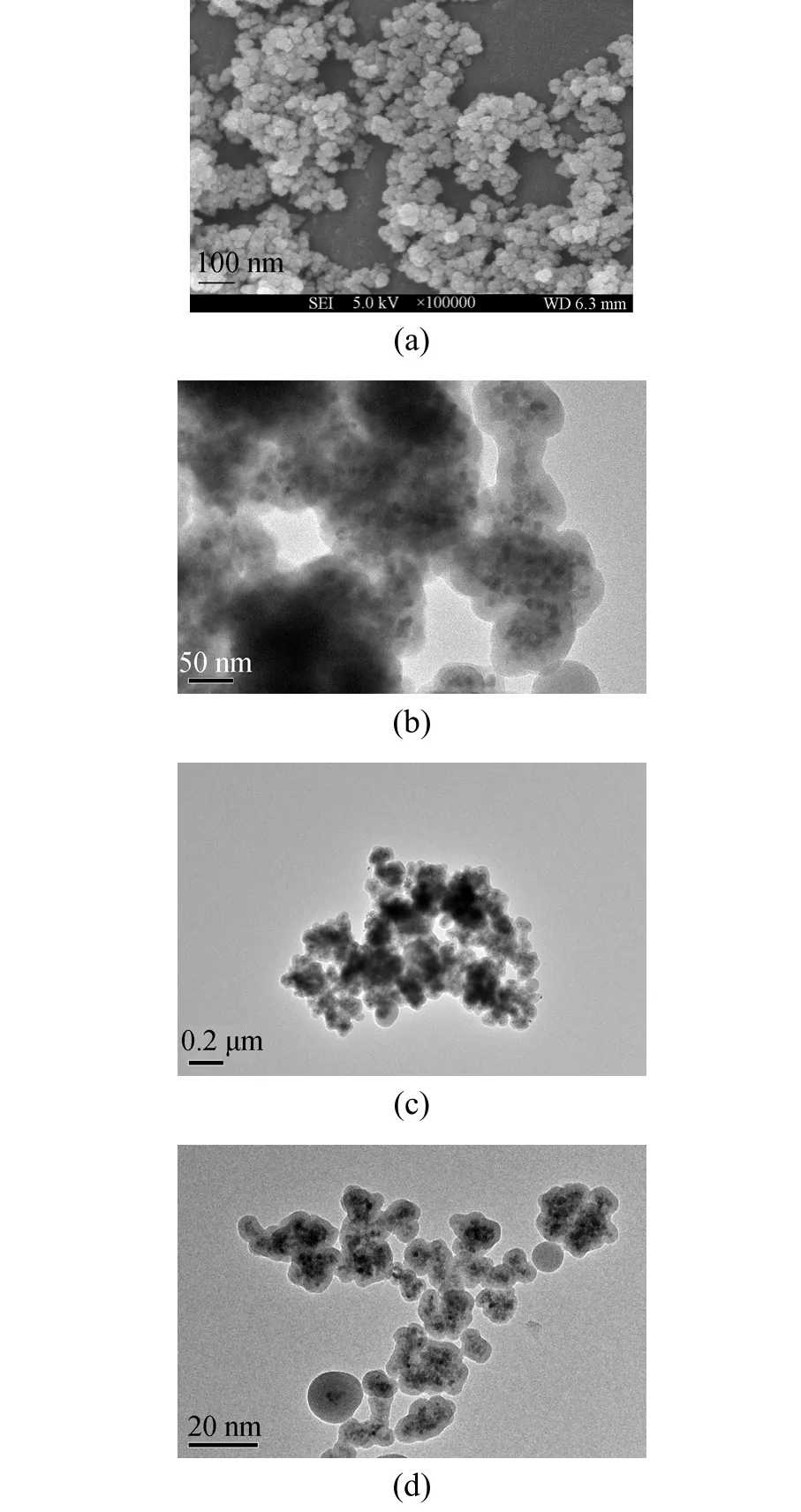
Figure 1 (a) SEM of naked Fe3O4 nanoparticles; (b) TEM of magnetic silica nanoparticles; (c) TEM of magnetic silica nanoparticles supported ionic liquids [C1C(S)Im]Cl;and (d) TEM of magnetic nanoparticles immobilized penicillin G acylase
To obtain magnetic Fe3O4, the co-precipitation method was adopted in this work because this technique is probably the simplest and most efficient way [22].The morphology of Fe3O4particles is shown in Fig. 1(a). It can be seen that naked Fe3O4nanoparticles aggregated seriously. This may be due to that naked Fe3O4nanoparticles have a strong dipole interaction and a high surface energy [22]. According to statistical results from different regions in SEM photographs, the particle size of Fe3O4ranged from 7 to 14 nm and the average size was 10 nm (Fig. 2), smaller than the critical size (25 nm) of superparamagnetism.
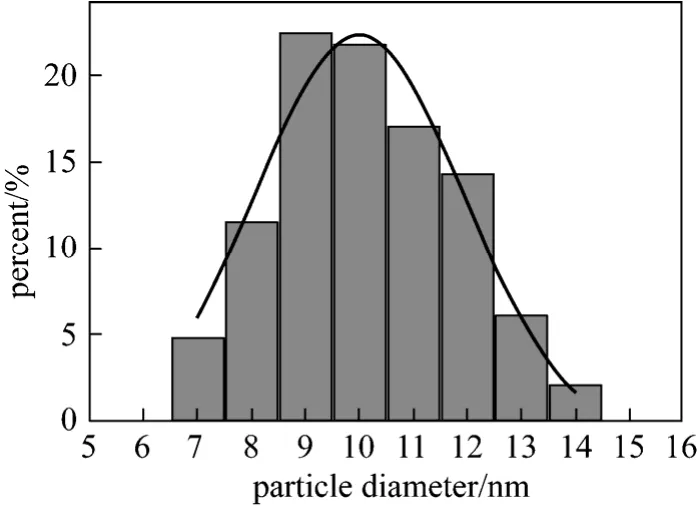
Figure 2 Size distribution of Fe3O4 nanoparticles
The introduction of silica shell on the surface of Fe3O4particles mainly for: (1) the prevention of aggregating of Fe3O4particles and (2) the presence of silanol groups on particle surface. The prevention of aggregating may be contributed to particle sizes increase, shielding the magnetic dipole interaction, and modifying the surface with negative charges. The silanol groups can react with the ethoxy groups in IL,which makes IL molecules immobilized on the surface chemically. Fig. 1 (b) shows the TEM of MSNPs, in which the core-shell structure of MSNPs can be obviously seen. The average diameter of MSNPs was about 90 nm.
FTIR spectra of Fe3O4and MSNPs were shown in Fig. 3. The strong bond at 585 cm-1in curve (a)corresponds to Fe-O vibrations [23]. Bonds at 3425 and 1632 cm-1represent the stretching and bending vibrations of OH on the surface of Fe3O4, respectively [16].The new peak at 1400 and 1100 cm-1in curve (b) represents SiO bonds, while the same peaks as in curve (a)at 3425 and 1632 cm-1correspond to the stretching and bending vibrations of OH on the surface of MSNP. This means the existence of abundant SiOH groups on the surface. The weak peak at 800 cm-1is the characteristic peak of SiO Fe, which implies that SiO2is chemically bonded to Fe3O4[24].
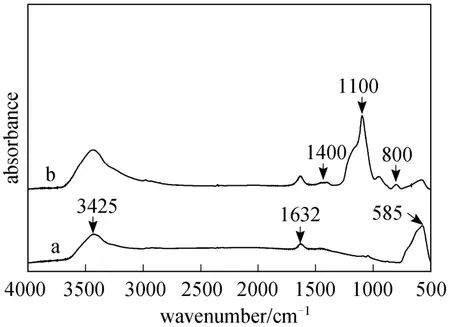
Figure 3 FTIR spectra of (a) Fe3O4 and (b) magnetic silica nanoparticles
Ionic liquid was loaded on the surface of MSNPs through reaction between ethoxy groups on IL molecules and silanol groups on MSNPs surface (illustrated in Scheme 2). Fig. 1 (c) shows the TEM photographs of MNP-IL. It can be seen that particles appear to a little aggregation after modified with IL, but not as serious as that in naked Fe3O4. This may be due to that the layer of IL may interact with each other [16].Fig. 4 presents the FTIR spectra of IL and MNPs-IL.Comparing with the spectra of MSNPs without modifiers (curve a), spectrum of MNP-IL (curve b) presents two new peaks at 2973 and 2929 cm-1corresponding to the stretching vibrations of CH3and CH2in IL,while new peaks at 1463 and 1381 cm-1are their bending vibrations. Accordingly, it can be sure that IL has been immobilized successfully on the surface of MSNPs.
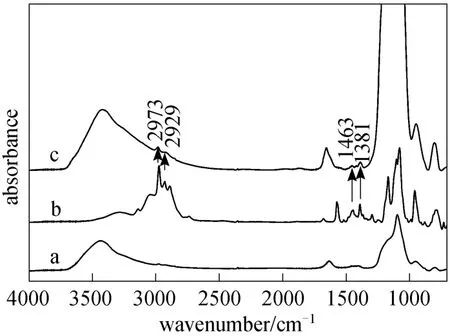
Figure 4 FTIR spectra of (a) magnetic silica nanoparticles;(b) ionic liquids [C1C(S)Im]Cl; (c) magnetic silica nanoparticles modified with [C1C(S)Im]Cl
The magnetic properties of Fe3O4and MNPs-IL are shown in Fig. 5. There was no hysteresis in the magnetization, meaning that there would be no magnetic interactions among particles in a zero-field environment. It can be seen that the two samples are superparamagnetic. The saturation magnetization of Fe3O4was turned from 63.667 A·m2·kg-1(curve 1) to 26.888 A·m2·kg-1when it was coated with silica and then IL (curve 2). Despite of this, it is found that the MNPs-IL are still very sensitive to external magnetic field and can be separated easily and rapidly.
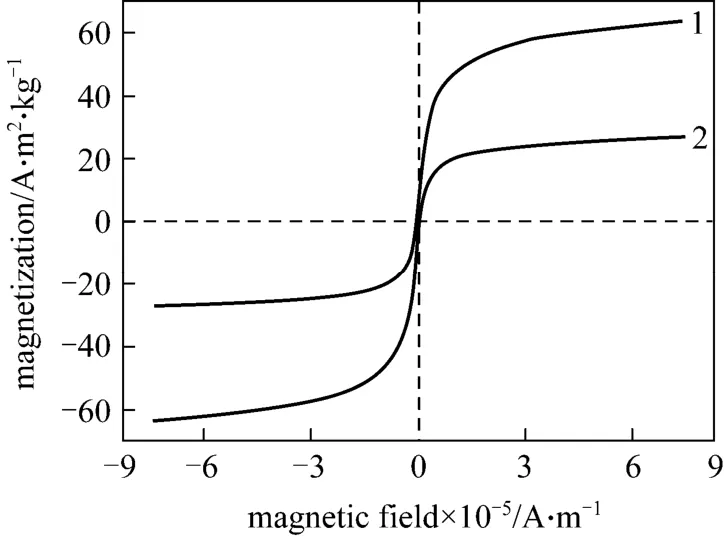
Figure 5 Magnetization curves of (1) Fe3O4 and (2) magnetic silica nanoparticles modified with [C1C(S)Im]Cl
3.2 Immobilization of PGA
PGA was successfully immobilized onto the surface of MNPs-IL. The adsorption isotherm is shown in Fig. 6. The PGA loaded amount increases with the initial concentration increase until the initial concentration of PGA comes to 2.1 mg·ml-1. The PGA loaded amount remains constant at about 209 mg·g-1(based on carrier) when the concentration of PGA is between 2.1 and 4.6 mg·ml-1. The enzyme activity reaches its maximum at 262 U·g-1(based on ImPGA) when the initial concentration of PGA is 1.71 mg·ml-1and decreases thereafter. It may be attributed to the steric hindrance of enzyme molecules on the surface of supporting particles, which is severe at high loading density [25].
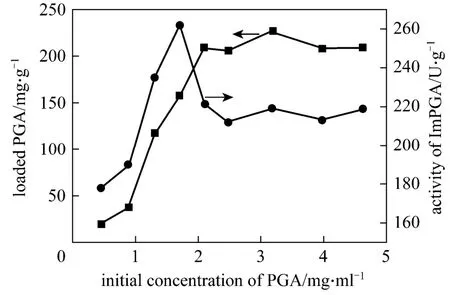
Figure 6 Effect of initial PGA concentration on loading density and enzyme activity■ loaded PGA; ● activity of ImPGA
3.3 Recovery performance of ImPGA
One of the most significant advantages of immobilizing enzyme is the recycling property. In this experiment, ImPGA was separated by magnetic separation, washed with phosphate buffer (0.1 mol·L-1, pH 9.0), and reused in the next batch. As can be seen in Fig. 7, the activity of ImPGA remains 75% of its initial activity after 5 cycles and 62% even after 10 cycles, while native PGA is difficult in recycling.
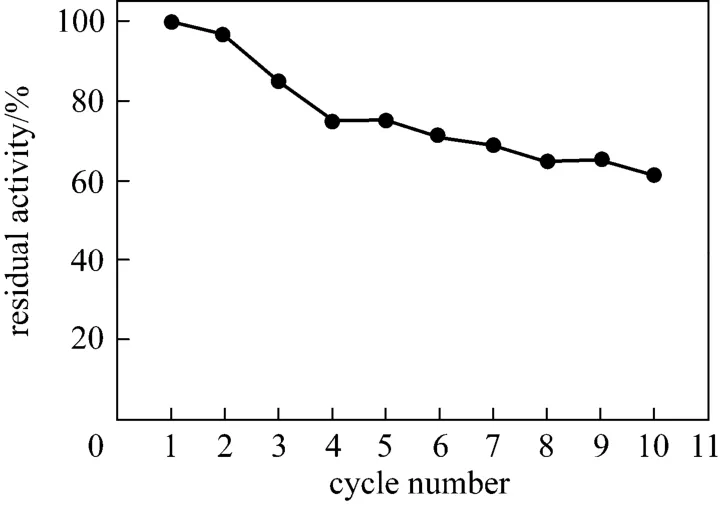
Figure 7 Reusability of immobilized PGA
This work has demonstrated that MNP-IL is suitable as carriers of PGA immobilization. The maximum PGA loaded amount [209 mg·g-1(based on carrier)] and enzyme activity [262 U·g-1(based on ImPGA)] are higher than that in the previous reports[4, 6]. However, in this study, we also found that the specific activity of the immobilized PGA decreased gradually comparing with the native form. The specific activity of native and immobilized PGA are 34 U·mg-1(based on PGA) and 2 U·mg-1(based on PGA),respectively. The apparent decrease of activity may be caused by the steric hindrance of enzyme molecules which decreases the contact chance between PGA and substrate molecules. Besides, the interaction between PGA and the carriers may also have a negative effect on the activity. However, ImPGA can be recycled and reused for many times, while it is very difficult to reuse native PGA. Immobilization can also fix PGA molecules on carriers and thus decrease or prevent the protein pollution after reaction, which simplifies the postpurification process. Furthermore, through optimizing the structures of ILs on the surface of carriers, it is possible to keep or improve the activity and stability of ImPGA. Thus, it is promising to construct new materials based on MNP-IL for immobilizing PGA and conduct detailed research on the effects of ILs on activity and stability of PGA molecules. Extensive work on PGA immobilization with MNP-IL is in progress.
4 CONCLUSIONS
Superparamagnetic Fe3O4nanoparticles with an average size of ~10 nm were prepared by modified co-precipitation method. After coated by silica shell and then modified with functionalized ILs, the obtained composite nanoparticles exhibited a typical core-shell structure and the average size of particles increased to ~90 nm, while the saturation magnetization decreased from 63.7 to 26.9 A·m2·kg-1. The MNP-ILs maintains the advantages of high specific area and sensitivity to external magnetic field. The biggest enzyme loaded amount was about 209 mg·g-1(based on carrier), and the highest enzyme activity of ImPGA was 262 U·g-1(based on ImPGA).
1 Xue, J.Y., Wang, A.M., Zhou, C., Shen, S.B., “Penicillin acylase immobilization depending on macromolecular crowding and catalysis in aqueous-organic medium”,Bioprocess Biosyst.Eng., 32 (8),765-772 (2009).
2 Brady, D., Jordaan, J., “Advances in enzyme immobilization”,Biotechnol.Lett., 31 (11), 1639-1650 (2009).
3 Chong, A.S.M., Zhao, X.S., “Design of large-pore mesoporous materials for immobilization of penicillin G acylase biocatalyst”,Catal.Today, 93 (95), 293-299 (2004).
4 Wang, W., Deng, L., Peng, Z.H., Xiao, X., “Study of the epoxydized magnetic hydroxyl particles as a carrier for immobilizing penicillin G acylase”, Enzyme Micrb. Technol., 40 (2), 255-261 (2007).
5 Hartmann, M., Jung, D., “Biocatalysis with enzymes immobilized on mesoporous hosts: the status quo and future trends”, J. Mater. Chem.,20 (5), 844-857 (2010).
6 Shi, B.F., Wang, Y.Q., Ren, J.W., Liu, X.H., Zhang, Y., Guo, Y.L.,Guo, Y., Lu, G.Z., “Superparamagnetic aminopropyl-functionalized silica core-shell microsphere as magnetically separable carriers for immobilization of penicillin G acylase”, J. Mol. Catal. B Enzym., 63(1/2), 50-56 (2010).
7 Liu, X.Q., Guan, Y.P., Shen, R., Liu, H.Z., “Immobilization of lipase onto micron-size magnetic beads”, J. Chromatogr. B, 822 (1/2),91-97 (2005).
8 Ma, Z.Y., Guan, Y.P., Liu, X.Q., Liu, H.Z., “Preparation and characterization of non-porous superparamagnetic microspheres with epoxy groups by dispersion polymerization”, Chin. J. Chem. Eng., 13(2), 239-243 (2005).
9 Lee, J., Isobe, T., Senna, M., “Preparation of ultrafine Fe3O4particles by precipitation in the presence of PVA at high pH”, J. Colloid Surf. Sci., 177 (2), 490-494 (1996).
10 Shi, B.F., Wang, Y.S., Guo, Y.L., Wang, Y.Q., Wang, Y., Guo, Y.,Zhang, Z.G., Liu, X.H., Lu, G.Z., “Aminopropyl-functionalized silicas synthesized by W/O microemulsion for immobilization of penicillin G acylase”, Catal. Today, 148 (1-2), 184-188 (2009).
11 Grazú, V., Abian, O., Mateo, C., Batista-Viera, F., Fernández-Lafuente,R., Guisán, J.M., “Stabilization of enzymes by multipoint immobilization of thiolated proteins on new epoxyl-thiol support”, Biotechnol.Bioeng., 90 (5), 597-605 (2005).
12 Seddon, K.R., Stark, A., Torres, M.J., “Influence of chloride, water,and organic solvents on the physical prpperties of ionic liquids”,Pure Appl. Chem., 72 (12), 2275-2287 (2000).
13 Fujita, K., MacFarlane, D.R., Forsyth, M., Yoshizawa-Fujita, M.,Murata, K., Nakamura, N., Ohno, H., “Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of oxo acid residues and kosmotropicity”, Biomacromolecules, 8 (7), 2080-2086 (2007).
14 Kaar, J.L., Jesionowski, A.M., Berberich, J.A., Moulton, R., Russell,A.J., “Impact of ionic liquid physical properties on lipase activity and stability”, J. Am. Chem. Soc., 125 (14), 4125-4131 (2003).
15 Lourenço, N.M.T., Barreiros, S., Afonso, C.A.M., “Enzymatic resolution of Indinavir precursor in ionic liquids with reuse of biocatalyst and media by product sublimation”, Green Chem., 9 (7), 734-736(2007).
16 Jiang, Y.Y., Guo, C., Xia, H.S., Mahmood, I., Liu, C.Z., Liu, H.Z.,“Magnetic nanoparticles supported ionic liquids for lipase immobilization enzyme activity in catalyzing esterification”, J. Mol. Catal.B Enzym., 58 (1-4), 103-109 (2009).
17 Tartaj, P., Serna, C.J., “Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites”, J. Am. Chem. Soc., 125(51), 15754-15755 (2003).
18 Valkenberg, M.H., deCastro, C., Holderich, W.F., “Immobilisation of chloroaluminate ionic liquids on silica materials”, Top. Catal., 14(1-4), 139-144 (2001).
19 Valkenberg, M.H., deCastro, C., Hölderich, W.F., “Friedel-crafts acylation of aromatics catalysed by supported ionic liquids”, Appl.Catal., A General, 215 (1-2), 185-190 (2001).
20 Bradford, M.M., “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding”, Anal. Biochem., 72 (1-2), 248-254 (1976).
21 Shewale, J.G., Kumar, K.K., Ambekar, G.R., “Evaluation of determination of 6-aminopenicillanic acid by p-dimethylaminobenzaldedyde”,Biotechnol. Tech., 1 (1), 69-72 (1987).
22 Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Elst, L.V.,Muller, R.N., “Magnetic iron oxide nanoparticles synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications”, Chem. Rev., 108 (6), 2064-2110 (2008).
23 Bruce, I.J., Sen, T., “Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations”,Langmuir, 21 (15), 7029-7039 (2005).
24 Haddad, P.S., Duarte, E.L., Baptista, M.S., Goya, G.F., Leite, C.A.P.,Itri, R., “Synthesis and characterization of silica-coated magnetic nanoparticles”, Prog. Colloid Polym. Sci., 128, 232-238 (2004).
25 Guo, Z., Bai, S., Sun, Y., “Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres”, Enzyme Microb. Technol., 32 (7), 776-782 (2003).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Low-temperature Electrodeposition of Aluminium from Lewis Acidic 1-Allyl-3-methylimidazolium Chloroaluminate Ionic Liquids*
- In-situ Synthesis and Catalytic Properties of ZSM-5/Rectorite Composites as Propylene Boosting Additive in FluidCatalytic Cracking Process*
- Solvent Extraction of Yttrium by Task-specific Ionic Liquids Bearing Carboxylic Group*
- An Experimental Study of Liquid-Liquid Microflow Pattern Maps Accompanied with Mass Transfer*
- Liquid-Liquid-Liquid Three Phase Extraction Apparatus: Operation Strategy and Influences on Mass Transfer Efficiency*
- Micron-sized Magnetic Polymer Microspheres for Adsorption and Separation of Cr(VI) from Aqueous Solution*
