Syntheses,Crystal Structures and Electrochemical Properties of Cobalt and Nickel Complexes Containing Imidazole Ligand
2011-11-09WANGQingHuaYULiLiLIUQi2CHENGMeiLing
WANG Qing-HuaYU Li-LiLIU Qi*,,2CHENG Mei-Ling
(1School of Chemistry and Chemical Engineering and Jiangsu Province Key Laboratory of Fine Petro-chemical Technology,Changzhou University,Changzhou,Jiangsu 213164,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
Syntheses,Crystal Structures and Electrochemical Properties of Cobalt and Nickel Complexes Containing Imidazole Ligand
WANG Qing-Hua1YU Li-Li1LIU Qi*,1,2CHENG Mei-Ling1
(1School of Chemistry and Chemical Engineering and Jiangsu Province Key Laboratory of Fine Petro-chemical Technology,Changzhou University,Changzhou,Jiangsu213164,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing210093,China)
Two novel complexes[M(ImH)6](tfbdc)(M=Co,Ni;ImH=imidazole,tfbdc=2,3,5,6-tetrafluoroterephthalate) were synthesized and characterized by elemental analysis,IR spectra,thermogravimetric analysis,cyclic voltammetry,and X-ray single crystal structure analysis.Both[Co(ImH)6](tfbdc)(1)and[Ni(ImH)6](tfbdc)(2)all crystallize in the monoclinic system,space group P21/c,and with Z=2.Metal ions have all octahedral geometry coordinated by six N atoms from six imidazole molecules.The independent components,[M(ImH)6]2+cations and tfbdc anions are connected into a three-dimensional supramolecular network by N-H…O and C-H…F hydrogen bonds.CCDC:739086,1;739088,2.
cobalt complexes;nickel complex;imidazole;2,3,5,6-tetrafluoroterephthalate;crystal structure;electrochemical property
0 Introduction
Recently,much attention has been paid to the design and construction of metal-organic frameworks because of their interesting structural motifs[1],and potential applications in gas storage,catalysis, separations,fluorescent and magnetic molecular-based materials[2-6].
Imidazole (ImH)is of considerable interest as a ligand and its presence in many biological systems provides a potential binding site for metalions[7]. Imidazole itself is usually a unidentate ligand to formcomplexes with metal ions through its nitrogen atom.It has been reported that a large number of imidazole derivatives possess diverse pharmacological effects, including antiinflammatory,antimicrobial,antimalarial and antitumor activities[8].
On the other hand,aromatic multicarboxylic acid, such as 1,4-benzenedicarboxylic acid,1,3-benzenedicarboxylic acid,1,2,4,5-benzenetetracarboxylic acid and 2,3,5,6-tetrafluoroterephthalatic acid(H2tfbdc),are good ligands in the design of metal-organic materials due to its diverse coordination mode.Although H2tfbdc as well as carboxylate could form various coordination complexes with interesting structures[9-12],the coordination compounds containing H2tfbdc and ImH have not been reported so far.With the aim of understanding the coordination chemistry of H2tfbdc and ImH,we recently began to study on the assembly reactions of H2tfbdc and ImH with metal ions via general solution synthetic methods.Herein,we report the syntheses,crystal structures and electrochemical properties of two threedimensional supramolecularcompounds[M(ImH)6] (tfbdc)(M=Co,Ni).
1 Experimental section
1.1 Material
2,3 ,5 ,6 -tetrafluoroterephthalatic acid (H2tfbdc), M(Ⅱ)acetate tetrahydrate(M=Co,Ni),imidazole(ImH) and solvents were of reagent grade without further purification before use.
1.2 Physical measurements
The elemental analysis(C,H,N)was performed on a Perkin-Elmer 2400 SeriesⅡelement analyzer.FTIR spectra were recorded on a Nicolet 460 spectrophotometer in the form of KBr pellets.Single-crystal X-ray diffraction measurement of the title complex was carried out with a Bruker Smart Apex CCD diffractometer at 296(2)K.Thermogravimetric analysis(TGA) experiments were carried out on a Dupont thermal analyzer from room temperature to 800℃ under N2atmosphere at a heating rate of 10℃·min-1.The cyclic voltammogram experiments were carried out on a microcomputer-based electrochemical analyzer (Tianjin Lanlike Chemical and Electron High Technology Co. Ltd) in highly pure nitrogen atmosphere.A Pt-piece was employed as working electrode,a saturated calomel electrode (SCE)as reference electrode and a platinum wire as auxiliary electrode.The supporting electrolyte was 0.1 mol·L-1NaCl.The half wave potentials E1/2were obtained from (Epa+Epc)/2.
1.3 Preparation of[Co(ImH)6](tfbdc)(1)
Co(OAc)2·4H2O(0.024 9 g,0.1 mmol),H2tfbdc (0.0476 g,0.2 mmol)and ImH(0.0681 g,1 mmol)were added into a mixed solvent of 4 mL anhydrous methanol and 2 mL water and stirred for several minutes to afford an orange solution.The resulting solution was allowed to stand at ambient temperature for several days, yielding orange crystals.[0.0485 g,69%yield,(based on Co)].Anal.Calcd.for C26H24CoF4N12O4(%):C 44.39 (44.50),H 3.44(3.31),N 23.90(23.95).I.R.data(cm-1, KBr pellet):3203(s),3172(s),3071(m),2953(m),2865 (m),1 627(s),1 527(m),1 465(m),1 371(s),1 329(m), 1 257(m),1 248(m),1 169(m),1 130(w),1 093(m),1 069 (s),985(s),943(m),880(m),782(m),734(s),661(s),617 (s),475(m),408(w).
1.4 Preparation of[Ni(ImH)6](tfbdc)(2)
The same synthetic procedure as that for 1 was used except Co(OAc)2·4H2O was replaced by Ni(OAc)2·4H2O(0.0249 g,0.1 mmol).The blue single crystals of 2 suitable for X-ray analysis were obtained [0.0499 g, 71%yield,(based on Ni)].Anal.Calcd.For C26H24Ni F4N12O4(%):C 44.40(44.47),H 3.44(3.36),N 23.91 (23.93).I.R.data(cm-1,KBr pellet):3 138(s),2 947(m), 2 861(m),2 622(w),2 367(w),1 625(s),1 538(m),1 468 (s),1 360(s),1 326(m),1 255(m),1 169(m),1 145(w), 1 097(m),1 068(s),984(s),940(m),830(m),757(s),729 (s),664(s),616(s),468(m).
1.5 X-ray crystallography
Single-crystal X-ray diffraction measurement of 1 and 2 were carried out with a Bruker Smart Apex CCD diffractometer at 296(2)K.Intensities of reflections were measured using graphite-monochromatized Mo Kα radiation(λ=0.071073 nm)with the φ-ω scans mode in the range of 2.02°≤θ≤27.55°(for 1)and 2.02°≤θ≤27.63°(for 2).The structures were solved by direct methods using SHELXS97[13]computer program and refined by full-matrix least-squares methods on F2with the SHELXL-97 program package.Anisotropic thermal factors were assigned to all the non-hydrogen atoms.Hydrogen atoms were included in calculated position and refined with isotropic thermal parameters riding on the parent atoms.Crystallographic data parameters for structural analyses are summarized in Table 1,the selected bond lengths (nm)and bond angles(°)are given in Table 2,and the hydrogen bond distances and angles in the complex are presented in Table 3, respectively.
CCDC:739086,1;739088,2.
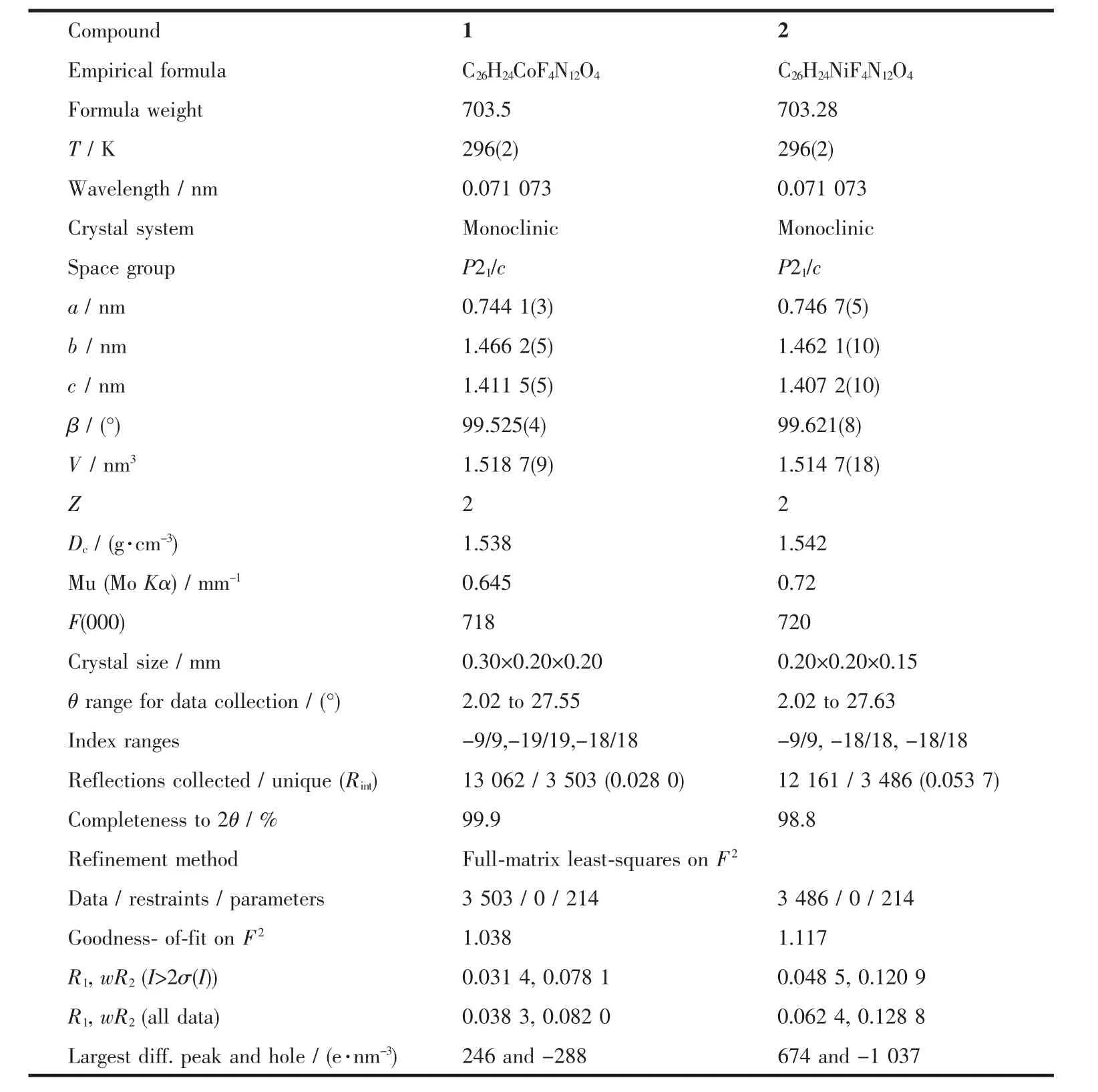
Table 1 Crystallographic data for complexes 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2

Continued Table 2
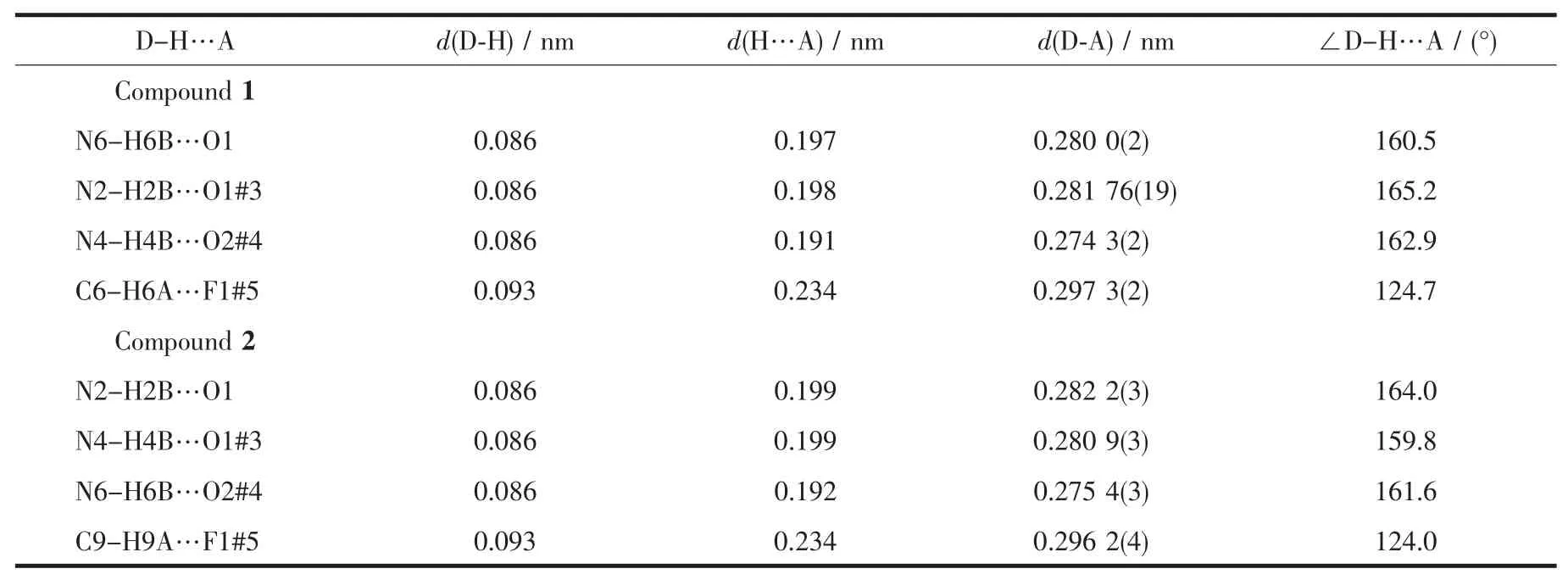
Table 3 Hydrogen bond distances and angles for complexes 1 and 2
2 Results and discussion
2.1 Infrared spectrum
The complexes 1 and 2 all have quite similar infrared spectra and reflects the binding patterns of ImH,and 2,3,5,6-tetrafluoroterephthalate moieties.Carboxylic group of the organic ligand in the compound is deprotonated,in agreement with the IR spectrum results,where no absorption peak around 1 730~1 700 cm-1for a protonated carboxylic group is observed.The strong absorption band in the 3600~3000 cm-1regions corresponds to ν(C-H)of the coordination ImH molecules[7].For[Co(ImH)6](tfbdc)(1),the strong peaks at 1627,1465 and 1371 cm-1are the νas(OCO),and νs(OCO)stretching mode of the coordinated tfbdc,while strong absorption at ca.985 cm-1is the δ(OCO)bent vibration of tfbdc[9].The peaks at 1527 cm-1is characteristic νas(C=N)of the coordinated ImH.For [Ni(ImH)6](tfbdc)(2),the strong peaks at 1 625,1 468 and 1360 cm-1are the νas(OCO),and νs(OCO)stretching mode of the coordinated tfbdc,while strong absorption at ca.984 cm-1is the δ(OCO)bent vibration of tfbdc.The peaks at 1538 cm-1is characteristic νas(C=N)of the coordinated ImH.
2.2 Description of crystal structures
Fig.1 shows a perspective view of the title compoundswith atomicnumberingscheme.The asymmetric unit of[M(ImH)6](tfbdc)(M=Co,Ni)consists of one-half of a monomeric[M(ImH)6]2+(M=Co,Ni) cation and a tfbdc anion,linked by electrostatic forces and hydrogen bonds,The other halves are generated by crystallographic inversion centres,while the metal ion lieson a crystallographic inversion centre.The coordination mode of the MⅡatom can be described as an MN6chromophore,with octahedral geometry.Six imidazole molecules are coordinated through their tertiary N atoms to each MⅡion and one tfbdc group is outside the coordination sphere,balancing the charge.

Fig.1 Molecular structure of the title compounds,dashed lines indicate intramolecular hydrogen bonds
For[Co(ImH)6](tfbdc),the Co1-N5,Co1-N1 and Co1-N3 bond distances are 0.217 85(14),0.220 88(14) and 0.214 55(13)nm,respectively(Table 2).All these bond distances are longer than the Co-N distances observed in catena-(tris(2,2′-Bi-imidazole-N,N′)-cobalt(Ⅱ)(μ2-2,2′-bi-imidazolato-N,N′,N″)-(μ2-benzene-1,3,5-tricarboxylato-O,O′)-cobalt(Ⅱ) dihydrate)[14].Allthe imidazole rings are planar.In the tfbdc moiety,the -COO-group is slightly twisted away from the aromatic ring,with the O1-C13-C10-C11 and O2-C13-C10-C12 torsion angles of 41.3(2)°and 39.4(2)°.For[Ni(ImH)6] (tfbdc),the Ni1-N5,Ni1-N1 and Ni1-N3 bond distances are 0.211 4(2),0.217 0(2)and 0.214 4(2)nm,respectively(Table 2).All these bond distances are longer than the Ni-N distances observed in Tris(2,2′-biimidazole) nickel(Ⅱ)phthalate correspondingly[15].All the imidazole rings are planar.In the tfbdc moiety,the-COO-group is slightly twisted away from the aromatic ring,with the O1-C13-C10-C11 and O2-C13-C10-C12 torsion angles of 41.0(4)°and 39.4(4)°.In each crystal structure,the independent components,[M(ImH)6]2+cations and tfbdc anions are connected by two kinds of hydrogen bonds (N-H…O and C-H…F)to form a three-dimensional supramolecular network,as shown in Fig.2.
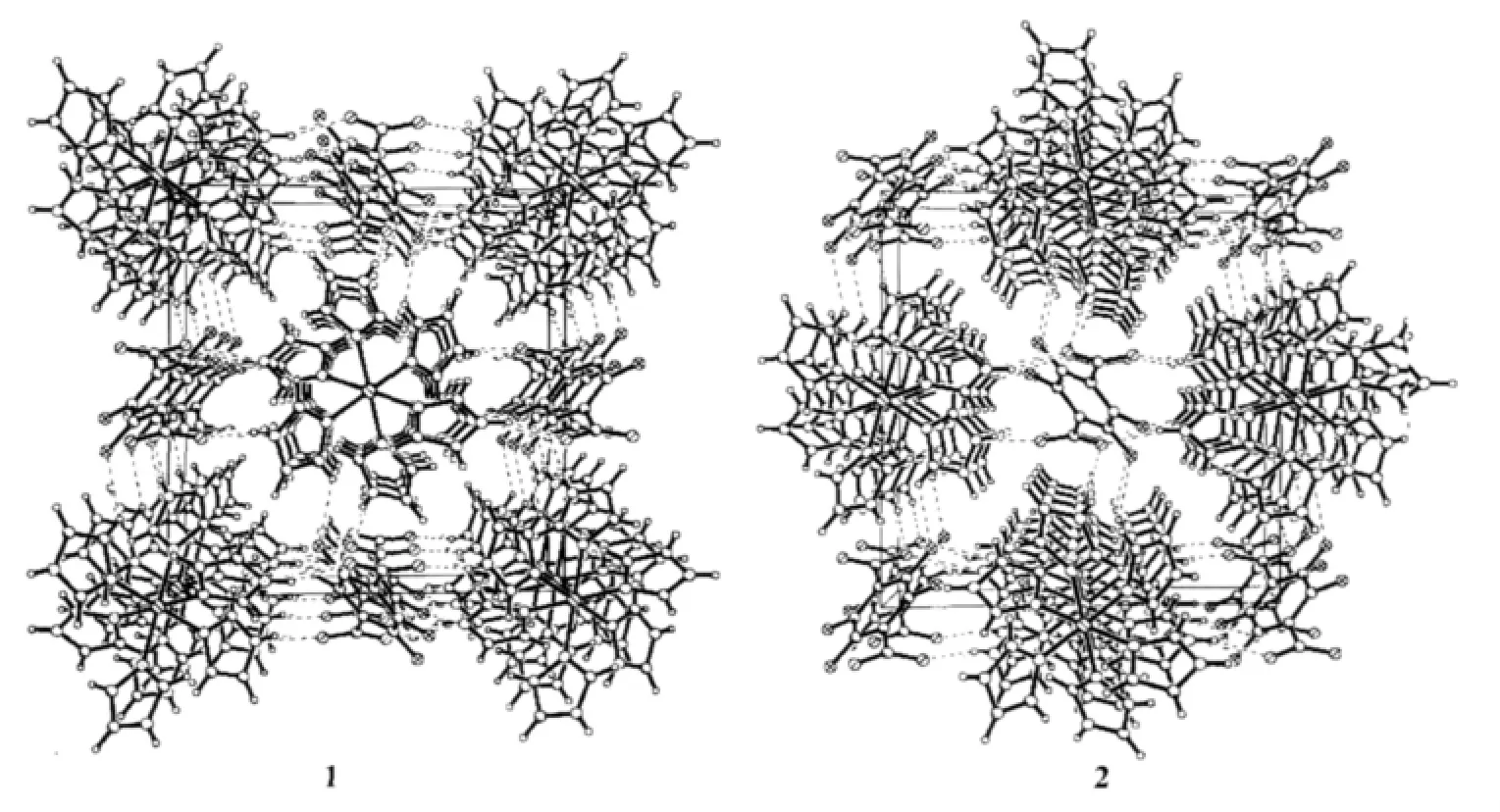
Fig.2 Views of the three-dimensional hydrogen-bonded networks of the title compounds,dashed lines indicate hydrogen bonds
2.3 Thermal stability
Thermalstability ofcoordination compound [Co(ImH)6](tfbdc)(1)hasbeen investigated by TG technique,The TG analysis shows that weight loss of [Co(ImH)6](tfbdc)begins at 120℃,and six Him molecules are lost from 120℃ to 196℃ (calculated, 58.06%;found,58.60%).The second weight loss of 30.65%at 196~800℃ corresponds to the loss of the tfbdc molecule (calculated 31.28%).The pyrolysis product is CoO(calculated,10.65%;found,10.75%).
Thermalbehaviorofcoordination compound [Ni(ImH)6](tfbdc)(2)is quite similar to 1.The TG analysis shows that weight loss of[Ni(ImH)6](tfbdc) begins at 135℃,and six ImH molecules are lost from 135 to 212℃ (calculated,58.08%;found,59.60%).The second weight loss of 30.46%at 212~800℃corresponds to the loss of the tfbdc molecule(calculated 31.30%).The pyrolysis product is NiO(calculated, 10.63%;found,9.94%).
2.4 Electrochemical property
Using water as solvent,the concentration of [Co(ImH)6](tfbdc)and[Ni(ImH)6](tfbdc)were 0.550 mmol·L-1and 0.540 mmol·L-1,respectively.The redox behavior of the[M(ImH)6](tfbdc)(M=Co,Ni)complexes were studied by cyclic voltammetry (CV).Cyclic voltammograms of compounds[Co(ImH)6](tfbdc)and [Ni(ImH)6](tfbdc)are shown in Fig.3 and Fig.4 respectively,which are quite similar,during scanning from -1.1 to 1.1 V in 100 mV·s-1,the cyclic voltammogram curve only has an oxidation-reduction peak,which corresponds to M(Ⅱ)/M(Ⅱ)redox process[16-18],For[Co(ImH)6] (tfbdc),Epa=0.30 V,Epc=0.028 V,ΔE=0.272 V.The results show that electron transfer of Co(Ⅱ) between Co(Ⅱ) in electrolysis is quasi-reversible process.For [Ni(ImH)6](tfbdc),Epa=0.22 V,Epc=-0.1 V,ΔE=0.23 V, which show that electron transfer of Ni(Ⅱ)between Ni(Ⅱ) in electrolysis is quasi-reversible process,too.

Fig.3 Cyclic voltammogram of[Co(ImH)6](tfbdc)(1)in aqueous solution

Fig.4 Cyclic voltammogram of[Ni(ImH)6](tfbdc)(2)in aqueous solution
3 Conclusion
We have synthesized two novel complexes [Mn(ImH)6](tfbdc)(M=Co,Ni)via general solution synthetic methods.They consist of two independent units:the monomeric cation[M(ImH)6]2+and the tfbdc anion.The[M(ImH)6]2+cations and tfbdc anions are connected by N-H…O and C-H…F hydrogen bonds to form a three-dimensional supramolecular architecture.Electrochemical property of the complex shows that electron transfer of between M(Ⅱ)and M(Ⅱ) (M=Co,Ni) in electrolysis is quasi-reversible process.
[1]Férey G,Mellot-Draznieks C,Serre C,et al.Acc.Chem.Res., 2005,38:217-225
[2]Shi X,Zhu G,Fang Q,et al.Eur.J.Inorg.Chem.,2004,1:185-191
[3]Eddaoudi M,Kim J,Rosi N,et al.Science,2002,295:469-472
[4]Seo J S,Whang D,Lee H,et al.Nature,2000,404:982-986
[5]Rowsell J L C,Millward A R,Park K S,et al.J.Am.Chem. Soc.,2004,126:5666-5667
[6](a)Milon J,Daniel M,Kaiba A,et al.J.Am.Chem.Soc.,2007, 129:13872-13878
(b)LIU Yu-Juan(刘玉娟),ZUO Jing-Lin(左景林),CHE Chi-Ming(支志明),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(4):610-614
[7](a)Wan J,Ye S J,Wen Y H,et al.Chin.J.Chem.,2003,21: 1458-1460
(b)LI Rong-Xun(李荣勋),DENG Yue-Yi(邓月义),LIU Bao-Cheng(刘保成),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(4):609-614
[8]Zhang S S,Niu S Y,Jie G F,et al.Chin.J.Chem.,2006,24: 51-58
[9]ZHU En-Jing(朱恩静),LIU Qi(刘琦),CHEN Qun(陈群),et al. Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(9): 1428-1433
[10]Zhu E J,Liu Q,Chen Q,et al.J.Coord.Chem.,2009,62:2449 -2456
[11]Kitaura R,Lwahofi F,Matsuda R,et al.Inorg.Chem.,2004, 43:6522-6524
[12]Chen B L,Yang Y,Zapata F,et al.Inorg.Chem.,2006,45: 8882-8886
[13]Sheldrick G M.SHELEXTL-97,Program for X-ray Crystal StructureDetermination,GöttingenUniversity,Germany,1997.
[14]Ding B B,Weng Y Q,Mao Z W,et al.Inorg.Chem.,2005,44: 8836-8845
[15]Yang L N,Li J,Zhang F X.Acta Cryst.,2005,E61:m2169-m2171
[16]Cańadas M,López-Torres E,Martínez-Arias A,et al. Polyhedron,2000,19(18/19):2059-2068
[17]Helena G M,Mendes P J,Romo D A.J.Organomet.Chem., 2005,690(17):4063-4071
[18]YU Li-Li(于丽丽),LIU Qi(刘琦),XI Hai-Tao(席海涛),et al. Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(4): 621-626
以咪唑为配体的钴和镍的配合物的合成、晶体结构及电化学性质
王庆华1于丽丽1刘 琦*,1,2程美令1
(1常州大学化学化工学院,江苏省精细石油化工重点实验室,常州 213164)
(2南京大学配位化学国家重点实验室,南京 210093)
合成了2种新颖的配合物[M(ImH)6](tfbdc)(M=Co,Ni;tfbdc=2,3,5,6-四氟对二苯甲酸根;ImH=咪唑),并通过元素分析,红外光谱,热重分析,循环伏安及X-射线单晶结构分析对其结构及性质进行了表征。2个配合物[Co(ImH)6](tfbdc)(1)和[Ni(ImH)6] (tfbdc)(2)都属单斜晶系,空间群为P21/c,且Z=2。每1个金属离子与来自6个咪唑分子的6个氮原子配位,形成八面体配位构型。独立组分,[M(ImH)6]2+阳离子和四氟对二苯甲酸阴离子之间通过两种氢键(N-H…O和C-H…F)连接形成了一种三维的超分子网络结构。
钴配合物;镍配合物;咪唑;2,3,5,6-四氟对二苯甲酸;晶体结构;电化学性质
O614.81+2;O614.81+3
A
1001-4861(2011)05-0989-07
2010-11-08。收修改稿日期:2011-01-27。
国家自然科学基金(No.20671045,20971060);南京大学配位化学国家重点实验室自然科学基金资助项目。*
。E-mail:liuqi62@163.com,Tel:0519-86330185;会员登记号:S060018987P。
猜你喜欢
杂志排行
无机化学学报的其它文章
- Synthesis of Porous Hydromagnesite Microspheres with Rosette-Like Morphology
- Hydrothermal Syntheses and Crystal Structures of Two Metal Complexes with Mixed 1,2,4-Benzenetricarboxylic Acid and 1,3,5-Tris(1-imidazolyl)benzene Ligands
- Effect of Synthesis Temperature on Structure and Ceramization Process of Polyaluminasilazanes
- Hydrothermal Syntheses,Crystal Structures and Luminescent Properties of Binuclear Zn(Ⅱ)and Co(Ⅱ)Complexes Assembled by 4-Carboxymethoxy Phenylacetic Acid
- Influence of Carbon Nanotube Content on Microstructures and Mechanical Properties of Carbon/Carbon Composite
- Thermal Stability and Antibacterial Activity of Phosphonium Salts Pillared Layered α-Zirconium Phosphates
