Hydrothermal Syntheses,Crystal Structures and Luminescent Properties of Binuclear Zn(Ⅱ)and Co(Ⅱ)Complexes Assembled by 4-Carboxymethoxy Phenylacetic Acid
2011-11-09FUJunDanGUOJingKeWENYiHang
FU Jun-Dan GUO Jing-Ke WEN Yi-Hang
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
Hydrothermal Syntheses,Crystal Structures and Luminescent Properties of Binuclear Zn(Ⅱ)and Co(Ⅱ)Complexes Assembled by 4-Carboxymethoxy Phenylacetic Acid
FU Jun-Dan GUO Jing-Ke WEN Yi-Hang*
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Institute of Physical Chemistry,Zhejiang Normal University,Jinhua,Zhejiang321004,China)
Two new binuclear complexes,[M2L2(phen)2(H2O)2](M=Zn,1;Co,2),assembled by 4-carboxymethoxy phenylacetic acid (H2L)and 1,10-phenanthroline (phen),have been synthesized under hydrothermal conditions. Single-crystal X-ray diffraction reveals complex 1 and 2 are isostructural,crystallized in monoclinic system,P21/c space group.The metal ion center is six-coordinated in a distorted octahedral geometry.Two neighboring center metal ions are linked together by two bridging L ligands forming a binuclear cage second building units(SBUs), and the SBUs are further linked to form 1D chain through intermolecular hydrogen bonds.In addition,π-π stacking interactions between the phen rings of the adjacent chains result in a 2D layer.The luminescence of complex 1 has also been investigated,and the result reveals that it displays luminescent property in the voilet region.CCDC:799358,1;799357,2.
4-carboxymethoxy phenylacetic acid;binuclear complex;hydrogen bonds;luminescence property
The rational design and construction of coordination polymers based upon assembly of metal ions and multifunctionalorganic ligandsis an interesting research field.This not only stems from their intriguing structural topologies but also from their potential application as functional materials[1-8].An effective and facile approach for the synthesis of such complexes is still the appropriate choice of well-designed organic ligands as bridges or terminal groups with metal ions or metal clusters as nodes[9-12].Among the various ligands, for commonly possessing a large conjugated π-electron system and multiple coordination modes,such asmonodentate,chelating and bridging,organic aromatic polycarboxylates have been extensively employed in the preparation of such metal organic compounds[13-15].In this paper,we chose another new flexibility aromatic polycarboxylates 4-carboxymethoxy phenylacetic acid (H2L)as the bridging ligand and 1,10-phenanthroline as a terminal ligand to generate two new coordination polymers,[Zn2L2(phen)2(H2O)2](1)and[Co2L2(phen)2(H2O)2](2),which were obtained by using hydrothermal technique and characterized by single crystal X-ray diffraction.
1 Experimental
1.1 Materials and measurements
The organic ligand 4-carboxymethoxy phenylacetic acid (H2L)was prepared according to a literature procedure based on 4-Hydroxyphenylacetic acid[16].All other reagents were purchased commercially and used as supplied.IR spectra were recorded on an FTIR-8700 spectrometer with KBr pellets in the range of 4 000~400 cm-1.Elemental analysis was performed on a PE-2400(Ⅱ) element analysis instrument.The crystal data collections were carried out on a Bruker SMART APEX-Ⅱ CCD diffractometer.Luminescence spectra were performed on a HITACHI F-2500 Fluorescence Spectrometer in solid state at room temperature.
1.2 Syntheses of complex
1.2.1 Synthesis of complex1
A mixture of 4-carboxymethoxy phenylacetic acid (0.1035 g,0.5 mmol),1,10-phenanthroline(0.079 3 g, 0.4 mmol),ZnSO4·7H2O(0.143 8 g,0.5 mmol),Na2CO3(0.053 g,0.5 mmol)and water-ethanol(18 mL,V/V=5∶1)was sealed in a 25 mL stainless-steel reactor with a Telflon liner and was heated at 433 K for 3 d.On completion of the reaction,the reactor was cooled slowly to room temperature and the mixture was filtered,giving colourless single crystals suitable for X-ray analysis in yield 85%.Elemental Anal.found(%): C,55.48;H,3.92;N,5.99.Calcd.for C44H36N4O12Zn2(%): C,56.01;H,3.85;N,5.94.IR (KBr,cm-1):3 244(w), 3062(m),1631(s),1509(s),1428(s),1221(s),1078(m), 857(m),728(s).
1.2.2 Synthesis of complex 2
A mixture of 4-carboxymethoxy phenylacetic acid (0.1035 g,0.5 mmol),1,10-phenanthroline(0.079 3 g, 0.4 mmol),CoSO4·7H2O(0.140 5 g,0.5 mmol),Na2CO3(0.053 g,0.5 mmol)and water-ethanol(18 mL,V/V=5∶1)was sealed in a 25 mL stainless-steel reactor with a Telflon liner and was heated at 433 K for 3 d.On completion of the reaction,the reactor was cooled slowly to room temperature and the mixture was filtered,giving red single crystals suitable for X-ray analysis in yield 40%.Elemental Anal.found(%):C, 56.30;H,3.91;N,6.21.Calcd.for C44H36N4O12Co2(%): C,56.79;H,3.90;N,6.02.IR (KBr,cm-1):3 299(w), 3018(m),1610(s),1502(s),1385(s),1200(s),1090(m), 819(m),778(s).
1.3 X-ray crystallography
Crystals of 1(0.24 mm×0.17 mm×0.10 mm)and 2 (0.49 mm×0.26 mm×0.14 mm)were mounted on glass fiber using epoxy resin.Data collection was performed on a Bruker SMART APEX-Ⅱdiffractometer equipped with a CCD detector(Mo Kα radiation,λ=0.071 073 nm).Data intensity was corrected by Lorentz-polarization factors and empirical absorption.The structure was solved by direct methods and expanded with Fourier techniques.Anisotropic displacement parameters were applied to all non-hydrogen atoms in full-matrix leastsquares refinements based on F2.The hydrogen atoms were assigned with isotropic displacement factors and included in the final refinement cycles by the use of geometrical restrains.All calculations were performed with SHELX-97 package[17].All pertinent crystallographic data for 1 and 2 are summarized in Table 1.The select bond distances and bond angles are listed in Table 2.
CCDC:799358,1;799357,2.

Table 1 Crystal data and structure refinement for two complexes
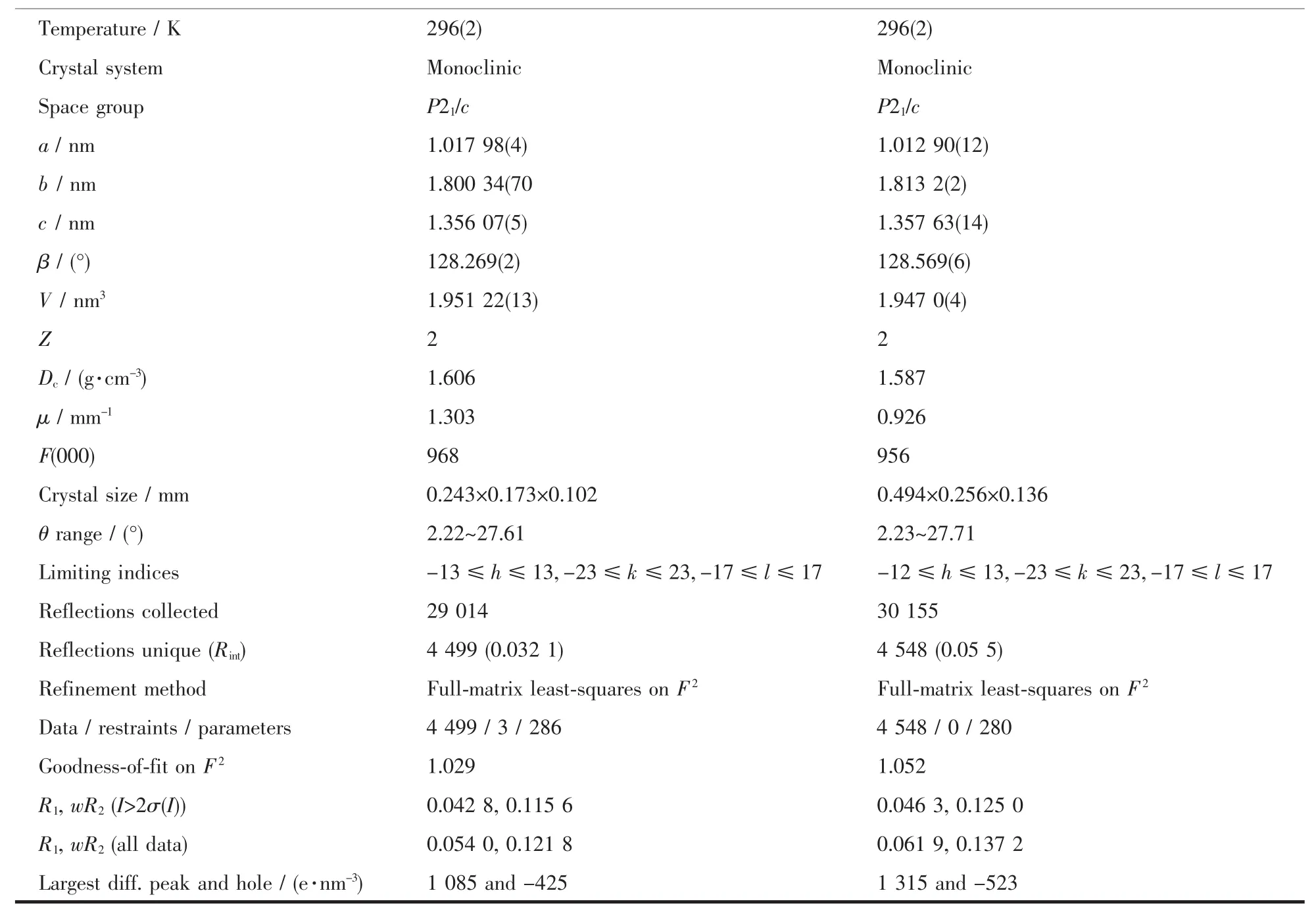
Continued Table 1

Table 2 Selected bond lengths(nm)and angles(°)for complex 1 and 2
2 Results and discussion
2.1 Crystal structure of the complex
The X-ray diffraction study shows that complex 1 and 2 are isostructural,crystallizes in a centrosymmetric space group P21/c,and the structure motif is composed of two metal atoms,two 4-carboxymethoxy phenylacetate ligands,two 1,10-phenanthroline ligands and two water molecules.As representative,the structure of complex 1 is described here in detail.
As shown in Fig.1,The center Zn(Ⅱ) is sixcoordinated and exhibits a distorted octahedral coordination geometry form by two nitrogen atoms from the phen ligand(Zn-N 0.216 9(2)and 0.209 5(2)nm), four oxygen atoms from four bridging L ligands and one water molecule(Zn-O 0.200 2(4)~0.216 9(2)nm).As to L ligand,the carboxylic groups are all deprotonated, and two carboxylate groups represent different coordination modes:mondentate and bidentate,each L2-ligand acts as a tridentate linker.Owing to flexibility of L ligand,The two neighboring Zn(Ⅱ) center are linked together by two bridging L ligands forming a binuclear cage with Zn…Zn separation of 0.819 1(7)nm.Meanwhile,phen ligands as terminal ligand exist on sides of cage,The dihedral angle between the two phen rings of cage is 0.194(42)°.
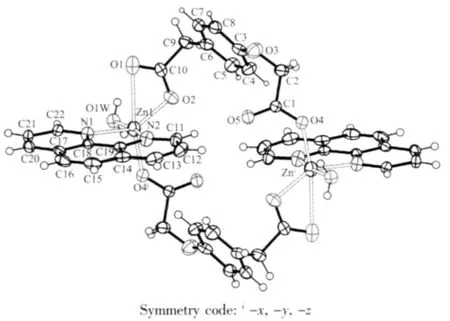
Fig.1 Coordination environment of the Znatom in the complex 1 showing 30%probability ellipsoids and the atom-labeling scheme
The weak intermolecularforces are usually important in the syntheses of coordination polymers[18-19]. There are persistent intermolecular hydrogen bonds involving lattice water molecules and corresponding carboxylate oxygen atoms in the neighboring cage.The hydrogen-bonding distances are listed in Table 3.The O-H…O hydrogen bonds link the neighboring cage to yield 1D double chain structure with a cavity along c axis(Fig.2a).In addition,there are relatively short distance(centroid separation=0.350 16(1)nm and interplanar spacing=0.330 64(4)nm) between the adjacent phen-ring planes of the adjacent double chains,implying strong π-π stacking interactions in complex 1.All these hydrogen bonds and π-π stacking interactions,resulting in a 2D layer framework along the(001)plane and lead the whole framework structure system stable(Fig.2b).
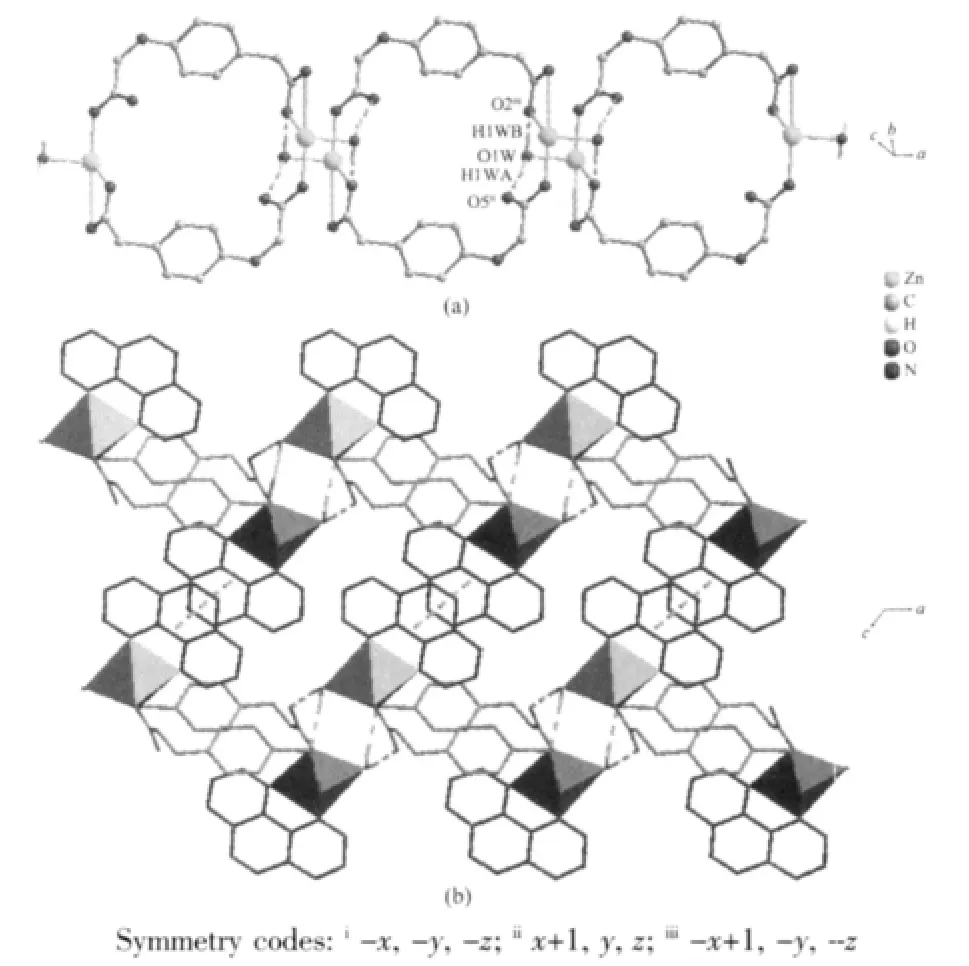
Fig.2 (a)View of the double chains of complex 1(phen are omitted for clarity);(b)View of 2D layer of complex 1(intermolecular forces are depicted as dashed lines:green is hydrogen bonds and yellow is π-π stacking interactions)
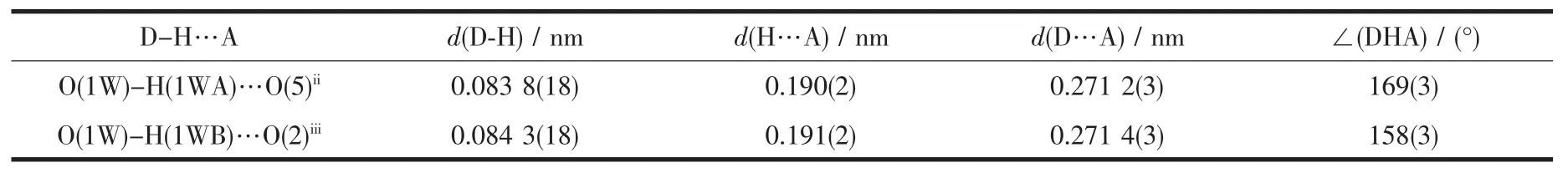
Table 3 Hydrogen-bonding parameters for 1
2.2 Luminescence property
Emission spectra for complex 1 and free ligand H2L were investigated in the solid state at room temperature.As indicated in Fig.3,H2L shows a certain emission with maximum at 298 nm upon excitation at 280 nm and complex 1 has a broader peak emission with maximum at 463 nm when irradiated with 350 nm.The free phen ligand displays fluorescent properties in the solid state at room temperature with the broader weak emission being located at 200~560 nm,the relatively strong emission wavelength is at 390 nm[20].Compared to free H2L and phen ligands,complex 1 has a remarkable lower energy emission shift,which may be due to mainly the chelating of the ligand to the metal center[21-23],and the use of second ligands of the Zn(Ⅱ)centers because the behavior of luminescence in d10complexes is closely associated with the coordination environments of the metal centers[24].
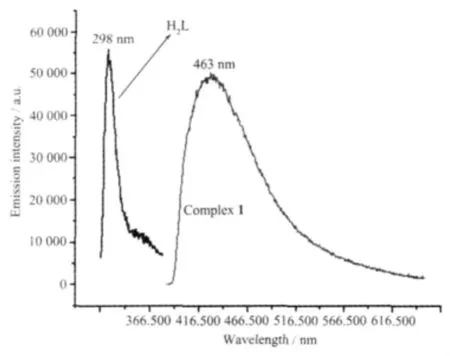
Fig.3 Fluorescence emission spectrum of complex 1 and H2L ligand in the solid state at room temperature
[1]Zhao X,Xiao B,Fletcher A J,et al.Science,2004,306:1012-1015
[2]Li Y W,Yang R T.J.Am.Chem.Soc.,2006,128:726-727
[3]Kubota Y,Takata M,Matsuda R,et al.Angew.Chem.Int.Ed., 2005,44:920-923
[4]Gao X M,Li D S,Wang J J,et al.CrystEngComm,2008,10: 479-482
[5]Chen X N,Zhang W X,Chen X M,et al.J.Am.Chem.Soc., 2007,129:15738-15739
[6]Kuppler R J,Timmons D J,Fang Q R,et al.Coord.Chem. Rev.,2009,253:3042-3066
[7]Xu G J,Zhao Y H,Shao K Z,et al.J.Mol.Struct.,2010,983(1/ 2/3):93-98
[8]Ma L F,Liu B,Wang L Y,et al.Dalton Trans.,2010,39:2301-2308
[9]Company A,G′omez L,Valbuena J M L,et al.Inorg.Chem., 2006,45:2501-2508
[10]Gu J Z,Lu W G,Jiang L,et al.Inorg.Chem.,2007,46:5835-5837
[11]Lu W G,Gu J Z,Jiang L,et al.Cryst.Growth Des.,2008,8: 192-199
[12]Ma L F,Wang L Y,Lu D H,et al.Cryst.Growth Des.,2009,4: 1741-1749
[13]Vodak D T,Braun M E,Kim J,et al.Chem.Commun.,2001: 2534-2535
[14]Yan B,Xie Q Y.Inorg.Chem.Commun.,2003,6:1448-1450
[15]Yan B,Xie Q Y.J.Mol.Struct.,2004,688:73-78
[16]Fu J D,Wen Y H.Acta Cryst.,2011,E67:o167
[17]Sheldrick G M.SHELXS-97 and SHELXL-97,Program for the solution and the Refinement of Crystal Structure, University of Göttingen,Germany,1997.
[18]Beatty A M.Coord.Chem.Rev.,2003,246:13l-143
[19]Brunsveld L,Folmer B J B,Meijer E W,et al.Chem.Rev., 2001,101:4071-4097
[20]LI Wei(李薇),LI Chang-Hong(李昶红),YANG Ying-Qun(李颖群),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2008,24(12):2060-2064
[21]Liu C S,Shi X S,Li J R,et al.Cryst.Growth Des.,2006,6(3): 656-663
[22]Zheng S L,Tong M L,Tan SD,et al.Organometallics,2001, 20:5319-5325
[23]Zhang L Y,Zhang J P,Chen X M,et al.Cryst.Growth Des., 2006,6(7):1684-1689
[24]Yin J L,Feng Y L,Lan Y Z.Inorg.Chim.Acta,2009,362: 3769-3776
4-氧乙酸苯乙酸构筑的双核Zn(Ⅱ)和Co(Ⅱ)配合物的合成,晶体结构及荧光性能
傅君丹 郭静轲 温一航*
(浙江师范大学物理化学研究所,浙江省固体表面反应化学重点实验室,金华 321004)
用水热法合成得到2个新配合物,[M2L2(phen)2(H2O)2](L=4-氧乙酸苯乙酸,phen=1,10-邻菲咯啉,M=Zn,1;Co,2),并测定了它们的晶体结构。配合物1和2为异质同晶,单斜晶系,P21/c空间群。中心金属M为六配位,相邻的M(Ⅱ)通过2个桥连的配体形成双核笼状次级结构单元,通过氢键和π-π堆积弱作用链接形成二维层结构。通过对配合物1的固态荧光测试,结果表明其在紫光区显示发光效应。
4-氧乙酸苯乙酸;双核配合物;氢键;荧光
O614.81+2;O614.24+1
A
1001-4861(2011)05-0996-05
2010-11-15。收修改稿日期:2011-01-08。
浙江师范大学博士启动基金资助项目。
*通讯联系人。E-mail:wyh@zjnu.edu.cn;会员登记号:S060017686M。
猜你喜欢
杂志排行
无机化学学报的其它文章
- Influence of Carbon Nanotube Content on Microstructures and Mechanical Properties of Carbon/Carbon Composite
- Hydrothermal Syntheses and Crystal Structures of Two Metal Complexes with Mixed 1,2,4-Benzenetricarboxylic Acid and 1,3,5-Tris(1-imidazolyl)benzene Ligands
- Effect of Synthesis Temperature on Structure and Ceramization Process of Polyaluminasilazanes
- Thermal Stability and Antibacterial Activity of Phosphonium Salts Pillared Layered α-Zirconium Phosphates
- Synthesis of Porous Hydromagnesite Microspheres with Rosette-Like Morphology
- Syntheses,Crystal Structures and Electrochemical Properties of Cobalt and Nickel Complexes Containing Imidazole Ligand
