Copper Containing SBA-15 Prepared through pH Modification Method and Its Catalytic Activity for N2O Decomposition
2012-01-03ew
, , , ew 2,,
(1 Faculty of Mechanical Engineering, Universiti Teknikal Malaysia Melaka, Melaka 76100, Malaysia;2 Faculty of Industrial Science and Technology, Universiti Malaysia Pahang, Kuantan 26300,Malaysia;3 College of Chemistry and Materials ,South-Central University for Nationalities, Wuhan 430074, China;4 Faculty of Chemical and Natural Resources Engineering, Universiti Malaysia Pahang, Kuantan 26300, Malaysia)
Nitrous oxide is considered an environmental pollutant because it is a relatively strong greenhouse effect gas and contributes towards the destruction of ozone in the stratosphere. The continuous increase of its concentration, due to both natural and anthropogenic sources and its long atmospheric residence time (about 150 years), requires the development of efficient solutions for its abatement. N2O emissions from automobile engines are also significant, particularly under lean burn conditions and when aged catalytic converters are used[1]. It is also produced in the catalytic deNOxprocess and is infact the main by-product when Pt-based catalysts were used[2].
Currently, due to the increasing concern over environmental issues, studies on N2O have been oriented towards the development of catalytic systems for its elimination. Various types of catalysts have been reported to be active for the decomposition of nitrous oxide including oxides of the transition metal of group VIII[3]. Copper based supported catalysts that are active for direct decomposition of N2O have been reported by Dandekarand Vannice[4]. Examples of other catalysts are Cu used in monolithic system[5], on different types of zeolites[6],on various types of perovskites[7]and also in modified silica[8].Meanwhile,Cu-SBA-15 samples prepared through the impregnation method have been found by Chmielarz et al.[9]to be the most active catalysts for the N2O decomposition.
Mesoporous molecular sieve have large surface area and uniform mesoporous channels, and these are advantageous characteristics of a catalytic support. SBA-15 is a new type of mesoporous silica molecular sieves with uniform hexagonal channels ranging from 5 to 30 nm, thick walls (3.1-6.4 nm), and with higher hydrothermal stability than those of MCM-41[10].
In recent years, supporting transition metal on molecular sieves have attracted increasing attentions because the supported SBA-15 can be more active compared to siliceous SBA-15 due to the active sites created by metal incorporation. Several successful examples of transition metal incorporated molecular sieves catalysts includes Ti/SBA-15 for oxidation of styrene catalysts[11], Zr/SBA-15 support for Fischer-Tropsch synthesis catalysts[12], Ce/SBA-15 as support for oxidation of benzene[13], and B/SBA-15 as a catalysts in isopropylation of naphthalene[14].
The pure-silica mesoporous molecular sieves show low catalytic activity due to the absence of heteroatom active sites. The introduction of heteroatoms into the framework of mesoporous silica molecular sieves is important to increase its activity. The simple way to functionalise the silica of SBA-15 is to introduce active metal ions in the silica matrix either by pre-synthesis or by post-synthesis process of SBA-15. However, according to Mu et al[13]it is very difficult to incorporate metal ions into SBA-15 directly under the strong acidic conditions, as the metal ions will exist as cations and the formation of corresponding oxo species will be difficult and so is the formation of metal—O—Si bonds for effective addition in the structure. During the preparation of metal incorporated SBA-15 via post-synthetic methods, metal oxides are often formed in the channels and/or on the external surfaces.
Recently, Shah et al[15]synthesized Cu containing mesoporous silica (type SBA-16)by internal pH-modification method using hexamethylenetetramine (HMTA)as a chelating agent and pH-modifier. During the hydrothermal step, the pH value of the hydrolyzed silica gradually increased due to the dissociation of HMTA into NH3, and resulted in an interaction between oxo metal species and silica gel to form M—O—Si bond. Based on that, we tried to synthesize copper containing SBA-15 mesoporous materials directly under mild acidic conditions and using hexamethylenetetramine (HMTA)to adjust the internal pH. The synthesized materials were characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM)equipped with EDX for elemental composition analysis, Fourier-transform infrared spectroscopy (FT-IR)and tested for catalytic activity for the decomposition of N2O.
1 Experimental
1.1 Materials
All chemicals and reagents used were commercial samples and used without further purification. Tetraethyl orthosilicate (TEOS - Aldrich)and copper nitrate (Cu (NO3)2·3H2O - Merck)were used as silicon and copper sources respectively. Triblock poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide)(P123, EO20-PO70-EO20,MAV=5800, Aldrich)was used as template.
1.2 Synthesis of Cu/SBA-15
Cu/SBA-15 with different Si/Cu molar ratios were prepared similar to the procedure reported previously by Shah et al[15]with some alterations as follows: 2.0 g of P123 was dissolved in 100 mL of HCl solution (0.1 mol/L)until a clear solution is obtained. Then the acidic solution of Cu (NO3)2·3H2O (to obtain Si/Cu molar ratios of 10, 25, 50, 75 and 90)and HMTA (HMTA/Cu molar ratio 1∶1)were added into the above mixture. Then 9.2 mL of TEOS was added drop-wise into this mixture while stirring continuously. The final mixture was stirred for 24 h at 35 ℃ and transferred into a PP bottle and then undergoes hydrothermal process at 100 ℃ for 2 days. The resultant solid product was filtered, washed with distilled water, dried and calcined at 550 ℃ for 6 h (at heating rate of 2 ℃/min).
1.3 Characterisation
X-ray powder diffraction (XRD)pattern for the calcined samples were recorded with an X′Pert PRO XRD diffractometer using CuKα (λ=0.1541 nm). The lower and wider scan ranges were 0.5° - 5° and 10° - 80° respectively and accumulated with 0.016° steps. The crystallite phase was estimated using the data of Joint Committee on Powder Diffraction Standards (JCPDS).The structure and morphology of the calcined SBA-15 and Cu/SBA-15 materials were studied using Zeiss scanning electron microscope, Evo 50, equipped with EDX for elemental analysis. Transmission electron microscopy (TEM)characterization of the samples was carried out using an FEI Tecnai G2 instrument. The samples were dispersed in ethanol and dropped onto copper grids for observation.Infrared spectra were acquired using the KBr technique with 2%(wt)of the material. The equipment used was a PerkinElmer Spectrum One FT-IR.
1.4 N2O decomposition
The catalytic activity experiments for N2O decomposition were carried out in an alumina tube (4.76 mm i.d.)micro-reactor. A 500.0 mg sample of catalyst was set in the reactor and the reaction temperature was monitored by a thermocouple inserted inside the catalyst bed. The reaction unit was equipped with mass flow controllers and product analysis was performed with on-line gas chromatograph (Micro GC 3000 series)equipped with two columnsin series (molecular sieve 5A and OV-1 column)and TCD detector. The catalyst bed was heated to 400 ℃ in a 25 mL/min helium gas flow and held at that temperature for 1 hour. The reaction gas composed of 1% N2O in helium at a total flow rate of 100 mL/min.
2 Results and dishcussions
2.1 pH value
The pH value is an important factor for the synthesis of the metal-containing mesoporous materials. As the SBA-15 hexagonal phase is synthesized at strongly acidic condition (pH<1), it is difficult to synthesize copper-containing SBA-15 due to the high solubility of the copper precursors, which hinders its incorporation into the silica walls of SBA-15. In this experiment HMTA plays an important role for introducing copper into SBA-15 silica framework. During hydrothermal synthesis at 100 ℃, HMTA dissociates to release NH3and increases the internal pH value of the mixture. This was confirmed by monitoring the pH of the gel that increased slightly from the initial pH value (pHa), to hydrolysis (pHb)and gradually hydrothermal process (pHc- in Table 1), especially at higher concentration of HMTA.HMTA therefore acted as pH-modifier and helps to introduce more copper into SBA-15 framework without affecting its structural organization. HMTA also acts as a complexing agent similar to methyl amine, hydrogen peroxide, acetylacetone or oxalic acid (Shah et al)[15].
2.2 Characterisation
To detect the content of copper in SBA-15 mesoporous materials, an elemental analysis by SEM-EDX was carried out for the calcined samples, and the results are presented in Table 1. These results indicate that the Cu/Si ratio based on EDX analysis is much lower than that present in synthesis solution. However, the content of Cu in SBA-15 gradually increases with the increase of Cu/Si ratio in the synthesis solution. The pH value during hydrothermal process (pHc)may still be below the isoelectric point of silica (pH < 2)which is considered as strong acidic condition. It is very difficult to incorporate Cu ions into SBA-15 framework directly[13]. However, when pHc> 2 for Cu/SBA-15 (1∶10)sample, rapid incorporation of Cu may take place producing 1∶21 molar ratio of Cu/Si. Further research is needed to confirm this assertion.
Tab.1 Summaries of sample preparation, pH values, element analysis by EDX, d-spacing and unit-cell
In Fig.1 the small-angle XRD pattern of Cu/SBA-15 shows defined peaks between 0.5° and 5.0°, which confirm the 2Dhexagonal p6 mm structure of SBA-15 based on the well-known peak (100)and two weak peaks (110)and (200)according to Meynen et al[10]. The effects of Cu content on the d spacing and hexagonal unit cell constant (ao)of the samples are summarized in Table 1. The five diffraction peaks shift to lower angle with the existence of copper. The results also show that thedspacing andaovalue of SBA-15 generally increase with an increase in Cu content. According to Mu et al[13], the lengths of metal—O bonds are larger than that of Si—O bond, thus their incorporation into the framework of SBA-15 may increase the unit-cell parameterao. Also similar results were reported for the incorporation of other metal ions into the silicate framework by Eswaramoorthi and Dalai[14]and Dai et al[16]. Hence, the increase of lattice spacing in the presence of Cu, suggested that the copper ions have been incorporated into the framework of SBA-15.

2θ/(°) 2θ/(°)
The wide-angle XRD pattern of Cu/SBA-15 in Fig.1 shows the presence of an amorphous silica phase defined peaks between 10° and 80°, with a broad diffraction peak observed around 2θ= 24° (JCPDS 13-0026, 51-1592 and 56-0505). No clear characteristic peaks of crystalline Cu were observed in XRD patterns of samples, except for Cu/SBA-15 (1∶10)sample which exhibits peaks at 2θof 32.5, 35.5, 38.7, 48.7, 53.4, 58.3, 61.5 and 68.0°due to extra-framework CuO phase (JCPDS 65-2309 and 80-1268).This indicates that the copper oxide was also disperse on the surface of channel wall. However the small-angle XRD (in Fig.1)show the typical highly ordered hexagonal structure characteristic of SBA-15. Therefore, it can be inferred that the high Cu/Si molar ratio content enclosed with HMTA have some of Cu ion incorporated in SBA-15 and some dispersed in the matrices.
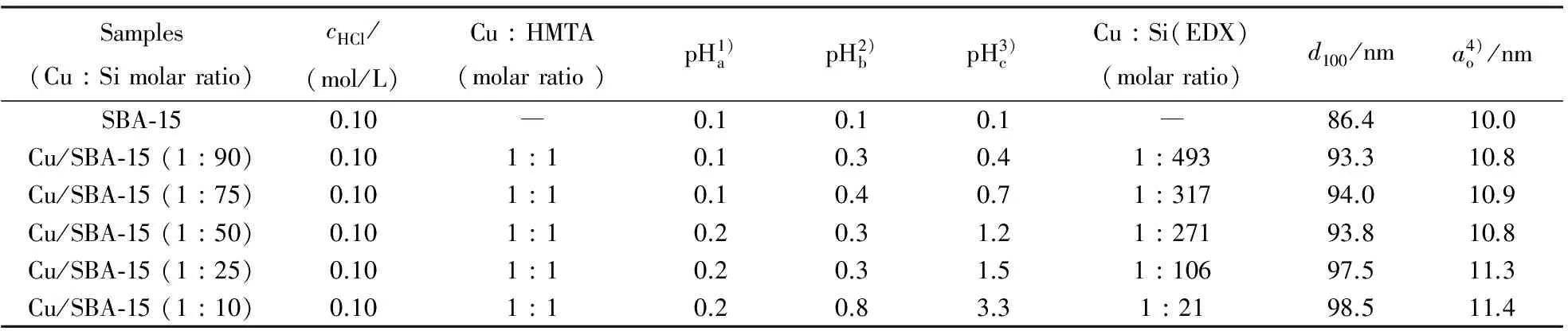
Fig.2 illustrates the SEM images and EDX pro-files for pure SBA-15 and different Cu-SBA-15 samples. The SEM image shows the curved morphology of the Cu/SBA-15 materials, rope-like aspect of as long as several hundred micrometers and width of less than 10 μm for all the samples except Cu/SBA-15 (1∶50)which is slightly smaller. It is also observed that many strip-like structures with relatively uniform sizes are aggregated into rope-like macrostructures. The micrograph of pure SBA-15 shows the presence of large pores while that of Cu/SBA-15 with 1∶10 ratio shows smooth surfaces suggesting that CuO form layers on the SBA-15 structure. To confirm the distribution of the particle size of copper on the SBA-15 support, TEM experiments were carried out. As shown in Fig.3a, the mesoporous structure was perfectly preserved, suggesting the well-preserved structure of SBA-15. Meanwhile Fig.3b and 3c for Cu containing SBA-15 (1∶50)and (1∶10)shows the dispersed copper particles was done on the framework of SBA-15. But the typical pore structure shows slightly less degree of mesoporous ordering based on some of surface was destroyed, perhaps caused by the increase pH value during the hydrothermal process and formation of CuO.

(a)SBA-15; (b)Cu/HMTA/SBA-15 (1∶1∶10); (c)Cu/HMTA/SBA-15 (1∶1∶25); (d)Cu/HMTA/SBA-15 (1∶1∶50);(e)Cu/HMTA/SBA-15 (1∶1∶75); (f)Cu/HMTA/SBA-15 (1∶1∶90)in 0.1 mol/L HCl
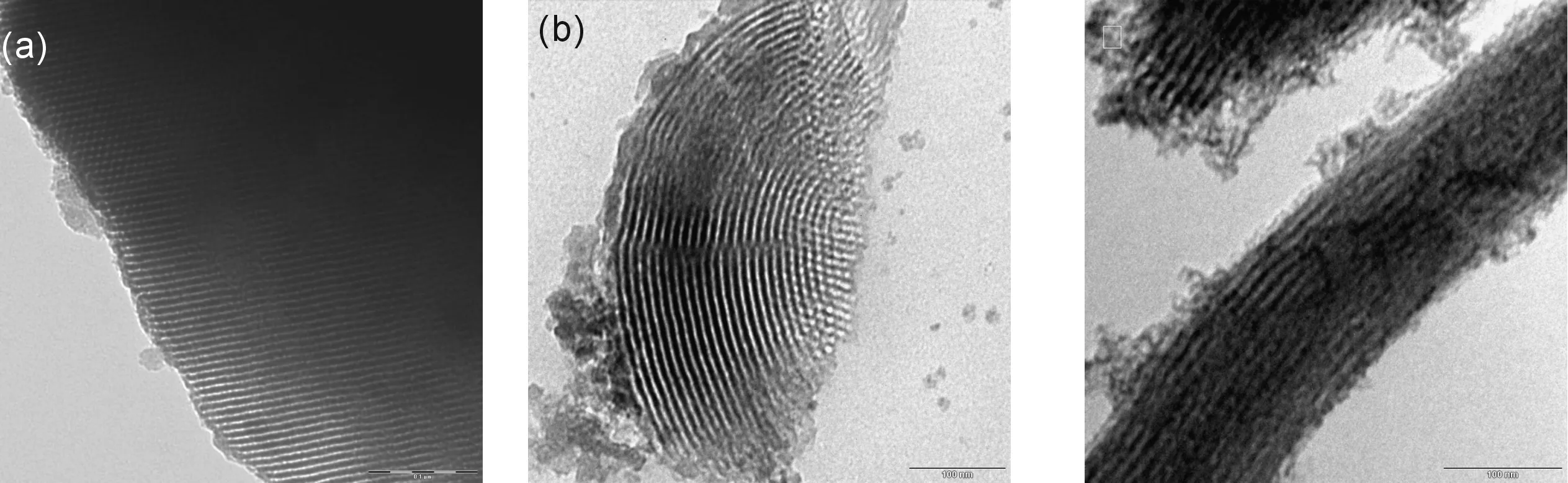
(a)SBA-15; (b)Cu/HMTA/SBA-15 (1∶1∶50); (c)Cu/HMTA/SBA-15 (1∶1∶10)
Meanwhile, Table 2 summarized the elemental composition of samples determined using EDX analysis. The analysis shows Cu/Si ratio increasing with increase of copper contents and also amount of HMTA used. However, Cu/Si molar ratio based in EDX analysis is much lower than the ratio use in the synthesis except for Cu/SBA-15 (1∶10). The EDX analysis of this sample shows relatively higher copper content suggesting that the SBA-15 has been fully occupied by CuO that it is now precipitating on the surface of the silicate.

Tab.2 EDX analyses of sample
The FT-IR spectra of pure SBA-15 and different Cu-SBA-15 samples are shown in Fig.4. In the framework region (700-1400 nm), the vibration band at 1082 cm-1(for pure SBA-15)is assigned tovas(Si-O-Si), while for Cu/SBA-15, this band shifts toward the lower wave number 1075 cm-1. Generally speaking, this shift toward the lower wave number is an indication of the incorporation of Cu ions into the framework of SBA-15[13]. For Cu/SBA-15, the band at ca. 960 cm-1can be assigned to avas(Si-O-Cu)vibration present in the framework of SBA-15[15]. However, a band at ca. 965 cm-1is also observed in the pure SBA-15. Reported by Mu and friends[13]this band can be assigned to the Si-O stretching vibrations of the Si-O-R+groups, as R+=H+groups are present in the calcined state. Therefore, this band cannot be taken as proof of Cu incorporation in the framework of SBA-15 because it can be interpreted in terms of the overlapping of both Si-OH groups and Si-O-Cu bonds vibrations. It is also observed that thevas(Si-O-Cu)and/orv(Si-OH)band intensity increases with respect to that ofvas(Si-O-Si)and/orv(Si-OH)band when Cu are incorporated. This may be taken as an evidence of the incorporation of the Cu metal into the framework of mesoporous materials. In all spectra, the band at ca. 800 cm-1is assigned to the symmetric Si-O-Si stretching vibration[16].

Wavenumber/cm-1
2.3 Catalytic activity
The activity profiles of the catalysts for catalytic decomposition of N2O are shown in Fig.5. The Cu-containing samples have been found to be significantly more active than pure SBA-15. The activity seems to increase with the increase in Cu incorporated into SBA-15. N2O decomposition on Cu/SBA-15 (1∶10)is significantly higher than that over SBA-15 at 200 ℃ and achieved 50% N2O conversion at 600℃. These data indicate that the presence of CuO on SBA-15 enhance N2O decomposition.

Temperature/℃
3 Conclusions
A direct synthesis pathway under acidic conditions by "pH modification method" produced ordered hexagonal mesoporous structures Cu/SBA-15. Incorporation of Cu in SBA-15 framework is successfully achieved by internal adjustment of pH through the use of HMTA. The presence of a CuO at high Cu/Si ratio does not disrupt the mesoporous order but enlarge the unit cell parameters. The decomposition of N2O on Cu/SBA-15 was investigated. Cu-containing SBA-15 samples have been found to be significantly more active towards N2O decomposition compared to that of pure SBA-15.
[1]Koike Odaka M N, Suzuki H. Infuence of catalyst deactivation on N2O emissions from automobiles[J]. Chemosphere - Global Change Science, 2000,2∶ 413-423.
[2]Burch R, Ramli A. A kinetic investigation of the reduction of NO by CH4on silica and alumina-supported Pt catalysts[J]. Applied Catalysis B: Environmental, 1998, 15: 63-73.
[3]Louis-Rose I, Me′thivier C, Ve′drine J C,et al. Reduction of N2O by NH3on polycrystalline copper and Cu(110): A combined XPS, FT-IRRAS and kinetics investigation[J]. Applied Catalysis B: Environmental, 2006,62: 1-11.
[4]Dandekar A, Vannice M A. Decomposition and reduction of N2O over copper catalysts[J]. Applied Catalysis B: Environmental, 1999,22: 179-200.
[5]Boissel V , Tahir S, Koh C. Catalytic decomposition of N2O over monolithic supported noble metal-transition metal oxides[J]. Applied Catalysis B: Environmental, 2006,64: 234-242.
[6]Smeets P, Sels B, Leeman Vanteeffelen, R, et al. The catalytic performance of Cu-containing zeolites in N2O decomposition and the influence of O2, NO and H2O on recombination of oxygen[J]. Journal of Catalysis, 2008, 256:183-191.
[7]Alini S, Basile F, Blasioli S, et al. Development of new catalysts for N2O-decomposition from adipic acid plan [J]. Applied Catalysis B: Environmental, 2007, 70: 323-329.
[8]Bennici S, Gervasini A. Catalytic activity of dispersed CuO phases towards nitrogen oxides (N2O, NO, and NO2)[J]. Applied Catalysis B: Environmental, 2006, 62: 336-344.
[9]Chmielarz L, Ku′strowski P, Kruszec M ,et al. Nitrous oxide reduction with ammonia and methane over mesoporous silica materials modified with transition metal oxides[J]. Journal of Porous Materials , 2005,12: 183-191.
[10]Meynen V, Cool P, Vansant E F. Verified syntheses of mesoporous materials[J]. Microporous and Mesoporous Materials, 2009, 125: 170-223.
[11]Chen Y, Huang Y,Xiu J, et al. Direct synthesis, characterization and catalytic activity of titanium-substituted SBA-15 mesoporous molecular sieves [J]. Applied Catalysis A: General, 2004, 173: 185-191.
[12]Tao C, Li J, Zhang Y, et al. Effect of isomorphic substitution of zirconium on mesoporous silica as support for cobalt Fischer-Tropsch synthesis catalysts [J]. Journal of Molecular Catalysis A: Chemical, 2010, 331: 50-57.
[13]Mu Z, Li J J, Tian H, et al. Synthesis of mesoporous Co/Ce-SBA-15 materials and their catalytic performance in the catalytic oxidation of benzene [J]. Materials Research Bulletin, 2008, 43: 2599-2606.
[14]Eswaramoorthi I, Dalai A K. Synthesis, characterisation and catalytic performance of boron substituted SBA-15 molecular sieves [J]. Microporous and Mesoporous Materials, 2006, 93:. 1-11.
[15]Shah A T, Li B, Abdalla Z E A. Direct synthesis of Cu-SBA-16 by internal pH-modification method and its performance for adsorption of dibenzothiophene [J]. Microporous and Mesoporous Materials, 2010, 130: 248-254.
[16]Dai Q, Wang X, Chen G, et al. Direct synthesis of cerium(III)-incorporated SBA-15 mesoporous molecular sieves by two-step synthesis method[J]. Microporous and Mesoporous Materials, 2007, 100:268-275.
