Central Properties of the Metabolites ofHouttuynia cordataThunb.Populations from Different Altitudes in Guizhou
2011-10-13YANGZhannanPENGQuancaiLUOShiqiongYUZhengwenZHAOChaoZHUGuoshengGUIYangXIAPinhua
YANG Zhan-nan,PENG Quan-cai,,LUO Shi-qiong,YU Zheng-wen,ZHAO Chao,ZHU Guo-sheng,GUI Yang,XIA Pin-hua
(1. Key Laboratory for Information System of Mountainous Area and Protection of Ecological Environment of Guizhou Province,Guizhou Normal University, Guiyang 550001, China;2. School of Life Sciences, Guizhou Normal University, Guiyang 550001,China;3. Guizhou Key Laboratory of Agricultural Biotechnology, Guiyang 550006, China)
Central Properties of the Metabolites ofHouttuynia cordataThunb.Populations from Different Altitudes in Guizhou
YANG Zhan-nan1,PENG Quan-cai1,2,LUO Shi-qiong2,YU Zheng-wen2,ZHAO Chao1,ZHU Guo-sheng3,GUI Yang3,XIA Pin-hua1
(1. Key Laboratory for Information System of Mountainous Area and Protection of Ecological Environment of Guizhou Province,Guizhou Normal University, Guiyang 550001, China;2. School of Life Sciences, Guizhou Normal University, Guiyang 550001,China;3. Guizhou Key Laboratory of Agricultural Biotechnology, Guiyang 550006, China)
The chemical composition and volatile compound content ofHouttuynia cordatain Guizhou province, China, were investigated at different altitudes. Altitudes were characterized by the evolution of chlorogenic acid, rutin, quercetin, quercitrin,hyperoside, kaempferol, isorhamnetin and quercetin-3-O-β-D-glucoside as well as volatile components. Chlorogenic acid and all flavonoids were analyzed by HPLC. The volatile extracts obtained by steam distillation were analyzed using HS-SPME-GC/MS. The chlorogenic acid and flavonoid content ofH. cordataremained high at 1400-1500 m and 500-600 m, respectively.The essential oil content remained high at 600-700 m. Pharmacological 2-undecanone varied with increasing altitude, and remained high at 700-800 m. The contents of monoterpene (pinene), sesquiterpene (caryophyllene-(Ⅱ)), fatty ketone(2-undecanone), fatty acid (caprinic acid) and fatty acid ester (pentadecanoic acid,2,6,10,14-tetramethyl-,methyl ester) changed significantly at different altitudes.
gas chromatography-mass spectrometry (GC-MS);headspace solid-phase microextraction (HS-SPME);high performance liquid chromatography (HPLC);Houttuynia cordataThunb.;metabolites;property;altitudes
Houttuynia cordataThunb. is both an edible and a medicinal plant in China, and is one of the most derived genera in Saururaceae[1]. According to the Florae Reipublicae Popularis Sinicae,H. cordataThunb. is the only species in the genus Houttuynia[2-3].H. cordatais mainly distributed in Eastern Asia. While in China, this species is mainly distributed in the middle, southeastern and southwestern provinces and regions, including Guizhou, Sichuan, andChongqing. Historically, traditional Chinese medicines have played an important role in clinical therapy. Due to the high pharmacological activity, low toxicity and rare complications of these medicines[4], there has been more and more interest on the health aspects ofH. cordataconsumption in recent years, However,H. cordatais a medicinal plant of the Saururaceae family, and has been used to clear heat, counteract toxins, evacuate pus, relieve stranguria and alleviate edema due to the presence of its many bioactive ingredients,especially chlorogenic acid and flavonoids. The presence of these volatile components makes the isolation and measurement of these constituents as well as the quality control of crude drugs and medical preparations extremely difficult. At present the main method used in the determination of chlorogenic acid and flavonoids in medicinal plants is generally high-performance liquid chromatography (HPLC)[5-10].Traditionally, the analysis of volatile compounds from traditional Chinese medicines is usually preceded by the extraction of essential oil by steam distillation using GC-MS, which often requires a large amount of sample and takes several hours to complete. The complex and time-consuming process for the preparation of samples sometimes further complicate the analytical results due to the influencing factors involved. Headspace solid-phase microextraction (SPME)developed by Pawliszyn and coworkers in 1989, is a solventless extraction technique widely used in the application of extraction from plants, food, biological and environmental samples[11-13]. The central properties of the metabolites ofH.cordataneed to be understood using modern analytical instruments and techniques. Differences in the growing conditions ofH. cordatainfluence the evolution of metabolite content, and these influencing factors are very complicated.The content of 2-undecanone, rutin, quercentin and queritrin fromH. cordatahave been analyzed from different areas[14-15],however, the properties of the metabolites fromH. cordataunder different environmental factors have rarely been systemically investigated.
This study investigated the content of chlorogenic acid,flavonoids and volatile compounds inH. cordatapopulations from different altitudes in Guizhou province, China by gas chromatography-mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC).
1 Materials and Methods
1.1 Plant materials
H. cordatasamples were harvested from eleven counties in Guizhou province, in the southwest region of China.The samples were harvested in August 2007 (Table 1).
1.2 Chlorogenic acid and flavonoids
1.2.1 Sample preparation for HPLC analysis
After being air-dried and crushed into a powder, 0.5 g of theH. cordatasample was accurately weighed then extracted with 2 × 30 mL of methanol by sonication for 30 min. The samples were centrifuged (Model 80-2, Jinda, Jiangsu) for 8 min at 4000 r/min. The extracts were combined and concentrated to about 3.5 mL at 40 - 50 ℃, and then diluted to 4.0 mL with methanol. The upper phase was filtered through a Minisart filter (RC4, Sartorius, Hanbang, Jiangsu). Peak identification was performed by standard addition methods.
1.2.2 HPLC conditions
The HPLC system LC-20AT series (Shimadzu, Japan),equipped with Chem Station software (Shimadzu), which comprised two pumps, an online vacuum degasser, a thermostatted column compartment and a diode array detector, was used for chromatographic analysis. All separations were carried out on a reversed-phaseC18column (4.6 mm × 250 mm,5μ m). A linear gradient elution using eluent A (water) and eluent B (methanol) was used for the separations. The elution programme was well optimized and was conducted as follows: an isocratic elution of 40% B for the first 10 min, a linear gradient of 40%-90% B for 10-80 min, and an isocratic elution of 90% B for the next 10 min. After holding the solvent composition of 90% B for a further minute the column was returned to its starting condition. The solvent flow rate was 0.6 mL/min and the detection wavelength was set at 320 nm, the injection volume was 20μL, and the column temperature was maintained at 40 ℃.
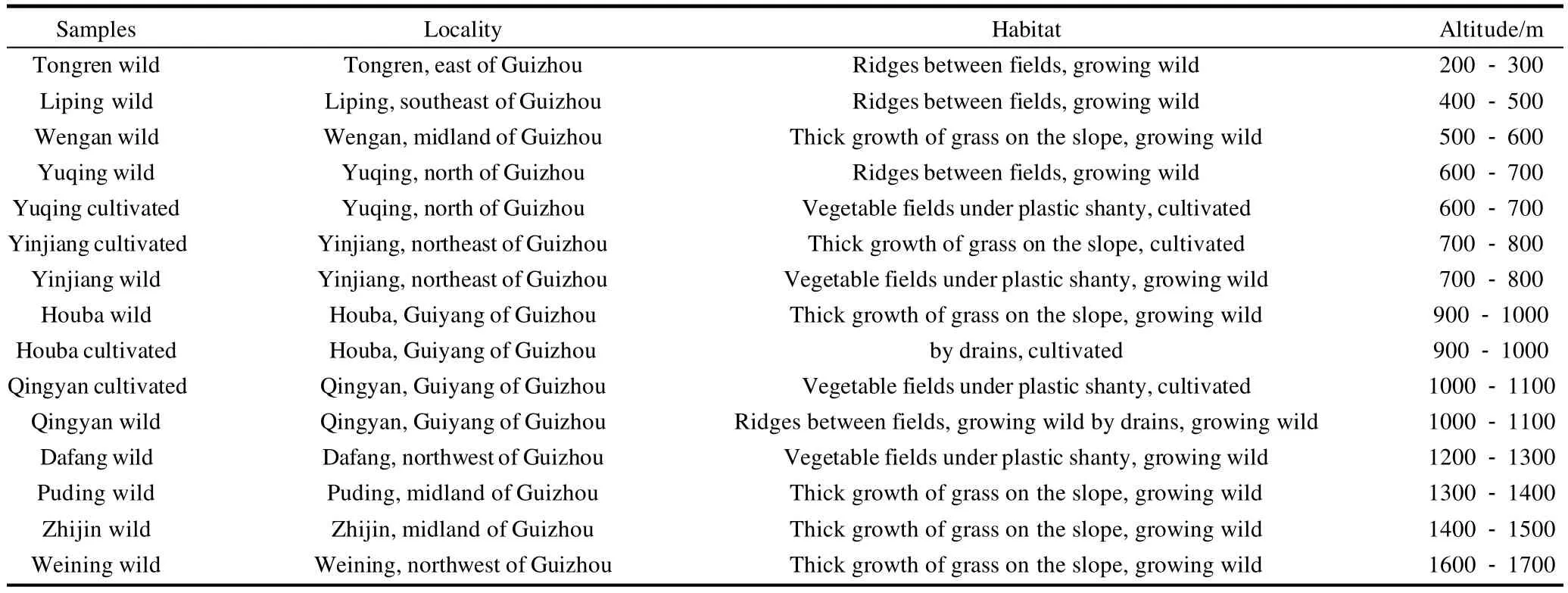
Table 1 The sources of the germplasm resources of the genusHouttuynia
After extraction using the technique outlined in 1.2.1,the samples were then filtered through a 0.45μm membrane before HPLC analysis. In the present work, the percentage extraction of chlorogenic acid and flavonoids was defined as follows: percentage extraction of chlorogenic acid and flavonoids (m/m) = mass of chlorogenic acid and flavonoids extracted/mass of material (H. cordatasample).
1.3 Volatile composition
1.3.1 Steam distillation extraction
A 1000 g sample ofH. cordatawas milled into a crude powder and transferred into a 5000 mL distillation flask, 2500 mL of water was added and the mixture was distilled for 4 h.Oil was collected from the condenser and was diluted with 5 mL of ether. The extracts were then dried with anhydrous sodium sulfate. The essential oil was stored at - 20 ℃ until analysis. The yield of oil was calculated based on the fresh plant weight.
1.3.2 HS-SPME extraction
The manual SPME holder was used with a 100μm polydimethylsiloxane fibre assembly (Supelco, Bellefonte,USA). Before use, the fibre was conditioned as recommended by the manufacturer. The extraction experiments were carried out following the method of literature[16].H. cordatasamples (2.0 g) with a particle size of 125μm were hermetically sealed in a 4 mL vial, the SPME fibre was then suspended in the HS and equilibrated for 20 min in a thermostatic bath, which was set at 80 ℃.
1.4 GC-mass spectral analyses
HS-SPME extraction samples were analyzed by GC-MS,using a Shimazu, QP-2010 system, with a DB-5 (30 m×0.25 mm, 0.5 μm) column. Operating conditions for GC were as follows: helium (99.999%) was used as the carrier gas at a constant flow rate of 0.78 mL/min; temperature of injector 250 ℃ and detector 260 ℃ and split 1:10. Temperature programming was: 40 ℃, 5min; 40-90 ℃, 2 ℃/min; 100-160 ℃, 3 ℃/min; 160-220 ℃, 5 ℃/min. The mass spectral analyses were performed at 70 eV. The volatile substances inH. cordatawere identified, by means of comparison only,through mass spectra, GC-MS database and Kovats index retention[17]. For the Kovats index, a standard mixture C8to C24was used under the same conditions as the samples. The results of volatile composition analyses are shown in Table 2.
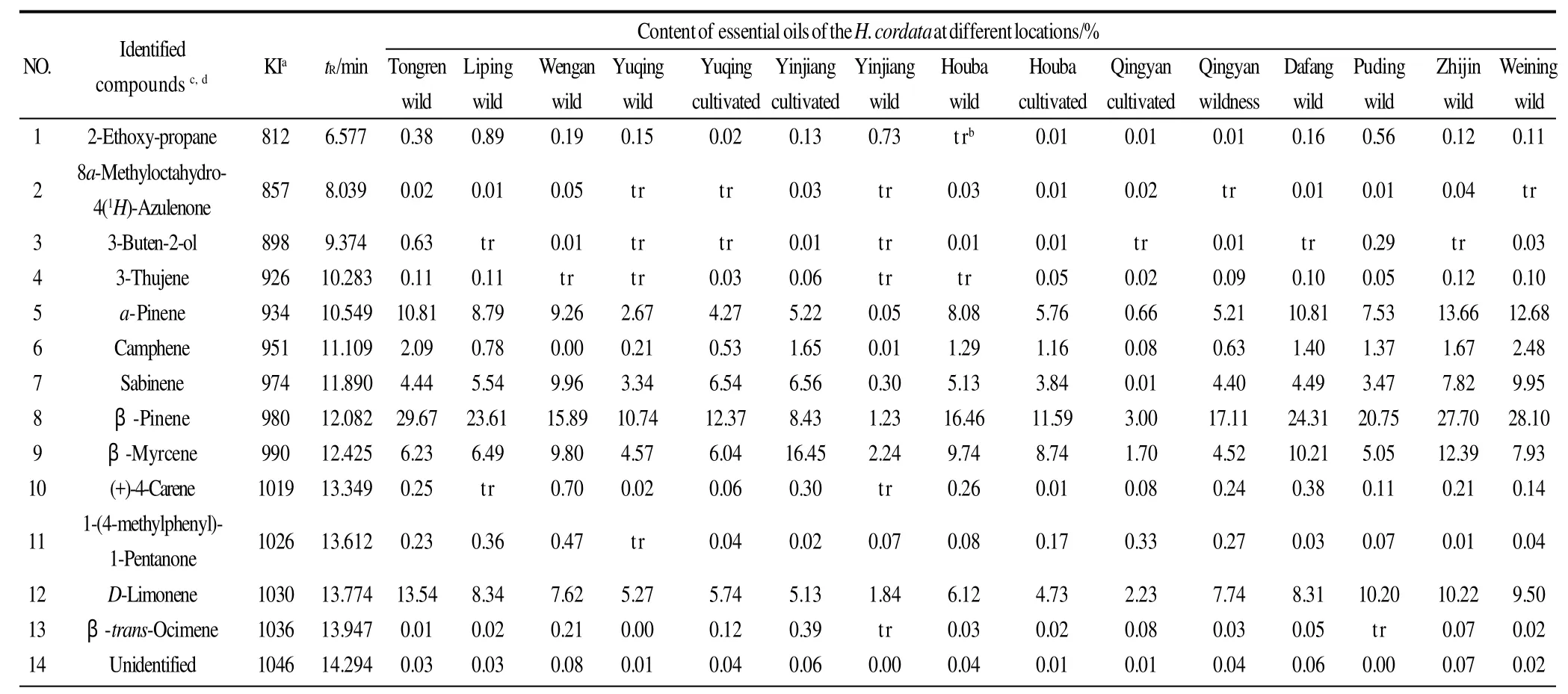
Table 2 Essential oils percentages and probable volatile compounds in theH. cordatasamples
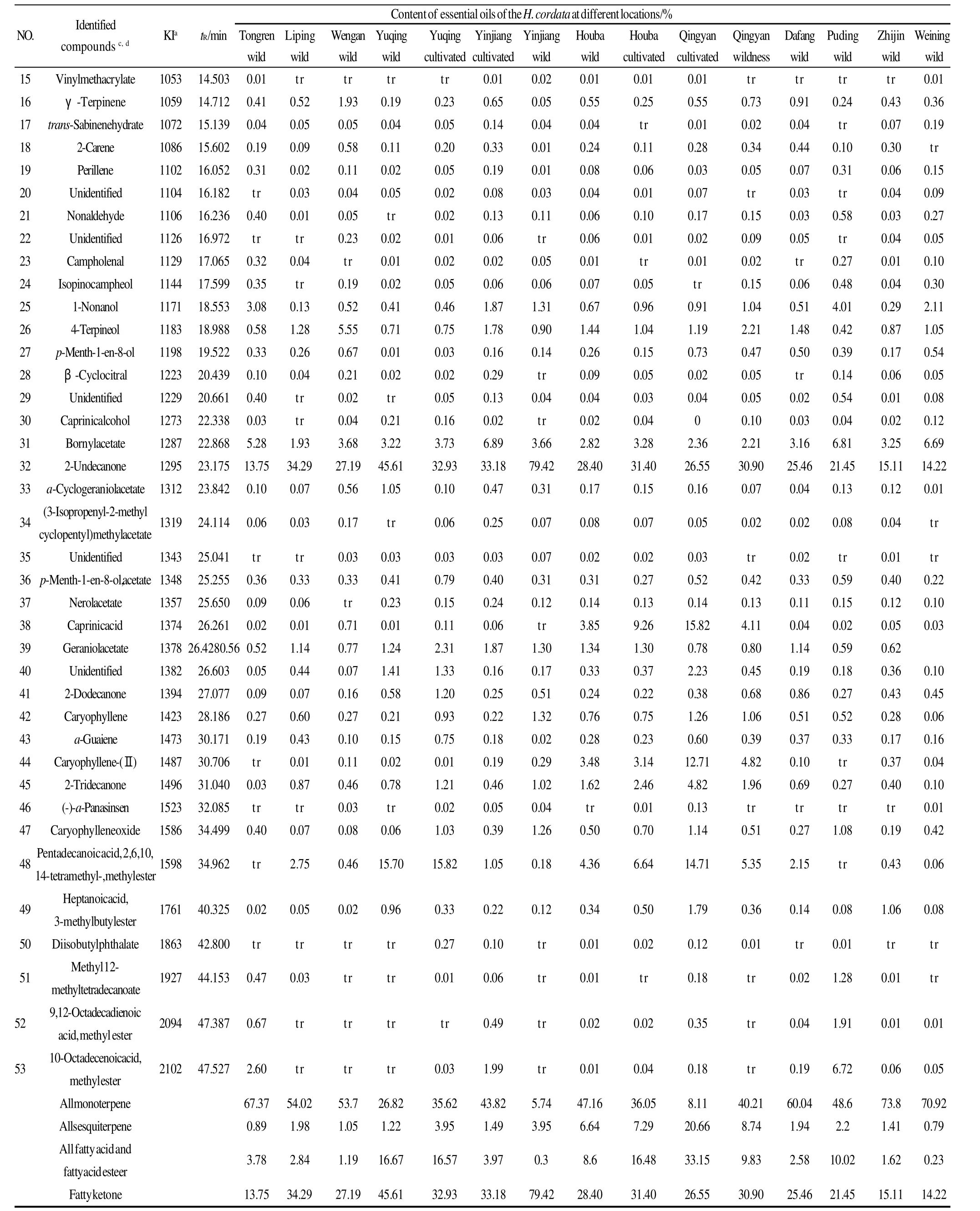
Table 2 (to be continued)
The ion currents generated depended on the characteristics of the compound and for this reason the quantification was not completely true. The results obtained by GC-MS may be used for biodiversity characteristics of the investigated plant, as well as for quantitative comparisons between the different groups of metabolites contained in the plants.This method is suitable for comparing the chemical composition of different organisms, because the deviations caused by the differences in the intensity of the mass spectral fragmentation will be identical[21].
2 Results and Discussion
2.1 Accumulation of chlorogenic acid and flavonoids
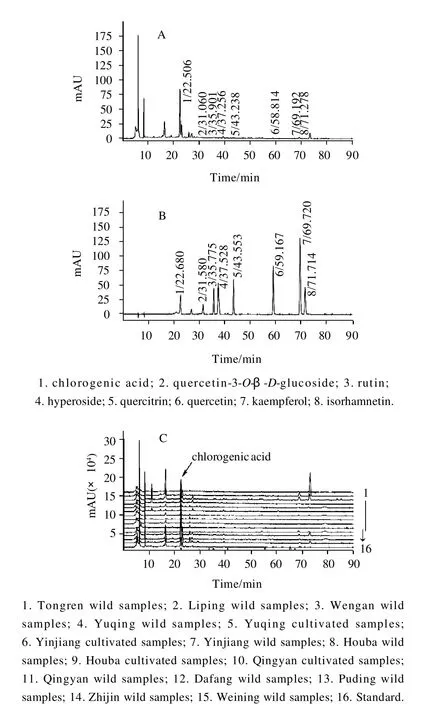
Fig.1 Dafang wild samples’representative chromatogram ofH. cordatasamples (A), chromatogram of standard (B), additional chromatogram ofH. cordatasamples and standard (C)
Fig.1 shows representative chromatograms ofH. cordatasamples and the standard. Observations on the growth pattern ofH. cordataat different altitudes from 200-300 m to 1600-1700 m and on the concentrations of chlorogenic acid and flavonoids in all plants were recorded on randomly selected plants from 15 sites in Guizhou province, China, in August 2007. The concentration of chlorogenic acid and flavonoids fromH. cordataare summarized in Fig. 2 (A) and (B).
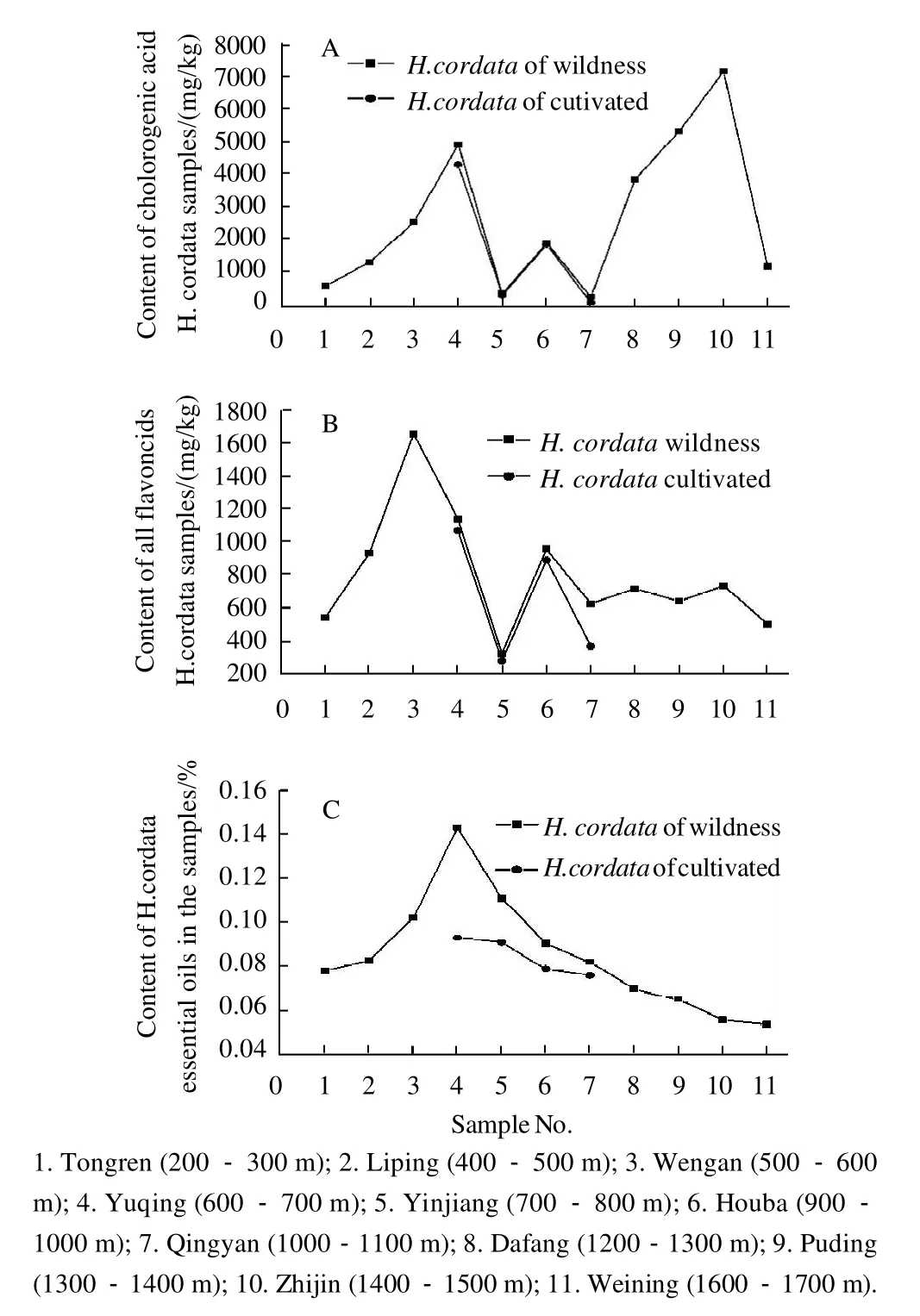
Fig.2 Comparison of chlorogenic acid content(A), flavonoids content(B), essential oils content(C) inH. cordata samples at different altitudes
It can be seen from Fig. 2 (A) that there was an obvious increase in the concentrations of chlorogenic acid in all plants from Tongren (200-300 m altitude) to Yuqing (600-700 m altitude), where the concentrations increased from 534.2 mg/kg to 4880.7 mg/kg. However, these concentrations rapidly decreased at Yinjiang (700-800 m altitude), and the concentrations of chlorogenic acid from wild samples in this area deceased to 297.2 mg/kg. At Houba (900-1000 m altitude), the concentrations of chlorogenic acid in wild samples were 1834.3 mg/kg. At Qingyan (1000-1100 maltitude), the concentrations of chlorogenic acid decreased rapidly and were 193.0 mg/kg in wild samples. Chlorogenic acid concentrations were increased at Qingyan (1000-1100 m) to Zhijin (1400-1500 m), and the concentrations in wild samples increased to 7121.3 mg/kg. These concentrations decreased rapidly at Weining (1600-1700 m), and were 1103.2 mg/kg in wild samples. The trend in chlorogenic acid concentrations in wild samples ofH. cordatawas consistent with cultivated samples ofH. cordata, with no obvious differences in concentrations.
It can be seen from Fig. 2 (B) that the concentration of flavonoids in all plan ts rapidly increased from Tongren(200-300 m altitude) to Wengan (500-600 m altitude),where the concentrations of all flavonoids increased from 539.2 mg/kg to 1649.2 mg/kg in wild samples at Wengan (500-600 m altitude), and the concentrations of all flavonoids decreased to 326.8 mg/kg in wild samples at Yinjiang(700-800 m altitude). As altitude increased at Houba(900-1000 m), the concentrations of all flavonoids showed another obvious increase, however, the concentrations of all flavonoids showed no further obvious increase when altitude continuously increased up to 1600-1700 m at Weining. The change in concentrations of all flavonoids from wild samples ofH. cordatawas consistent with cultivated samples ofH. cordata, with no obvious differences. Preliminary results also showed that the concentration of each flavonoid was not related to the altitude at which samples ofH.cordatawere collected. In addition, the concentrations of chlorogenic acid and flavonoids in wild samples ofH.cordatawere higher than those in cultivated samples. Liu et al[15]reported that variations in the quercetin content ofH.cordatapopulations from different altitudes showed that there were significant differences in the quercetin content of populations from different altitudes, however, the relationship between quercetin content and altitude was not significant.Chen et al[22]reported that the difference in quercetin content in the germplasm ofH. cordataof different origin was significant.Liu et al[23]reported that the concentrations of rutin and quercetin in wildH. cordatasamples were higher than those in cultivated samples. This finding is consistent with previous observations,and may be related to environment, growth micro-environment and the complicated mountainous region.
2.2 Volatile composition analysis
2.2.1H. cordataessential oil accumulation
Fig. 2 (C) shows that the content of essential oils inH.cordatacontinuously increased in wild samples from Tongren(200-300 m altitude) to Yuqing (600-700 m altitude). The highest content of essential oils inH. cordatawas 0.143%from wild samples in Yuqing (600-700 m altitude). The content of essential oils then continuously decreased with increasing altitude, and the lowest content was 0.054% when altitudes increased to 1600-1700 m (wild samples at Weining). In addition, the yield of essential oils from wild samples at Yuqing, Yinjiang, Houba, and Qingyan were 0.143%, 0.111%, 0.0905%, and 0.082%, respectively. The yield of essential oils in cultivated samples at Yuqing, Yinjiang,Houba, and Qingyan were 0.0931%, 0.091%, 0.0079%, and 0.076%, respectively. The results also showed that there were significant differences in the content of essential oils in populations from different altitudes, and that the concentrations of essential oils in wild samples ofH. cordatawere higher than those in cultivated samples.
2.2.2 Volatile composition ofH. cordataessential oil analysis The total ion chromatograms ofH. cordataessential oils showed that the oils contained 53 components. The identified compounds and relative quantitative distribution are shown in Table 2. The compounds were identified not by standard samples but by mass spectra, database, Kovats index retention, and the literature. Additional chromatograms ofH. cordataessential oils in 15 samples are shown in Fig. 3.
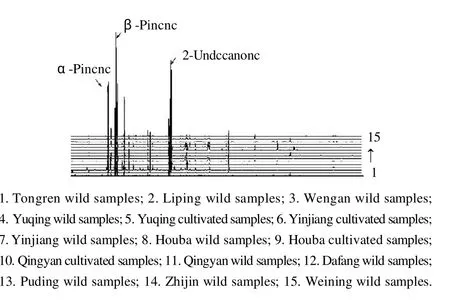
Fig.3 H. cordataessential oils additional chromatographs ofH. cordatasamples
It can be seen from Table 2 that the chemical composition ofH. cordataessential oils were consistent, however, the relative percentage of chemicals showed remarkable differences at different altitudes. The relative percentage ofβpinene inH. cordataessential oil from wild samples at Tongren (200-300 m altitude) was 29.67%, however, the relative percentage of beta-pinene inH. cordataessential oil from wild samples at Yinjiang (700-800 m altitude) wasonly 1.23%. The relative percentage of 2-undecanone inH.cordataessential oil from wild samples at Tongren was only 13.75%, however, the relative percentage of 2-undecanone inH. cordataessential oil from wild samples at Yinjiang was 79.42%. A little caprinic acid was detected inH. cordataessential oil from wild samples at Houba (900-1000 m altitude), however, caprinic acid inH. cordataessential oil from cultivated samples at Qingyan (1000-1100 m altitude)was 15.82%. A little caryophyllene-(Ⅱ) was detected inH. cordataessential oil from wild samples at Tongren,however, caryophyllene-(Ⅱ) inH. cordataessential oil from cultivated samples at Qingyan was 12.71%. A little pentadecanoic acid, 2,6,10,14-tetramethyl-, methyl ester was detected inH. cordataessential oil from wild samples at Tongren and Puding (1300-1400 m altitude), however,pentadecanoic acid, 2,6,10,14-tetramethyl-, methyl ester inH. cordataessential oil from cultivated samples at Qingyan was 14.71%.
Table 2 shows that the compounds found in the essential oils were mainly monoterpenes, sesquiterpenes, fatty ketones, fatty acids and fatty acid esters.α-pinene,βpinene, sabinene andβ-myrcene were the predominant monoterpenes in the 15 samples with the exception of wild samples at Yinjiang and cultivated samples at Qingyan.Caryophyllene-(Ⅱ) and caprinic acid were the predominant sesquiterpenes and fatty acids at Qingyan and Houba. The highest concentrations of these chemicals were 12.71% and 15.82%, respectively obtained from cultivated samples at Qingyan. Pentadecanoic acid, 2,6,10,14-tetramethyl-, methyl ester was the predominant fatty acid ester at Yuqing,Qingyan, and Houba, and the highest concentration of 15.82%was obtained from cultivated samples at Yuqing.
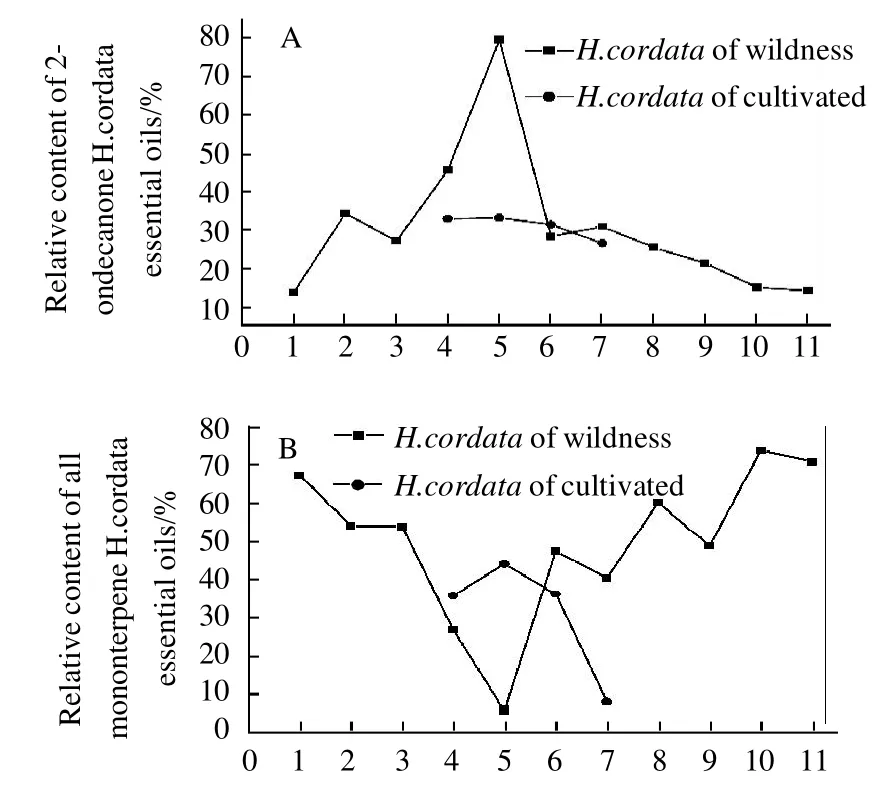
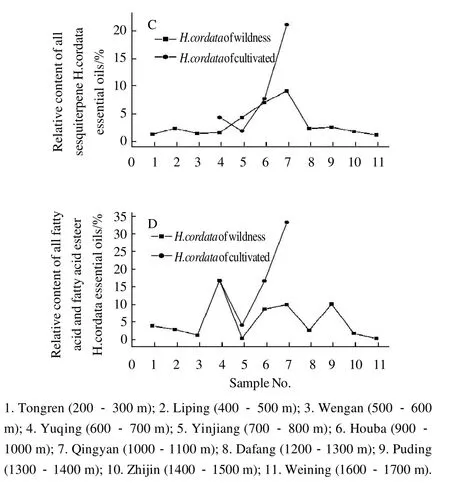
Fig.4 Comparison of relative contents of 2-undecanone, all monoterpenes, all sesquiterpenes, and all fatty acid and fatty acid esters inH. cordatasamples at different altitudes, respectively
The main component in the essential oil of this medicinal plant was decanoyl acetadehyde, which is known to have pharmacological effects[24-25]. Fig. 4 (A) shows that the relative percentage of 2-undecanone increased from 13.75% to 79.42%when the altitude increased from 200-300 m at Tongren to 700-800 m at Yinjiang with the exception being Wengan(500-600 m). The relative percentage of 2-undecanone progressively decreased with increasing altitude except at Qingyan (1000-1100 m) to 13.75% at Weining (1600-1700 m). A comparison of wild and cultivated samples showed that the relative percentage of 2-undecanone was similar in wild and cultivated samples. The relative percentage of 2-undecanone from wild samples at Houba was 28.40%, and was 31.4% in cultivated samples. The relative percentage of 2-undecanone from wild samples at Yuqing was 45.61%, and was 32.93% in cultivated samples. These figures for wild and cultivated samples at Qingyan were 30.90% and 26.55% respectively and were 79.42% and 33.10%, respectively at Yinjiang. Yang et al[26]reported that the concentrations of 2-undecanone fromH. cordatadiffered with latitude, however,no relationship between high and low latitude was observed.
Fig. 4 (B) shows that the relative percentage of monoterpene from wild samples ofH. cordatadecreased from 67.37% to 5.74% when the altitude increased from 200-300 m at Tongren to 700-800 m at Yinjiang. The trend in relativepercentage of monoterpene from wild samples ofH. cordatathen increased with increasing altitude. However, the cultivated samples did not follow this trend. Fig. 4 (C) and Fig. 4(D) show the relationship between the percentage of monoterpene, fatty acid and fatty acid ester fromH. cordataand altitudes, however, the relationship is not obvious.
A comparison of the concentrations of monoterpene,sesquiterpene, fatty ketone (2-undecanone), fatty acid and fatty acid ester from the samples (Fig. 4), shows that the relative concentration of fatty ketone from wild samples ofH. cordataat Yinjiang was highest at 79.42%, however,monoterpene, sesquiterpene, and fatty acid plus fatty acid ester concentrations were low at only 5.74%, 3.95%, and 0.30%, respectively. In some samples the relative proportion of monoterpene was high while in others the relative proportion was low. For example, the relative proportion of monoterpene from wild samples at Tongren was 67.37%, while the relative proportions of sesquiterpene, fatty acid and fatty acid ester were 0.89%, 378% and 13.75%, respectively. When the relative proportions of fatty acid and fatty acid ester were high, monoterpene, sesquiterpene and fatty ketone (2-undecanone) were low. For example, wild samples at Qingyan showed that the relative proportion of fatty acid and fatty acid ester was 33.15%, but the relative proportions of monoterpene, sesquiterpene and fatty ketone (2-undecanone)were only 8.11%, 20.66% and 26.55%, respectively. When the relative proportions of monoterpene and sesquiterpene decreased, fatty ketone (2-undecanone), fatty acid and fatty acid ester increased. For example, the relative proportions of monoterpene and sesquiterpene from wild samples at Yuqing were only 26.82% and 1.22%, respectively, but fatty ketone (2-undecanone) and fatty acid plus fatty acid ester were 45.61% and 16.67% respectively. Liang et al[16]reported that decanoyl acetaldehyde may be converted into 2-undecanone via both oxidation and decarboxylation. Eleftherios et al[27]also reported that monoterpene oxides were translated. Findings from this study show that, to a certain extent, biosynthesis of monoterpene, sesquiterpene, fatty ketone, fatty acid and fatty acid ester can be related to altitude. These results are consistent with those reported by Liu et al[14-15].
3 Conclusion
In the present work it was observed that the content of chlorogenic acid, flavonoids, and essential oils inH. cordatavaried with increasing altitude. Chlorogenic acid content ofH. cordataremained high at altitudes of 1400-1500 m. The content of all flavonoids, essential oils and 2-undecanone inH. cordataremained high at 500-600 m, 600-700 m and 700-800 m, respectively. It was further noted that wild and cultivated samples ofH. cordataat the same altitude showed a difference in essential oils and their compounds. In addition,the content of each flavonoid significantly changed inH.cordatasamples, and the chemical compounds in the essential oils also changed. If these metabolites can reflect the evolution and distribution ofH. cordata, further work on these aspects may be worth investigating.
[1] LIANG Hanxing. On the evolution and distribution in Saururaceae[J].Acta Bot Yunnanica, 1995, 17: 255-267.
[2] TSENG Yungchien. Florae reipublicae popularis sinicae (Tomus 20-1)[M]. Beijing: Science Press, 1982: 8.
[3] FANG Wenpei. Flora Sichuanica(vol. 1)[M]. Chengdu: Sichuan People Press, 1981: 126-127.
[4] WEN Kuoching, HUANG Chengyu, LU Fenling. Determination of baicalin and puerarin in traditional Chinese medicinal preparations by high-performance liquid chromatography[J]. J Chromatogr A, 1993, 631(1/2): 241-250.
[5] BROLIS M, GABETTA B, FUZZATI N, et al. Identification by highperformance liquid chromatography-diode array detection-mass spectrometry and quantification by high-performance liquid chromatography-UV absorbance detection of active constituents ofHypericum perforatum[J].J Chromatogr A, 1998, 825(1): 9-16.
[6] KEINANEN M, JULKUNEN-TIITTO M. High-performance liquid chromatographic determination of flavonoids inBetula pendulaandBetula pubescensleaves[J]. J Chromatogr A, 1998, 793: 370-377.
[7] MERKEN H M, BEECHER G B. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones[J]. J Chromatogr A, 2000, 897: 177-184.
[8] XU Xueqin, YE Hezhi, QI Xiuzhen, et al. Determination of quercetin and rutin inSaururus chinensis(Lour.) Baill by RP-HPLC[J]. J Fuzhou Univ: Nat Sci, 2002, 30: 870-872.
[9] ALONSO-SALCES R M, BARRANCO A, CORTA E, et al. A validated solid-liquid extraction method for the HPLC determination of polyphenols in apple tissues: comparison with pressurised liquid extraction[J]. Talanta, 2005, 65: 654-662.
[10] ZUO Yuegang, CHEN Hao, DENG Yiwei. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector[J]. Talanta, 2002, 57:307-316.
[11] JAYATILAKA A, POOLE S K, POOLE C F, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupledcolumn gas chromatography[J]. Anal Chim Acta, 1995, 302: 147-162.
[12] CAI Jibao, LIU Baizhan, SU Qingde. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components[J]. J Chromatogr A, 2001, 930: 1-7.
[13] ARTHUR C L, PAWLISZYN J. Solid phase microextraction with thermal desorption using fused silica optical fibers[J]. Anal Chem, 1990,62: 2145-2148.
[14] LIU Lei, WU Wei, ZHENG Youliang, et al. Variations on the chemicalcomponents of the volatile oil ofHouttuynia cordataThunb. populations from different valleys and altitudes of Mt. Emei[J]. Acta Ecologica Sinica, 2007, 27(6): 2239-2250.
[15] LIU Lei, WU Wei, ZHENG Youliang, et al. Variations on the quercetin content ofHouttuynia cordataThunb. populations from different altitudes of Mt. Emei[J]. Lisizhen Medicine and Materia Medica Research,2007, 18(7): 1601-1603.
[16] LIANG Minmin, QI Meiling, ZHANG Changbin, et al. Gas chromatography-mass spectrometry analysis of volatile compounds fromHouttuynia cordataThunb. after extraction by solid-phase microextraction, flash evaporation and steam distillation[J]. Analytica Chimica Act, 2005, 531: 97-104.
[17] CHYAU C C, CHEN S Y, WU C M. Differences of volatile and nonvolatile constituents between mature and ripe guava (Psidium guajavaLinn.) fruits[J]. Journal of Agricultural and Food Chemistry, 1992, 40(5): 846-849.
[18] LU Hongmei, WU Xianjin, LIANG Yizeng, et al. Variation in chemical composition and antibacterial activities of essential oils from two species ofHouttuyniaThunb.[J]. Chem Pharm Bull, 2006, 54(7): 936-940.
[19] XU Chengjian, LIANG Yizeng, CHAU F T. Identification of essential components ofHouttuynia cordataby gas chromatography/mass spectrometry and the integrated chemometric approach[J]. Talanta, 2005, 68(1): 108-115.
[20] QI Meiling, GE Xiaoxia, LIANG Minmin, et al. Flash gas chromatography for analysis of volatile compounds fromHouttuynia cordataThunb.[J]. Analytica Chimica Acta, 2004, 527: 69-72.
[21] GEORGIEVA E, HANDJIEVA N, POPOV S, et al. Comparative analysis of the volatiles from flowers and leaves of threeGentianaspecies[J]. Biochemical Systematics and Ecology, 2005, 33(9): 938-947.
[22] CHEN Li, ZHENG Youliang, WU Wei, et al. Comparative study on content of quercetin in the germplasm ofHouttuynia cordataThunb. of different origin[J]. Chin J Pharm Anal, 2007, 27(8): 1232-1235.
[23] LIU Siman, BIAN Qingquan. Determination of rutin and quercetin ofHouttuynia cordataThunb. by RP-HPLC[J]. Chinese Traditional Patent Medicine, 2006, 28(7): 1057-1059.
[24] LI Shuang, YU Qinghai, JIN Peike. Studies on the effective, constituent pharmacological effects and clinical application ofHouttuynia cordra[J].J Shenyang Pharm Univ, 1997, 14(2): 144-147.
[25] SHIMURA M, ZHOU Y, ASADA Y, et al. Inhibition of Vpr-induced cell cycle abnormality by quercetin: a novel strategy for searching compounds targeting Vpr[J]. Biochem Biophys Res Commun, 1999, 261(2): 308-316.
[26] YANG Jian, GUO Shutai, YANG Xueli, et al. Quality value ofHouttuynia cordataThunb. different places[J]. Modern Traditional Chinese Medicine,2007, 27(6): 62-63.
[27] ELEFTHERIOS A, TARANTILIS P A, HARIZANIS P C. Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatographic-mass spectrometric analysis[J]. Food Chemistry, 2007, 100: 396-404.
贵州不同海拔鱼腥草代谢产物主要特征
杨占南1,彭全材1,2,罗世琼2,余正文2,赵 超1,朱国胜3,桂 阳3,夏品华1
(1.贵州师范大学 贵州省山地环境信息系统与生态环境保护重点实验室,贵州 贵阳 550001;2.贵州师范大学生命科学学院,贵州 贵阳 550001;3.贵州省农业生物技术重点实验室,贵州 贵阳 550006)
对贵州不同海拔鱼腥草中绿原酸、芦丁、槲皮素、槲皮苷、金丝桃苷、山奈素、异鼠李素、槲皮素-3-O-β-D-葡萄糖苷以及挥发性化学成分行研究,通过HPLC分析绿原酸和黄酮类化合物,挥发性化学成分利用顶空固相微萃取结合气-质联用(HS-SPME-GC/MS)进行分析,总挥发油的含量用水蒸气蒸馏提取获得。结果表明:鱼腥中绿原酸和黄酮的含量分别在海拔1400~1500m和500~600m有较高的含量;鱼腥草精油在海拔600~700m有较高的含量,甲基正壬酮含量随海拔的增加而变化在700~800m有较高的含量;单萜(蒎烯)、倍半萜(石竹烯(Ⅱ))、脂肪酮(甲基正壬酮)、脂肪酸(羊油酸)和脂肪酸酯(2,6,10,14-四甲基十五酸甲酯)的含量在不同的海拔有显著的差异。关键词:气相色谱-质联用仪(GC-MS);顶空固相微萃取(HS-SPME);高效液相色谱(HPLC);鱼腥草;代谢产物;特征;海拔
TS252.1
A
1002-6630(2010)16-0261-09
2009-05-14
贵州省教育厅自然科学基金项目(黔科教2006306)
杨占南(1974—),男,副教授,硕士,主要从事分析化学和植物次生代谢产物的环境信息研究。E-mail:yangzhannan@163.com
