中药砂仁挥发油化学成分及其抗菌活性
2011-12-22张生潭王兆玉汪铁山李苗霞林敬明
张生潭,王兆玉,汪铁山,李苗霞,林敬明*
1南方医科大学珠江医院药剂科,广州510282;2广东药学院生命科学与生物制药学院,广州510006
Introduction
Fructus Amomi is the ripened fruits of several plants of genus Amomum(Zingiberaceae)including A.villosum Lour,A.villosum Lour.Var.xanthioides T.L.Wu et Senjen and A.Iongiligulare T.L.Wu.[1]It has been used as an ingredient in traditional chinese medicine for eliminating dampness,promoting appetite,warming spleen and anti diarrhea,etc.[2]In addition,it is also a key flavoring in both food and drink.These activities have been linked to the presence of essential oil.[3]
In this study,an essential oil was extracted from the seeds and husks of Fructus Amomi by using Bligh-Dyer method and Hydrodistillation method respectively.One hundred and thirty-eight compositions were analyzed and identified by GC-MS from the four samples.Thirtyfive compositions such as neophytadiene,globulol,germacrene D,fluorenone,bicyclogermacrene,piperitol,tricosane,tetracosane,etc were first identified in this plant.Compared to conventional hydrodistillation meth-od,21 kinds of new chemical compositions were identified.And the extraction efficiency of Bligh-Dyer method is much higher than that of hydrodistillation method.The results of pharmacological activity showed that the essential oil of Fructus Amomi had significant inhibitory effects for chosen bacteria and fungi.In this paper,a new application for Fructus Amomi is provided in antibiotics and food preservation,while related scientific theory and experimental evidence are also presented for utilizing essential oil of Fructus Amomi in the pharmaceutical industry and food industry of development and utilization.
Materials and Methods
General
GC-MS system(GC 6890 series II;MSD 5973,Hewlett Packard,America),Rotary Evaporator(R-200,BUCHI company,Switzerland),ultrasonic cleaning equipment(KQ-600DE,Kunshan Ultrasonic equipment company,China),electronic scales(BL-2000S,setra company,America),Incubator(DNP-9162,Ningbo Southeast Instrument Company,China),biological safety cabinet(BHC-1300IIA/B3,Suzhou Antai Air Tech Co,Ltd,China);Antimicrobial susceptibility test discs (5×50 discs,Oxoid Ltd,England),Medium(Oxoid Ltd,England).
Plant Materials
Our research was carried out on Fructus Amomi,including seeds and husks of drying and ripening fruits.The Fructus Amomi was purchased in Guangzhou herbal medicine company.The original purchasing place was Guangdong province,China (Batch number: 20090601).
Microbiological Materials
Trichophyton rubrum CMCC(F)T1a,Trichophyton mentagrophyton CMCC(F)T5a,and Microsporum gypseum CMCC(F)M2a were purchased in Skin Research Institute of Chinese Academy of Medical Sciences.Escherichia coli ATCC 35218,Staphyloccocus aureus Rosenbach ATCC 29213,Enterococcus faecalis ATCC 29212 and Enterobacter cloacae ATCC 700323 were provided by Medical Testing Center,Zhujiang hospital.
Preparation and analysis of the essential oil
Hydrodistillation method
Seeds and husks of Fructus Amomi were peeled and crushed separately,with 24 mesh screen.One hundred grams of plant materials were distilled with water(1 L)for 6 h in an extraction equipment in accordance with the description of the Pharmacopoeia of the People’s Republic of China(2005)[4].The essential oil was collected and dried over anhydrous Na2SO4,then stored in sealed vials at 4℃.
Bligh-Dyer method
The plant materials(100 g)and 500 mL of extracting solvent(the volume ratio of methanol∶chloroform∶water was 1∶1∶0.5)were placed in a Erlenmayer flask (1500 mL)and soaked for three days.And then the flask was treated three times with ultrasonic(40 kHz) at 40±1℃ for 45 min.The extracts were centrifuged and the supernatants were combined.After evaporation of the solvent,the essential oil was collected and stored at 4℃.
Gas chromatography-mass spectrometry analysis
The essential oil extracts were analyzed by gas-chromatographically.The GC operating conditions were as follows:column temperature was increased from 50 to 250℃ at a rate of 5℃/min;injector temperature was set to 250℃,and the detector temperature was set to 280℃;the carrier gas was He at a rate of 1.0 mL/min.The MS operating conditions were as follows:carrier gas was He at a rate of 1.0 mL/min;mass spectrometry ionization energy was 70 eV,and electron multiplier tube voltage was 1905 V;the ion source temperature was set to 230℃,and the quadrupole temperature was set to 150℃;the mass scan range was 30-550 amu.The ethyl acetate solution of the essential oil was dissolved with 1 μL EtOAc and injected in split mode.The compositions in essential oil were identified by GCMS by mass fragmentation pattern and spectral comparison with standards in the Wiley 275 and Nist 05 libraries.
Determination of antibacterial activity
In vitro antibacterial activities of the essential oil extracts were evaluated by disc diffusion method using Mueller-Hinton Agar for bacteria and Sabouraud Agar for fungiwith determination ofinhibition zones (IZ).[5]A 100 μL suspension of tested bacteria and fungi(108CFU/mL)spreaded on Agar mediums.10 μL of essential oil was dropped on 6 mm filter papers and place on the Agar surface.Standard antibiotic and black control disks were used as reference.Plates were incubated at 28℃ for 96 h for fungi and at 37℃ for 24 h for bacteria,respectively.The inhibiton zones were measured in diameters.The scale of measurement was the following:>15 mm zone was strongly inhibitory,10-15 mm zone moderately inhibitory,and <10 mm zone weakly inhibitory.
Determination of Minimum Inhibitory Concentration(MIC)
The MIC were measured by micro dilution RPMI-1640 susceptibility assay recommended by NCCLS (2000).[6]The 96 well micro plate was used,each well contained 10 μL of bacteria RPMI-1640 liquid medium(bacteria concentration of 0.5-5×105CFU/ mL)and 90 μL of test essential oil solution.10 μL sterile normal saline was black control.An appropriate amount of Tween-80 was added to make it into a 5% solution.This concentration of Tween-80 did not affect the growth of bacteria and fungi.The final concentrations of the essential oil in wells ranged from 0.00977 mg/mL to 10.000 mg/mL.The incubation conditions were 72 h at 28℃ for fungi and 24 h at 37℃ for bacteria,respectively,and each treatment was performed in duplicate thrice.The lowest concentration of the test samples,which did not show any visual growth of tested organisms after macroscopic evaluation,was determined as MIC,which was expressed in mg/mL.
Determination of Minimum Bactericidal Concentration(MBC)
The MBC of essential oil were determined according to the MIC values.Of each well showing complete absence of growth,20 μL was transferred to agar plates and incubated 72 h at 28℃ for fungi and 24 h at 37℃ for bacteria,respectively.The lowest concentration of essential oil which no viable bacteria or fungi were identified was the MBC.
Results and Conclusions
Chemical compositions of the essential oil extracts One hundred and thirty-eight compositions were identified from the four essential oil samples(Table 1).The yields of essential oil from seeds and husks by hydrodistillation method were 3.57%(g/g)and 0.97% (g/g),respectively,while those by Bligh-Dyer method were 7.01%(g/g)and 5.37%(g/g),respectively.The main compositions of essential oil were bornyl acetate,camphor,borneol,α-pinene,β-pinene,and other components.The relative contents of compositions identified were accounted for 93.39%,92.18%,74.85%,and 69.85%in four essential oils,respectively,and those of total alkenes and ketones compositions were accounted for 10%-40%(Table 2).The TIC of oil samples shown in Figure 1-4.
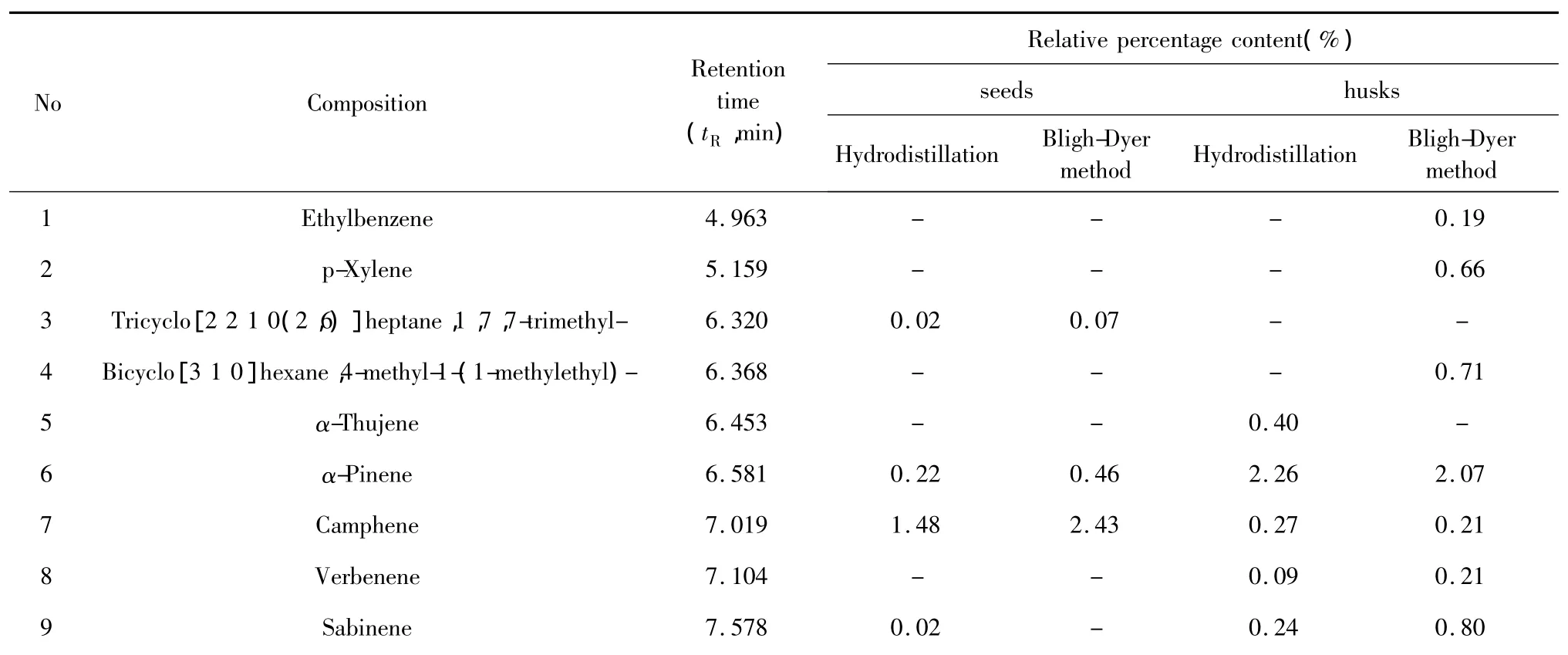
Table 1 Chemical constituents of the essential oil extracts of Fructus Amomi
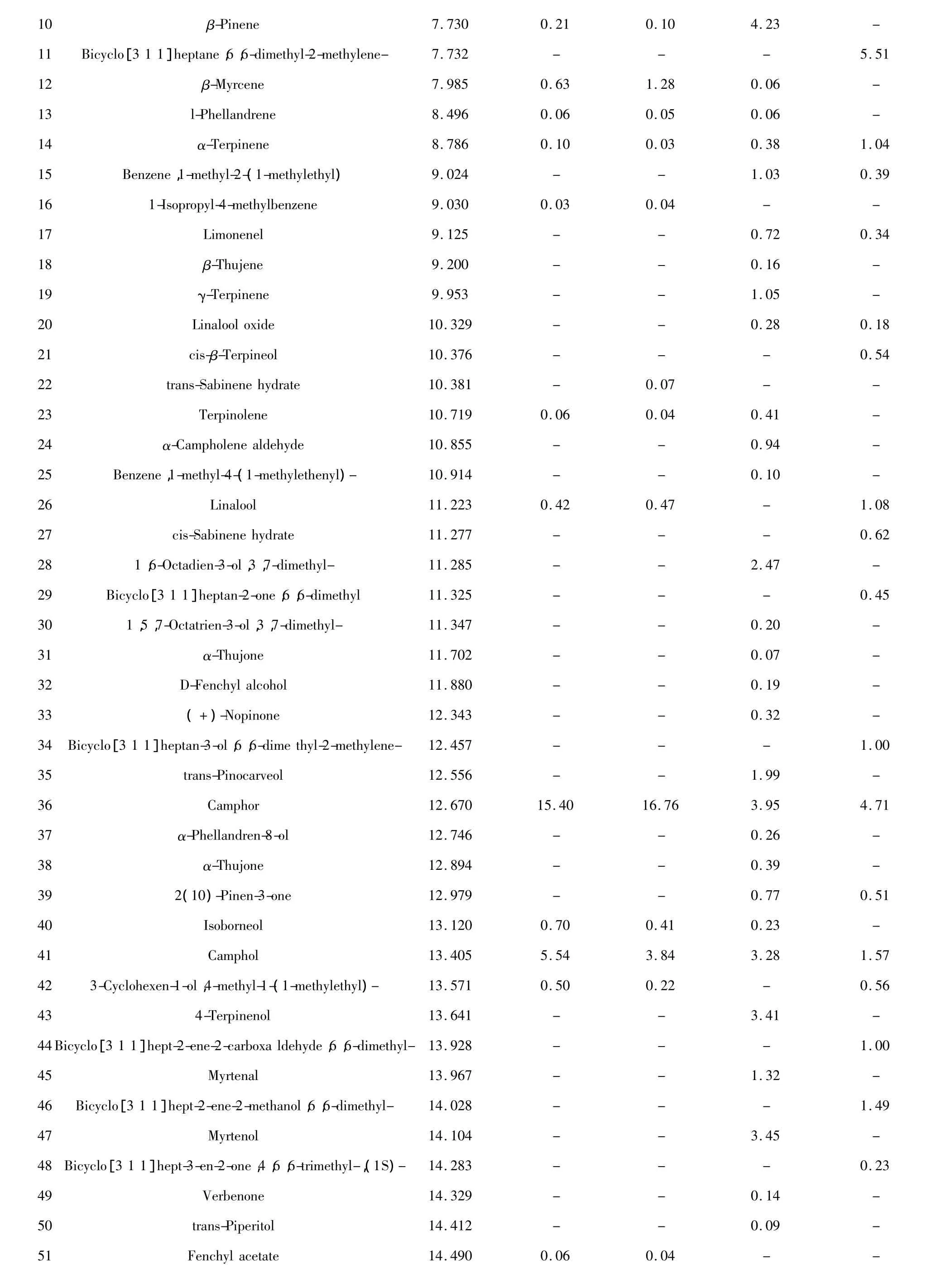
10 β-Pinene 7.730 0.21 0.10 4.23 -11 Bicyclo[3 1 1]heptane,6,6-dimethyl-2-methylene- 7.732 - - - 5.51 12 β-Myrcene 7.985 0.63 1.28 0.06 -13 l-Phellandrene 8.496 0.06 0.05 0.06 -14 α-Terpinene 8.786 0.10 0.03 0.38 1.04 15 Benzene,1-methyl-2-(1-methylethyl) 9.024 - - 1.03 0.39 16 1-Isopropyl-4-methylbenzene 9.030 0.03 0.04 - -17 Limonenel 9.125 - - 0.72 0.34 18 β-Thujene 9.200 - - 0.16 -19 γ-Terpinene 9.953 - - 1.05 -20 Linalool oxide 10.329 - - 0.28 0.18 21 cis-β-Terpineol 10.376 - - - 0.54 22 trans-Sabinene hydrate 10.381 - 0.07 - -23 Terpinolene 10.719 0.06 0.04 0.41 -24 α-Campholene aldehyde 10.855 - - 0.94 -25 Benzene,1-methyl-4-(1-methylethenyl)- 10.914 - - 0.10 -26 Linalool 11.223 0.42 0.47 - 1.08 27 cis-Sabinene hydrate 11.277 - - - 0.62 28 1,6-Octadien-3-ol,3,7-dimethyl- 11.285 - - 2.47 -29 Bicyclo[3 1 1]heptan-2-one,6,6-dimethyl 11.325 - - - 0.45 30 1,5,7-Octatrien-3-ol,3,7-dimethyl- 11.347 - - 0.20 -31 α-Thujone 11.702 - - 0.07 -32 D-Fenchyl alcohol 11.880 - - 0.19 -33 (+)-Nopinone 12.343 - - 0.32 -34 Bicyclo[3 1 1]heptan-3-ol,6,6-dime thyl-2-methylene- 12.457 - - - 1.00 35 trans-Pinocarveol 12.556 - - 1.99 -36 Camphor 12.670 15.40 16.76 3.95 4.71 37 α-Phellandren-8-ol 12.746 - - 0.26 -38 α-Thujone 12.894 - - 0.39 -39 2(10)-Pinen-3-one 12.979 - - 0.77 0.51 40 Isoborneol 13.120 0.70 0.41 0.23 -41 Camphol 13.405 5.54 3.84 3.28 1.57 42 3-Cyclohexen-1-ol,4-methyl-1-(1-methylethyl)- 13.571 0.50 0.22 - 0.56 43 4-Terpinenol 13.641 - - 3.41 -44Bicyclo[3 1 1]hept-2-ene-2-carboxa ldehyde,6,6-dimethyl- 13.928 - - - 1.00 45 Myrtenal 13.967 - - 1.32 -46 Bicyclo[3 1 1]hept-2-ene-2-methanol,6,6-dimethyl- 14.028 - - - 1.49 47 Myrtenol 14.104 - - 3.45 -48 Bicyclo[3 1 1]hept-3-en-2-one,4,6,6-trimethyl-,(1S)- 14.283 - - - 0.23 49 Verbenone 14.329 - - 0.14 -50 trans-Piperitol 14.412 - - 0.09 -51 Fenchyl acetate 14.490 0.06 0.04 - -
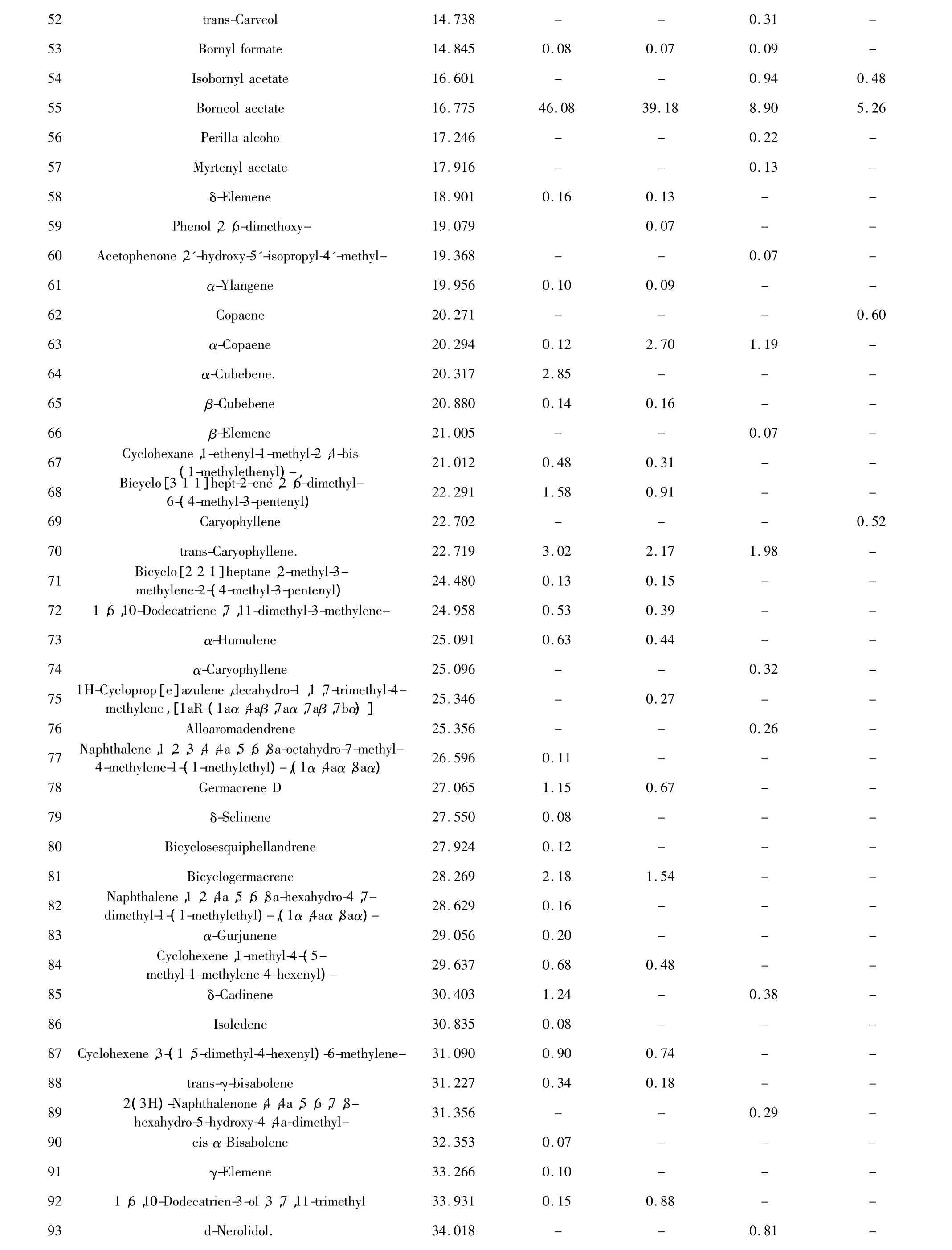
52 trans-Carveol 14.738 - - 0.31 -53 Bornyl formate 14.845 0.08 0.07 0.09 -54 Isobornyl acetate 16.601 - - 0.94 0.48 55 Borneol acetate 16.775 46.08 39.18 8.90 5.26 56 Perilla alcoho 17.246 - - 0.22 -57 Myrtenyl acetate 17.916 - - 0.13 -58 δ-Elemene 18.901 0.16 0.13 - -59 Phenol,2,6-dimethoxy- 19.079 0.07 - -60 Acetophenone,2'-hydroxy-5'-isopropyl-4'-methyl- 19.368 - - 0.07 -61 α-Ylangene 19.956 0.10 0.09 - -62 Copaene 20.271 - - - 0.60 63 α-Copaene 20.294 0.12 2.70 1.19 -64 α-Cubebene. 20.317 2.85 - - -65 β-Cubebene 20.880 0.14 0.16 - -66 β-Elemene 21.005 - - 0.07 -67 Cyclohexane,1-ethenyl-1-methyl-2,4-bis (1-methylethenyl)-, 21.012 0.48 0.31 - -68 Bicyclo[3 1 1]hept-2-ene,2,6-dimethyl-6-(4-methyl-3-pentenyl) 22.291 1.58 0.91 - -69 Caryophyllene 22.702 - - - 0.52 70 trans-Caryophyllene. 22.719 3.02 2.17 1.98 -71 Bicyclo[2 2 1]heptane,2-methyl-3-methylene-2-(4-methyl-3-pentenyl) 24.480 0.13 0.15 - -72 1,6,10-Dodecatriene,7,11-dimethyl-3-methylene- 24.958 0.53 0.39 - -73 α-Humulene 25.091 0.63 0.44 - -74 α-Caryophyllene 25.096 - - 0.32 -75 1H-Cycloprop[e]azulene,decahydro-1,1,7-trimethyl-4-methylene,[1aR-(1aα,4aβ,7aα,7aβ,7bα)] 25.346 - 0.27 - -76 Alloaromadendrene 25.356 - - 0.26 -77 Naphthalene,1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-,(1α,4aα,8aα) 26.596 0.11 - - -78 Germacrene D 27.065 1.15 0.67 - -79 δ-Selinene 27.550 0.08 - - -80 Bicyclosesquiphellandrene 27.924 0.12 - - -81 Bicyclogermacrene 28.269 2.18 1.54 - -82 Naphthalene,1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-,(1α,4aα,8aα)- 28.629 0.16 - - -83 α-Gurjunene 29.056 0.20 - - -84 Cyclohexene,1-methyl-4-(5-methyl-1-methylene-4-hexenyl)- 29.637 0.68 0.48 - -85 δ-Cadinene 30.403 1.24 - 0.38 -86 Isoledene 30.835 0.08 - - -87 Cyclohexene,3-(1,5-dimethyl-4-hexenyl)-6-methylene- 31.090 0.90 0.74 - -88 trans-γ-bisabolene 31.227 0.34 0.18 - -89 2(3H)-Naphthalenone,4,4a,5,6,7,8-hexahydro-5-hydroxy-4,4a-dimethyl- 31.356 - - 0.29 -90 cis-α-Bisabolene 32.353 0.07 - - -91 γ-Elemene 33.266 0.10 - - -92 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl 33.931 0.15 0.88 - -93 d-Nerolidol. 34.018 - - 0.81 -
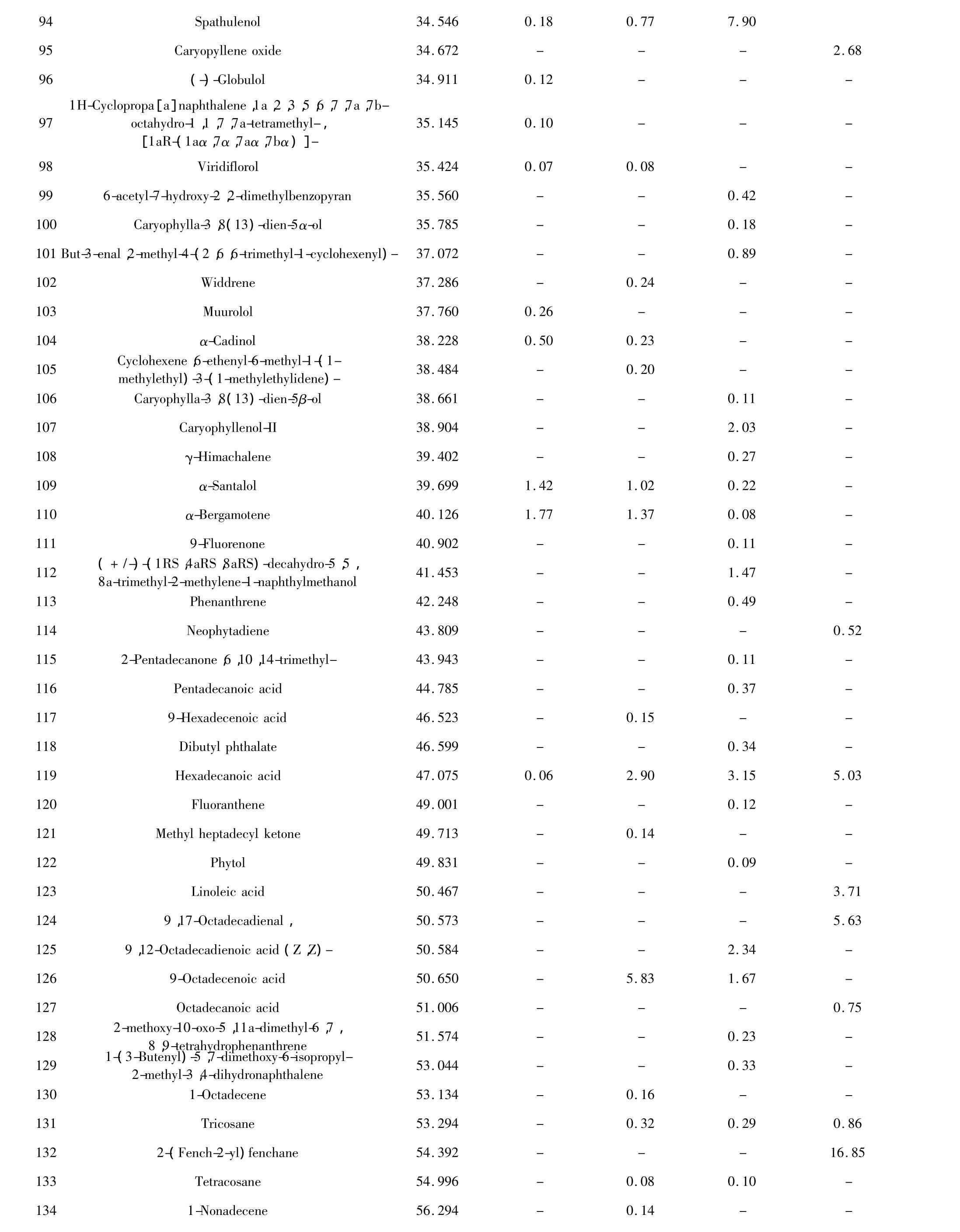
94 Spathulenol 34.546 0.18 0.77 7.90 95 Caryopyllene oxide 34.672 - - - 2.68 96 (-)-Globulol 34.911 0.12 - - -97 1H-Cyclopropa[a]naphthalene,1a,2,3,5,6,7,7a,7boctahydro-1,1,7,7a-tetramethyl-,[1aR-(1aα,7α,7aα,7bα)]-35.145 0.10 - - -98 Viridiflorol 35.424 0.07 0.08 - -99 6-acetyl-7-hydroxy-2,2-dimethylbenzopyran 35.560 - - 0.42 -100 Caryophylla-3,8(13)-dien-5α-ol 35.785 - - 0.18 -101 But-3-enal,2-methyl-4-(2,6,6-trimethyl-1-cyclohexenyl)- 37.072 - - 0.89 -102 Widdrene 37.286 - 0.24 - -103 Muurolol 37.760 0.26 - - -104 α-Cadinol 38.228 0.50 0.23 - -105 Cyclohexene,6-ethenyl-6-methyl-1-(1-methylethyl)-3-(1-methylethylidene)- 38.484 - 0.20 - -106 Caryophylla-3,8(13)-dien-5β-ol 38.661 - - 0.11 -107 Caryophyllenol-II 38.904 - - 2.03 -108 γ-Himachalene 39.402 - - 0.27 -109 α-Santalol 39.699 1.42 1.02 0.22 -110 α-Bergamotene 40.126 1.77 1.37 0.08 -111 9-Fluorenone 40.902 - - 0.11 -112 (+/-)-(1RS,4aRS,8aRS)-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthylmethanol 41.453 - - 1.47 -113 Phenanthrene 42.248 - - 0.49 -114 Neophytadiene 43.809 - - - 0.52 115 2-Pentadecanone,6,10,14-trimethyl- 43.943 - - 0.11 -116 Pentadecanoic acid 44.785 - - 0.37 -117 9-Hexadecenoic acid 46.523 - 0.15 - -118 Dibutyl phthalate 46.599 - - 0.34 -119 Hexadecanoic acid 47.075 0.06 2.90 3.15 5.03 120 Fluoranthene 49.001 - - 0.12 -121 Methyl heptadecyl ketone 49.713 - 0.14 - -122 Phytol 49.831 - - 0.09 -123 Linoleic acid 50.467 - - - 3.71 124 9,17-Octadecadienal, 50.573 - - - 5.63 125 9,12-Octadecadienoic acid(Z,Z)- 50.584 - - 2.34 -126 9-Octadecenoic acid 50.650 - 5.83 1.67 -127 Octadecanoic acid 51.006 - - - 0.75 128 2-methoxy-10-oxo-5,11a-dimethyl-6,7,8,9-tetrahydrophenanthrene 51.574 - - 0.23 -129 1-(3-Butenyl)-5,7-dimethoxy-6-isopropyl-2-methyl-3,4-dihydronaphthalene 53.044 - - 0.33 -130 1-Octadecene 53.134 - 0.16 - -131 Tricosane 53.294 - 0.32 0.29 0.86 132 2-(Fench-2-yl)fenchane 54.392 - - - 16.85 133 Tetracosane 54.996 - 0.08 0.10 -134 1-Nonadecene 56.294 - 0.14 - -

-No detected
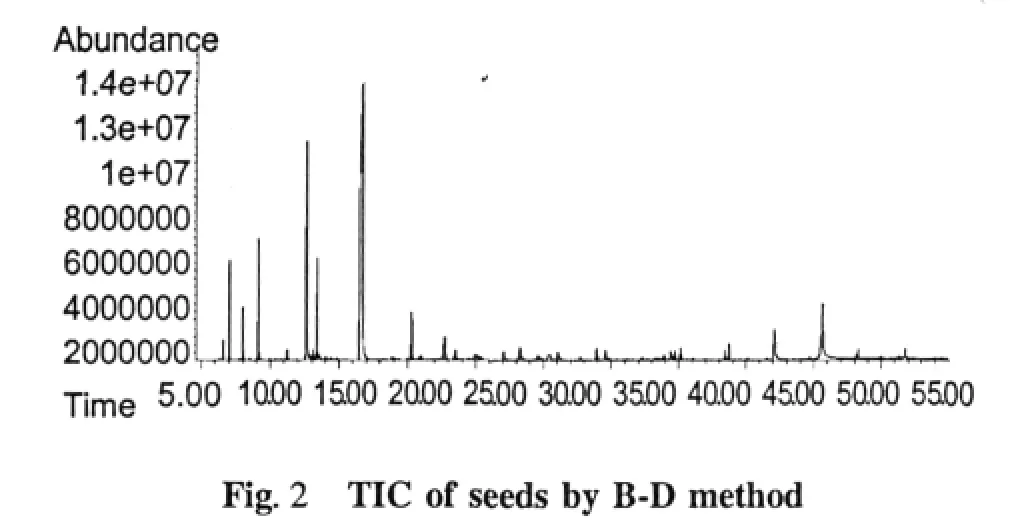
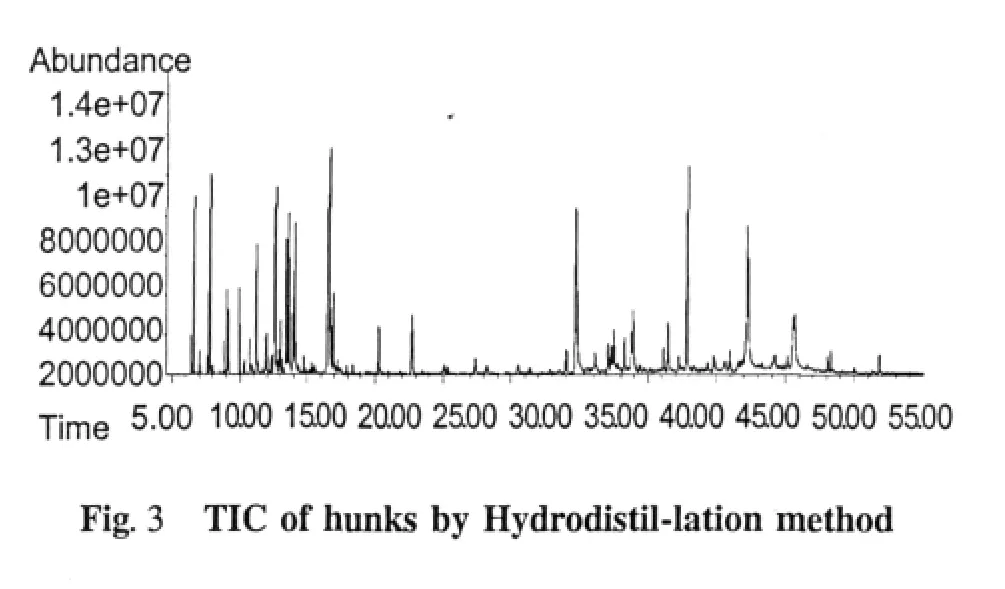
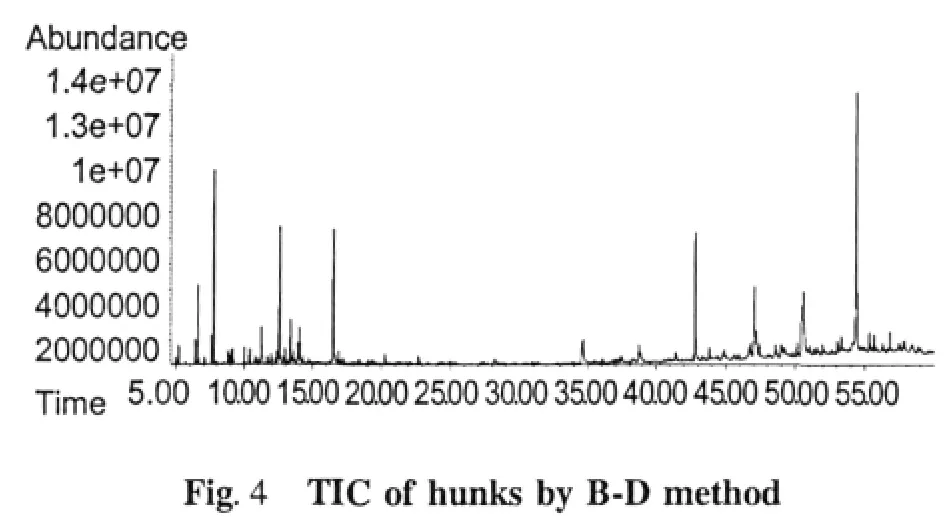
Table 2 Compositions in the different parts of Fructus Amomi by different extraction methods
Results of antibacterial activities of essential oil extracts
The antibacterial activity was assessed by evaluating the presence of IZ,MIC and MBC values.Results of IZ (Table 3)showed that the essential oil of Fructus Amomi had different antibacterial activities against of the three fungus and two bacterias tested.The inhibition zone size of different extracts for fungi and bacteria shown in Fig.5,the inhibitory effects shown in Fig.6.
Table 3 The bacterial inhibition zone of different extracts(±s,mm,n=3)
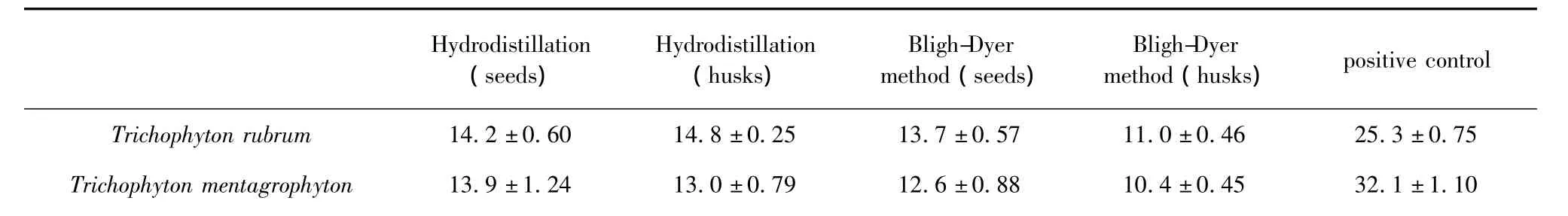
Table 3 The bacterial inhibition zone of different extracts(±s,mm,n=3)
Hydrodistillation (seeds) Hydrodistillation (husks) Bligh-Dyer method(seeds) Bligh-Dyer method(husks)positive control 75 Trichophyton mentagrophyton 13.9±1.24 13.0±0.79 12.6±0.88 10.4±0.45 32.1±1.10 Trichophyton rubrum 14.2±0.60 14.8±0.25 13.7±0.57 11.0±0.46 25.3±0.

-Indicate no antibacterial activity
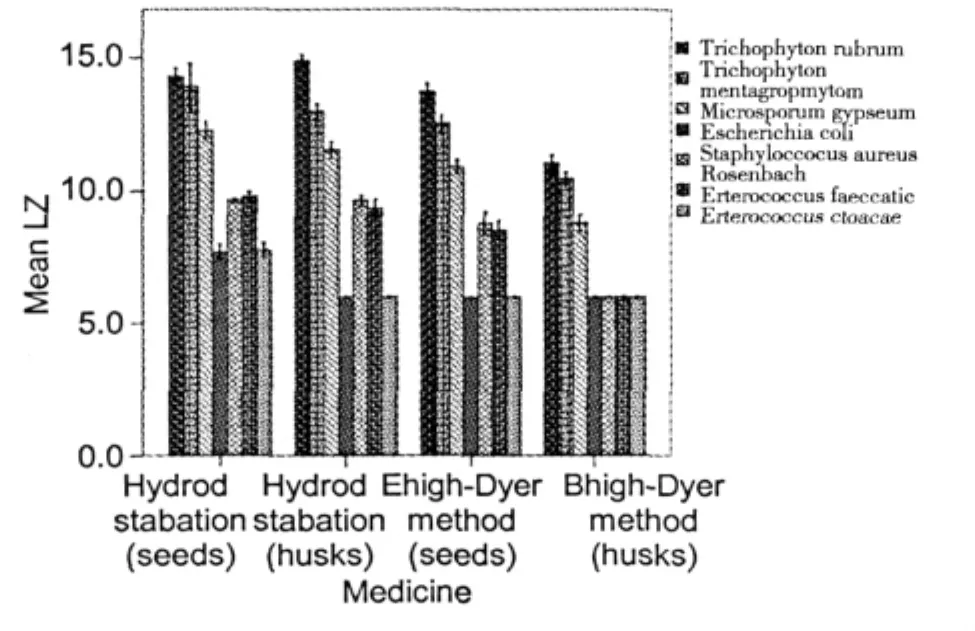
Fig.5 The inhibition zones of different extracts for fungi and bacteria
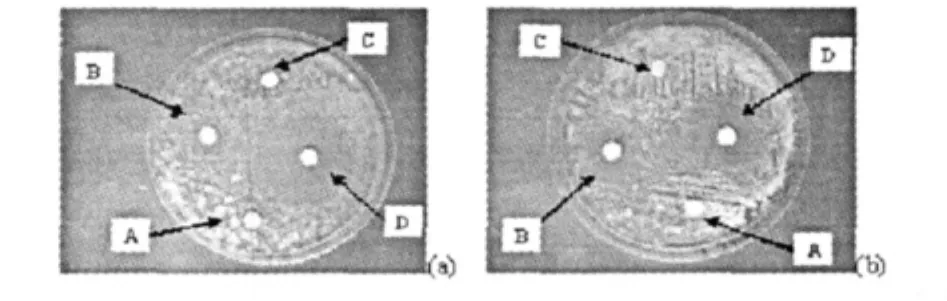
Fig.6 Effect of essential oil against Microsporum gypseum(a)and Trichophyton rubrum(b)
The MIC and MBC values for fungi and bacteria(table 4),which were sensitive to the essential oil of Fructus Amomi were in the range of 0.033 mg/mL→10.000 mg/mL and 0.210 mg/mL→10.000 mg/mL,respectively.
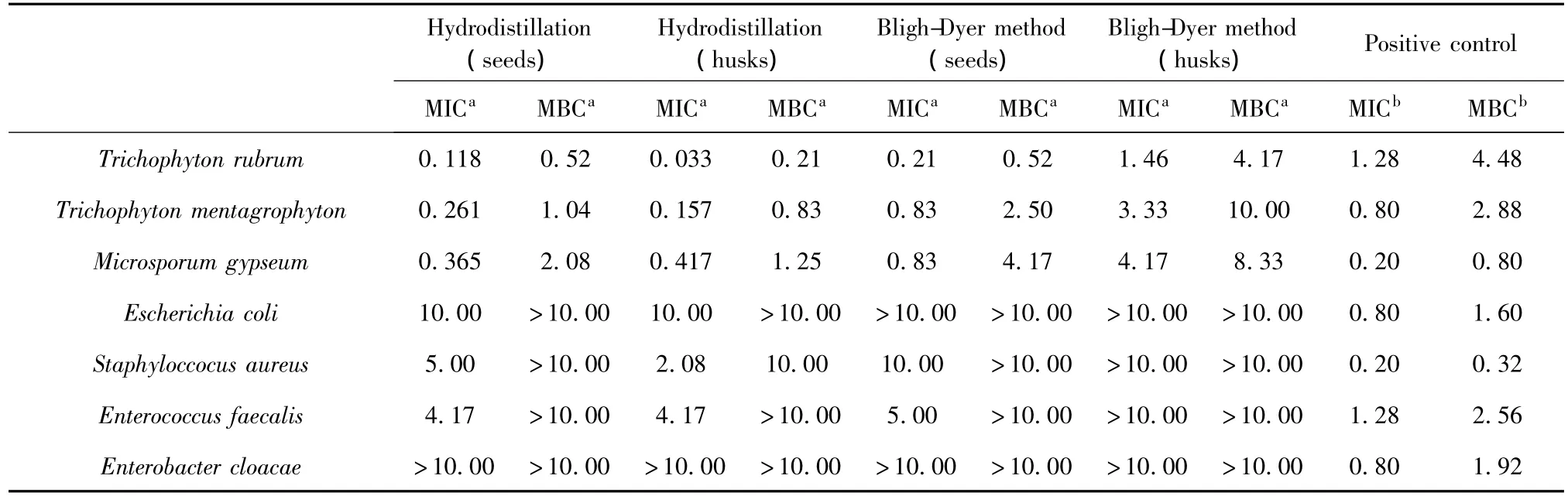
Table 4 The MIC and MBC of different extracts(n=3)
Conclusion
The results showed that the essential oil of Fructus Amomi had different inhibition for different fungi and bacteria.The inhibition for fungi was better than that for bacteria,and the inhibition for gram-positive bacteria was better than that for gram-negative bacteria.The antibacterial activity of essential oil from seeds was better than that from husks.And the antibacterial activity obtained by hydrodistillation method was stronger than that by Bligh-Dyer method,but the yield of essential oil was lower than that by Bligh-Dyer method.
Discussion
In recent years,several researchers had reported that the oxygenated mono-or sesquiterpenes and mono-or sesquiterpene hydrocarbons are the major components of the essential oil extracted from several plants which exhibit potential antibacterial activities.[7]
In literatures,it showed the essential oil of this plant had monoterpenes such as α-pinene,β-pinene,caryophyllene,cadinene,bergamotene,myrcene,α-cubebene,and borneol as the major components with strong antibacterial and antimicrobial activities.[8,9]These chemical components exert their antimicrobial activity on microorganisms through the disruption of bacteria membrane integrity.[10]Some essential oils exerts a greater inhibitory activity against Gram-positive bacteria.As it is reported that Gram-negative bacteria are more resistant to the plant-based essential oil[11],and these reports are in agreement with our findings.In addition,it was also possible that the minor components such as limonene,linalool oxide,verbenol,α-terpineol,myrtenol,farnesol,eugenol,caryphyllene oxide,etc,might be involved in some type of antibacterial synergism with other active components of essential oil.[12]While,as the differences of kinds and content of components in essential oil samples,there are some differences for the essential oil extracts in inhibitory activity.Bornyl acetate and camphor are main components in the essential oil,and their total proportion in seeds is up to 70%,whether bornyl acetate and camphor play a role in the antimicrobial process remains to be further studied.The results of table 2 and activity test showed that the antibacterial activity of essential oil had closely relation to the kinds and relative content of the total of vinyl compositions,which contains alkenes,alcohols and aldehydes.The more kinds and content of the essential oil contained,the stronger it performed for antibacterial activity.
Bligh-Dyer method[13]is used to extract the Lipids,A-mino acids and other components,and ultrasonic or microwave method could make higher extraction efficiency.At the same time,the component damage and loss are not easy,while as the organic solvent extraction,there may have a certain extract organic solvents residual.So in the actual process of production and application,we could select appropriate essential oil extracts and extraction methods according to the specific requirements.
The content of the essential oil is determined to be 4%-7%in Fructus Amomi,which indicated that the essential oil is an abundant natural resource for the pharmaceutical industry and food production processing industry.
1 The Pharmacopoeia Commission of PRC.Pharmacopoeia of the People’s Republic of China.Beijing:Chemical Industry Press,2005,177-177.
2 Hu YL,Zhang ZY,Lin JM.The research of the chemical composition and pharmacological on Fructus Amomi.J Chin Med Mat,2005,28(1),72-74.
3 Mei QX,Bi HX.Modern Pharmacology Manual.Beijing:China.Traditional Chinese Medicine Press,1999:207.
4 The Pharmacopoeia Commission of PRC.Pharmacopoeia of the People’s Republic of China.Beijing:Chemical Industry Press,2005,Appendix X 56.
5 Murray PR,Baron EJ,Pfaller MA,et al.Manual of clinical microbiology(6th ed.).Washington:ASM.1995.
6 National Committee for Clinical Laboratory Standard(NCCLS).Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically.Approved Standard,M7-A5,New Jersey,USA.2000.
7 Cakir A,Kordali S,Zengin H,et al.Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum.J Fla Frag,2004,19,62-68.
8 Deba F,Xuan TD,Yasuda M,et al.Chemical composition and antioxidant,antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn.Var.Radiata.Food Control,2008,19,346-352.
9 Qiu B,Lv Q,Bao FK,et al.GC-MS analysis and antimicrobial activity of essential oils from the fresh and dried roots of Codonopsis cordifolioidea.Nat Prod Res Dev,2010,22:445-449,465.
10 Knobloch K,Pauli P,Iberl B,et al.Antibacterial and antifungal properties of essential oil components.J Essent Oil Res,1989,1,603-608.
11 Reynolds JEF.Martindale the extra Harmacopoeia,31st ed.Royal Pharmaceutical Society of Great Britain,London.1996.
12 Marino M,Bersani C,Comi G.Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceace and Compositae.Int J Food Microbiol.2001,67,187-195.
13 Bligh ED,Dyer WJ.A rapid method for totall lipid extraction and purification.Can J Biochem physiol,1959,37,911-917.
