Roles of sulfonylurea receptor 1 and multidrug resistance protein 1 in modulating insulin secretion in human insulinoma
2011-07-07ChengJiangLiHuaLiZhouJunLiHongTianYaoRongSuandWenPengLi
Cheng-Jiang Li, Hua-Li Zhou, Jun Li, Hong-Tian Yao, Rong Su and Wen-Peng Li
Hangzhou, China
Original Article / Pancreas
Roles of sulfonylurea receptor 1 and multidrug resistance protein 1 in modulating insulin secretion in human insulinoma
Cheng-Jiang Li, Hua-Li Zhou, Jun Li, Hong-Tian Yao, Rong Su and Wen-Peng Li
Hangzhou, China
BACKGROUND:Sulfonylurea receptor 1 (SUR1) and multidrug resistance protein 1 (MRP1) are two prominent members of multidrug resistance proteins associated with insulin secretion. The aims of this study were to investigate their expression in insulinomas and their sole and synergistic effects in modulating abnormal insulin secretion.
METHODS:Fasting glucose, insulin and C-peptide were measured in 11 insulinoma patients and 11 healthy controls. Prolonged oral glucose tolerance tests were performed in 6 insulinoma patients. Insulin content, SUR1 and MRP1 were detected in 11 insulinoma patients by immunohistochemistry. SUR1 and MRP1 were also detected in 6 insulinoma patients by immunofluorescence.
RESULTS:Insulinoma patients presented the typical demonstrations of Whipple's triad. Fasting glucose of each insulinoma patient was lower than 2.8 mmol/L, and simultaneous insulin and C-peptide were increased in insulinoma patients. Prolonged oral glucose tolerance tests showed that insulin secretion in insulinoma patients were also stimulated by high glucose. Immunohistochemistry and immunofluorescence staining showed that SUR1 increased, but MRP1 decreased in insulinoma compared with the adjacent islets.
CONCLUSIONS:The hypersecretion of insulin in insulinomas might be, at least partially, due to the enrichment of SUR1. In contrast, MRP1, which is down-regulated in insulinomas, might reflect a negative feedback in insulin secretion.
(Hepatobiliary Pancreat Dis Int 2011; 10: 88-94)
sulfonylurea receptor 1; multidrug resistance protein 1; ATP-binding cassette transporters; insulinoma; insulin secretion
Introduction
Insulinomas are rare neuroendocrine tumors of pancreatic islet cells that can produce and secrete insulin. In contrast to normally differentiated betacells, insulinoma cells continue to secrete insulin and proinsulin at low blood glucose without fine regulation,[1]which results in hypoglycemia clinically. ATP-sensitive potassium (KATP) channels play crucial roles in insulin secretion. Sulfonylurea receptor 1 (SUR1) and multidrug resistance protein 1 (MRP1) are two prominent members of ATP-binding cassette transporters, which are associated with multidrug resistance in diabetes mellitus.[2]They are associated with insulin secretion via modulating KATP channels.[2,3]However, the underlying mechanisms of SUR1 and MRP1 in the autonomous insulin hypersecretion by insulinoma remain to be clarified.
SUR1 (ATP-binding cassette transporter C8, ABCC8) is a subunit of the pancreatic beta-cell KATP channels and plays a key role in the regulation of glucose-induced insulin secretion.[2-4]Mutations of SUR1 are associated with persistent hyperinsulinemic hypoglycemia of infancy as well as type 2 diabetes of adults.[5-7]MRP1 (ATP-binding cassette transporter C1, ABCC1) is also a regulator of insulin secretion related to KATP channel.[2,3,8]However, the role of SUR1 and MRP1 in insulin secretion by insulinoma, and whether they might influence insulin secretion synergistically warrant further investigation. The aim of this study was, therefore, to investigate the alteration of these two important multidrug resistance proteins in insulinoma of human subjects, which might help to understand theunderlying mechanisms whereby insulin secretion by insulinoma is unexhausted.
Methods
Recruitment of subjects
Eleven cases of insulinoma inpatients were recruited from Department of Endocrinology at the First Affiliated Hospital of Zhejiang University School of Medicine. The diagnosis of insulinoma was made according to the following criteria: the demonstration of Whipple's triad (i.e., biochemical hypoglycemia <2.8 mmol/L, symptoms consistent with hypoglycemia and reversal with carbohydrate replacement) and inadequately suppressed insulin levels (>3 μIU/ml). Computed tomography or magnetic resonance imaging was performed for the localization of insulinomas before operation. There were no related familial history, and the patients with insulinomas were also considered sporadic. Prolonged oral glucose tolerance tests (OGTTs) were performed in 6 insulinoma patients who could be intolerant to fasting for 8-10 hours. Blood samples were taken after an overnight fast. Fasting glucose was determined on a HITACH 7170 bio-chemicalautoanalyzer (Tokyo, Japan). Insulin and C-peptide were measured by direct chemiluminescence immunoassay using commercially available kits on the ADVIA Centaur®XP Immunoassay System (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Insulin release index was calculated according to the following formula: insulin (μIU/ml)/glucose (mg/dl). Fasting glucose, insulin and C-peptide were also measured in 11 age- and gender-matched healthy volunteers recruited from the community.
Immunohistochemistry staining
The patients were subjected to surgical operations, and the samples of insulinomas were fixed in 4% formalin and embedded in paraffin. Hematoxylin-eosin staining and immunohistochemistry for insulin, SUR1 and MRP1 were conducted in 11 patients. Sections were generally pretreated and incubated with the respective primary antibody at room temperature for 2 hours. The primary antibodies of mouse monoclonal anti-insulin (Fitzgerald Industries International, Concord, MA, USA), rabbit polyclonal anti-SUR1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit polyclonal anti-MRP1 (ABBIOTEC, San Diego, CA, USA) were diluted with Trisbuffered saline (TBS). The sections were then incubated with anti-mouse or anti-rabbit ENVISIONTM+System, HRP Labeled Polymer (DAKO, Carpinteria, CA, USA) as secondary antibody at room temperature for 30 minutes. Between all steps, the sections were rinsed three times with TBS. Diaminobenzidine (Sigma Chemical Co., Louis, MO, USA) was used for color development and hematoxylin was used to counterstain the specimen. The specificity of immunohistochemistry was confirmed by omitting the primary antibodies. Quantitative analysis was made using Image-Pro® Plus, and the results were expressed as the integrated option density within unit area.
Immunofluorescence staining
The fresh tissues of 6 insulinoma patients were stored at -80 ℃ for immunofluorescence staining. The cryosections of insulinoma and the adjacent normal pancreatic tissues (10 μm-thick) were fixed for 10 minutes in acetone at 4 ℃. The sections were then washed in phosphate buffered saline (PBS) three times for 15 minutes and blocked for 1 hour in PBS with 5% bovine serum albumin (BSA). The sections were incubated overnight at 4 ℃ in the respective primary antibody (rabbit polyclonal anti-SUR1 and anti-MRP1), and recovered to the room temperature. After 3 washes in PBS the sections were then incubated for 1 hour at room temperature with a diluted goat anti-rabbit Alexa Fluor 555-conjugated IgG (Jackson ImmunoResearch, West Grove, PA, USA). After 3 washes in PBS, several drops of 1∶1000 diluted DAPI (BIOMOL Research Laboratories, Plymouth, USA) were added to the sections to stain the cell nuclei blue. The stained sections were imaged on an Olympus IX81 epifluorescence microscope (Tokyo, Japan) using 555 nm illumination for Alexa Fluor and 390 nm illumination for DAPI. One section was used as a negative control (no primary antibody). Quantitative analysis was made using Image-Pro® Plus, and the results were expressed as the integrated option density within unit area.
Results
Clinical features
The clinical features of the subjects are shown in Table 1.Age and gender were matched between the healthy controls and insulinoma patients. The chief complaints ofinsulinoma patients were consistent with hypoglycemia symptoms such as dizziness, anxiety, tremulousness, pallor, palpitations, sweating, nausea, hunger, weakness, drowsiness, fatigue, visual disturbance, confusion, seizures and coma (Table 2), and they could be reversed immediately by the intake of sugars. Fasting glucose of each insulinoma patient was lower than 2.8 mmol/L, simultaneous insulin and C-peptide were much higher in the insulinoma patients, and as a result, insulin release index was significantly higher in the insulinoma patients compared with the healthy controls. Prolonged OGTT showed that insulin secretion in the insulinoma patients was also stimulated by high glucose level (Fig. 1).

Table 1. The clinical features of the subjects

Table 2. The clinical manifestations of the insulinoma patients
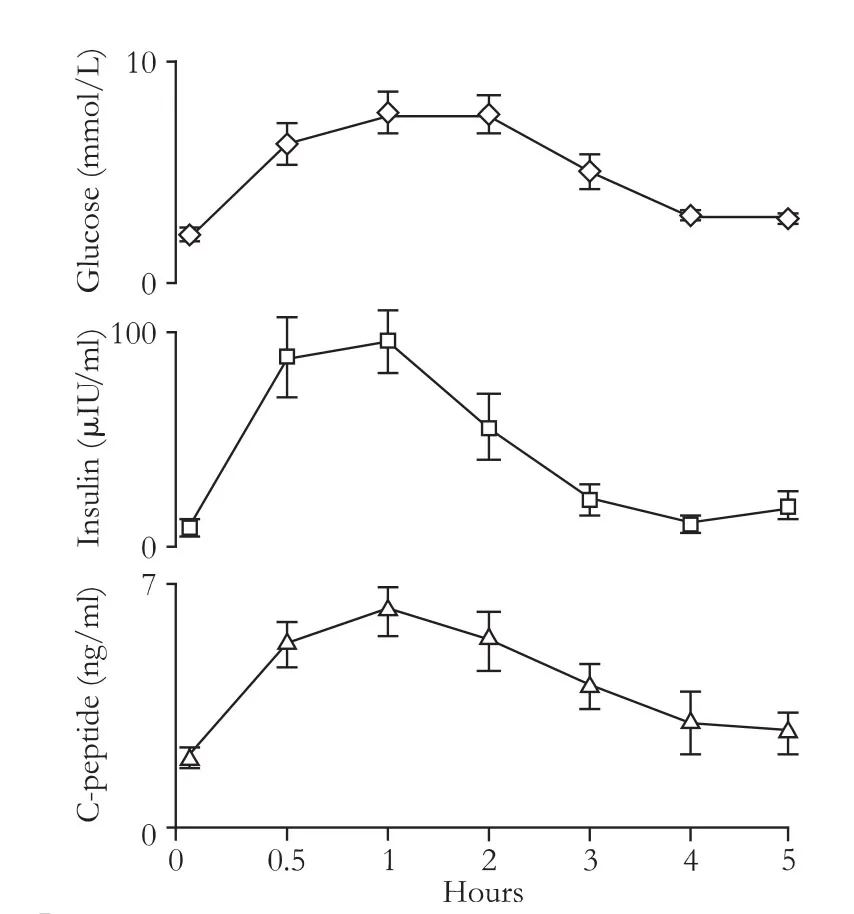
Fig. 1. Prolonged OGTT performed in insulinoma patients. Prolonged OGTT performed in insulinoma patients who could be intolerant of fasting for 8-10 hours. After fasting for 8-10 hours, 75 g glucose was taken orally, and blood was withdrawn at various time points. Blood glucose, insulin and C-peptide were measured. Data were expressed as mean±SEM (n=6).
General pathological findings
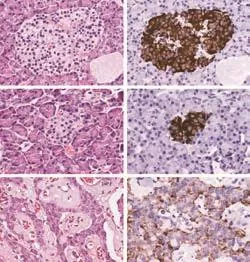
Fig. 2. Morphology and insulin content of insulinomas (original magnification ×200). Hematoxylin-eosin shows that outside the lesion of insulinoma, islets have a normal appearance (A). For those islets in the insulinoma patients with a long duration, the islets became small and irregular (C). In insulinomas, the normal pancreatic tissue disappears, and tumoral endocrine cells are organized in nests scattered throughout a fibro-vascular stroma (E). Immunohistochemistry for insulin shows that insulinomas are almost exclusively composed of β-cells, with a weak and variable insulin staining (F). Outside the lesion, insulin staining is much more intense within the islets (B and D).

Fig. 3. SUR1 and MRP1 measured by immunohistochemistry staining. The staining for SUR1 is homogeneous in the whole cytoplasm of endocrine cells, it is stronger in insulinoma (B, original magnification ×200) compared with the adjacent islets (A, original magnification ×200). The staining for MRP1 is also homogeneous in the whole cytoplasm of endocrine cells, it is weaker in insulinoma (D, original magnification ×100) compared with the adjacent islets (C, original magnification ×100). Quantitative analysis is made (E), data are expressed as mean± SEM (n=11), paired t test was performed. *: P<0.05, compared with the adjacent normal islets.
Insulinomas by hematoxylin-eosin staining showed the disappearance of the normal pancreatic tissue (Fig. 2E). Tumoral endocrine cells were organized in nests scattered throughout a fibro-vascular stroma. There were several islets outside the lesion, which had a normal appearance (Fig. 2A). For those with a long duration of the disease, the adjacent normal islets became small and irregular (Fig. 2C). Immunohistochemistry for insulin showed that insulinomas were almost exclusively composed of beta-cells, with a weak and variable insulin staining (Fig. 2F). Outside the lesion, insulin staining was much more intense within the adjacent islets (Fig. 2B and D).
Expression of SUR1 and MRP1
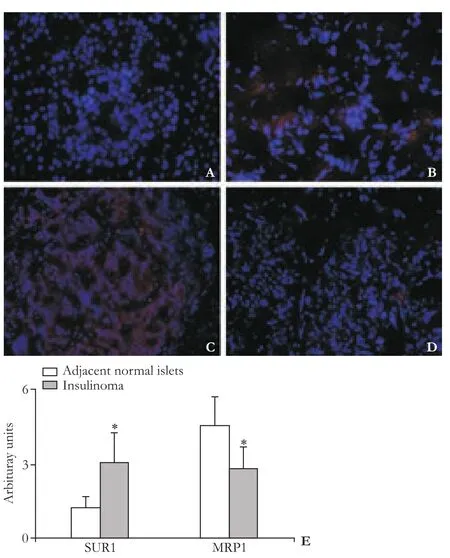
Fig. 4. SUR1, MRP1 measured by immunofluorescence staining (A-D, original magnification ×100). The staining for SUR1 is homogeneous in the whole cytoplasm of endocrine cells, this staining is stronger in insulinoma (B) compared with the adjacent islets (A). The staining for MRP1 is homogeneous in the whole cytoplasm of endocrine cells, this staining is weaker in insulinoma (D) compared with the adjacent islets (C). Red indicates fluorescence of Alexa Fluor 555, and blue indicates staining the cell nuclei by DAPI. Quantitative analysis is made (E), data are expressed as mean±SEM (n=6), paired t test was performed; *: P<0.05, compared with the adjacent normal islets.
Immunohistochemistry showed that SUR1 expression significantly increased in insulinomas compared with the adjacent islets (Fig. 3A and B), which was also confirmed by immunofluorescence staining (Fig. 4A and B). In contrast, MRP1 expression significantly decreased in insulinomas compared with the adjacent islets (Fig. 3C and D), which was also confirmed by immunofluorescence staining (Fig. 4C and D). The quantitative analysis (Fig. 3E and 4E) showed that the alteration of SUR1 and MRP1 was statistically significant (P<0.05).
Discussion
Insulinoma presents a variety of hypoglycemia symptoms including adrenergic, cholinergic and neuroglycopenic,[1]and they are easily mistaken as cardiovascular disorders, hysteria or neurological deficits. Hence there are missed cases frequently, and in this study, the longest duration of the disease was 10 years. Because recurrenthypoglycemia may lead to brain damage, it has crucial clinical relevance for the early diagnosis and treatment of insulinomas. In the present study, about two-thirds of the tumors were larger than 2 cm in diameter. However it was difficult to be localize small tumors less than 2 cm in diameter, and blind distal pancreatomies were not recommended.[1]Insulin levels were inadequately suppressed in the insulinoma patients at fasting status; however, prolonged OGTT tests showed that the insulin peak time and insulin increase were within normal ranges. It is of great interest to explore the underlying mechanisms of insulin hypersecretion by insulinoma, which might help to find interesting aspects in the timely diagnosis of insulinoma or to develop a drug for insulinomas that fail to be localized.
SUR1 is an important MRP that is associated with abnormal insulin secretion. The normal insulin secretion by beta-cells is controlled by a KATP channel, which is a hetero-octameric complex composed of four SUR1 subunits and four Kir6.2 subunits.[9-13]SUR1 plays a crucial role in modulating insulin secretion, and the mutations of SUR1 have been found to be associated with various conditions of inappropriate insulin secretion.[14-16]In this study, we found that SUR1 expression increased in insulinomas compared with the adjacent islets, which was consistent with the previous findings by Sempoux et al.[7]These data suggested that the hypersecretion of insulin by insulinoma might be, at least partially, due to the overexpression of SUR1.
SUR1 is also the best known candidate gene of the congenital persistent hyperinsulinemic hypoglycemia of infancy.[17-21]Strikingly, the alteration of SUR1 is not only responsible for the oversecretion of insulin, but also associated with insufficient insulin secretion. Recently, SUR1 mutations have also to be linked to the pathogenesis of adult diabetes[22-24]or neonatal diabetes.[25-29]The distinct SUR1 peptides may, therefore, plays unique roles in modulating insulin secretion.[30-32]SUR1 have been the targets of several important anti-diabetic drugs, especially sulphanylureas including glimepiride,[33-35]and the effective treatment with sulfonylureas in diabetic patients has been associated with SUR1 mutations.[36]Accordingly, further investigation on the underlying mechanisms of unexhausted insulin secretion mediated by SUR1 and other functional proteins might contribute to setting novel targets to treat the abnormal secretion of insulin such as insulinomas or diabetes millitus.
MRP1 is another MRP, which has also been identified in human pancreatic beta-cells, and associated with insulin secretion.[2,3]Since MRP1 serves as the regulator of Kir6.2 protein, the essential composition of KATP channel for insulin secretion, MRP1 and SUR1 may play a synergistic effect in inducing insulin secretion.[2,8]Previously it was unclear about the alteration of MRP1 in insulinoma of human subjects. In contrast to SUR1, MRP1 expression was decreased in insulinomas compared with the adjacent islets, suggesting that MRP1 did not play a crucial role in mediating the high insulin secretion by insulinomas.
The down-regulation of MRP1 in insulinomas might reflect an adaptive mechanism controlling the excessive secretion of insulin due to the enriched SUR1. It is not feasible to obtain normal tissues of islets from healthy volunteers, so it was uncertain in our study whether the expression of MRP1 in the adjacent normal islets was significantly altered in the insulinoma patients. However, MRP1 did express well in the adjacent normal islets. Thus the response of MRP1 in the loop of negative feedback seemed to be limited in the local over-secretary tumor cells. However, this feedback was obviously very limited to prevent hypoglycemia in these insulinoma patients. Dysregulation of insulin secretion in children with congenital hyperinsulinism might shift to the late development of diabetes;[5,37,38]however, insulin secretion by insulinomas was progressively aggressive and seemed to be never exhausted. Further investigation in vitro or in animal models is needed to be focused on how these multidrug resistance proteins or other criminal proteins modulate insulin secretion, which might be helpful to the diagnosis and treatment of insulinomas. It is good for developing novel anti-diabetic target by using the characteristics of active insulin secretion by insulinomas.
In conclusion, SUR1 and MRP1 are two multidrug resistance proteins that are involved in a variety of abnormal insulin secretion conditions. The enrichment of SUR1 in insulinomas might, at least partially, account for the over secretion of insulin. In contrast, MRP1, which is down-regulated, seems to be involved in rescuing the hypernomic insulin secretion, but insufficiently. Functional studies of these proteins might provide insights into the physiological and pathophysiological changes in insulinoma, which might help to treat insulinomas by efficiently inhibiting the high secretion, or to set up new targets to treat diabetes by using the trait of unexhausted secretion if it could be regulated appropriately.
Acknowledgements
We acknowledge the following colleagues at the First Affiliated Hospital, Zhejiang University School of Medicine for their generous help in completing this study: Dr. Ming-Zhi Xu for her help and suggestions. Dr. Hai-Yang Xie from Key Laboratory of Multi-organ Transplantation of Ministry of Public Health for his technical assistant, Dr. Shao-Hua Shi, Hua Guo, Xiao-FengTang from Department of Hepatobiliary and Pancreatic Surgery for collecting the insulinoma samples.
Funding:None.
Ethical approval:Not needed.
Contributors:ZHL proposed the study. LCJ and ZHL wrote the first draft. ZHL analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZHL is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Guettier JM, Gorden P. Insulin secretion and insulinproducing tumors. Expert Rev Endocrinol Metab 2010;5:217-227.
2 Koehn J, Fountoulakis M, Krapfenbauer K. Multiple drug resistance associated with function of ABC-transporters in diabetes mellitus: molecular mechanism and clinical relevance. Infect Disord Drug Targets 2008;8:109-118.
3 Bryan J, Aguilar-Bryan L. The ABCs of ATP-sensitive potassium channels: more pieces of the puzzle. Curr Opin Cell Biol 1997;9:553-559.
4 Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP 4th, Boyd AE 3rd, González G, et al. Cloning of the beta cell highaffinity sulfonylurea receptor: a regulator of insulin secretion. Science 1995;268:423-426.
5 Gussinyer M, Clemente M, Cebrián R, Yeste D, Albisu M, Carrascosa A. Glucose intolerance and diabetes are observed in the long-term follow-up of nonpancreatectomized patients with persistent hyperinsulinemic hypoglycemia of infancy due to mutations in the ABCC8 gene. Diabetes Care 2008;31: 1257-1259.
6 Tarasov AI, Nicolson TJ, Riveline JP, Taneja TK, Baldwin SA, Baldwin JM, et al. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes 2008;57:1595-1604.
7 Sempoux C, Guiot Y, Dahan K, Moulin P, Stevens M, Lambot V, et al. The focal form of persistent hyperinsulinemic hypoglycemia of infancy: morphological and molecular studies show structural and functional differences with insulinoma. Diabetes 2003;52:784-794.
8 Conseil G, Deeley RG, Cole SP. Role of two adjacent cytoplasmic tyrosine residues in MRP1 (ABCC1) transport activity and sensitivity to sulfonylureas. Biochem Pharmacol 2005;69:451-461.
9 Bellanné-Chantelot C, Saint-Martin C, Ribeiro MJ, Vaury C, Verkarre V, Arnoux JB, et al. ABCC8 and KCNJ11 molecular spectrum of 109 patients with diazoxide-unresponsive congenital hyperinsulinism. J Med Genet 2010;47:752-759.
10 Shimomura K, de Nanclares GP, Foutinou C, Caimari M, Castaro L, Ashcroft FM. The first clinical case of a mutation at residue K185 of Kir6.2 (KCNJ11): a major ATP-binding residue. Diabet Med 2010;27:225-229.
11 Sandal T, Laborie LB, Brusgaard K, Eide SA, Christesen HB, Søvik O, et al. The spectrum of ABCC8 mutations in Norwegian patients with congenital hyperinsulinism of infancy. Clin Genet 2009;75:440-448.
12 Smith AJ, Partridge CJ, Asipu A, Mair LA, Hunter M, Sivaprasadarao A. Increased ATP-sensitive K+channel expression during acute glucose deprivation. Biochem Biophys Res Commun 2006;348:1123-1131.
13 Miki T, Nagashima K, Seino S. The structure and function of the ATP-sensitive K+channel in insulin-secreting pancreatic beta-cells. J Mol Endocrinol 1999;22:113-123.
14 Cohen MM Jr. Persistent hyperinsulinemic hypoglycemia of infancy. Am J Med Genet A 2003;122A:351-353.
15 Chistiakov DA, Potapov VA, Khodirev DC, Shamkhalova MS, Shestakova MV, Nosikov VV. Genetic variations in the pancreatic ATP-sensitive potassium channel, beta-cell dysfunction, and susceptibility to type 2 diabetes. Acta Diabetol 2009;46:43-49.
16 Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 2007;81:375-382.
17 James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet 2009;46:289-299.
18 Damaj L, le Lorch M, Verkarre V, Werl C, Hubert L, Nihoul-Fékété C, et al. Chromosome 11p15 paternal isodisomy in focal forms of neonatal hyperinsulinism. J Clin Endocrinol Metab 2008;93:4941-4947.
19 Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest 2008;118: 2877-2886.
20 Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 2007;56: 2339-2348.
21 Cartier EA, Conti LR, Vandenberg CA, Shyng SL. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci U S A 2001;98:2882-2887.
22 Wang F, Han XY, Ren Q, Zhang XY, Han LC, Luo YY, et al. Effect of genetic variants in KCNJ11, ABCC8, PPARG and HNF4A loci on the susceptibility of type 2 diabetes in Chinese Han population. Chin Med J (Engl) 2009;122:2477-2482.
23 Hartemann-Heurtier A, Simon A, Bellanné-Chantelot C, Reynaud R, Cavé H, Polak M, et al. Mutations in the ABCC8 gene can cause autoantibody-negative insulin-dependent diabetes. Diabetes Metab 2009;35:233-235.
24 Laukkanen O, Pihlajamäki J, Lindström J, Eriksson J, Valle TT, Hämäläinen H, et al. Polymorphisms of the SUR1 (ABCC8) and Kir6.2 (KCNJ11) genes predict the conversion from impaired glucose tolerance to type 2 diabetes. The Finnish Diabetes Prevention Study. J Clin Endocrinol Metab 2004;89:6286-6290.
25 Støy J, Greeley SA, Paz VP, Ye H, Pastore AN, Skowron KB, et al. Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatr Diabetes 2008;9:450-459.
26 de Wet H, Rees MG, Shimomura K, Aittoniemi J, Patch AM, Flanagan SE, et al. Increased ATPase activity produced bymutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc Natl Acad Sci U S A 2007;104:18988-18992.
27 Vaxillaire M, Dechaume A, Busiah K, Cavé H, Pereira S, Scharfmann R, et al. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes 2007;56: 1737-1741.
28 Stanik J, Gasperikova D, Paskova M, Barak L, Javorkova J, Jancova E, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab 2007;92:1276-1282.
29 Babenko AP, Polak M, Cavé H, Busiah K, Czernichow P, Scharfmann R, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006;355: 456-466.
30 Pratt EB, Yan FF, Gay JW, Stanley CA, Shyng SL. Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure. J Biol Chem 2009; 284:7951-7959.
31 Klupa T, Kowalska I, Wyka K, Skupien J, Patch AM, Flanagan SE, et al. Mutations in the ABCC8 (SUR1 subunit of the K(ATP) channel) gene are associated with a variable clinical phenotype. Clin Endocrinol (Oxf) 2009;71:358-362.
32 Flanagan SE, Clauin S, Bellanné-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat 2009;30:170-180.
33 Aquilante CL. Sulfonylurea pharmacogenomics in Type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther 2010;8:359-372.
34 Xu H, Murray M, McLachlan AJ. Influence of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of sulfonylurea drugs. Curr Drug Metab 2009;10: 643-658.
35 Darendeliler F, Fournet JC, Baş F, Junien C, Gross MS, Bundak R, et al. ABCC8 (SUR1) and KCNJ11 (KIR6.2) mutations in persistent hyperinsulinemic hypoglycemia of infancy and evaluation of different therapeutic measures. J Pediatr Endocrinol Metab 2002;15:993-1000.
36 Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT; et al. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care 2008;31:204-209.
37 Abdulhadi-Atwan M, Bushman J, Tornovsky-Babaey S, Perry A, Abu-Libdeh A, Glaser B, et al. Novel de novo mutation in sulfonylurea receptor 1 presenting as hyperinsulinism in infancy followed by overt diabetes in early adolescence. Diabetes 2008;57:1935-1940.
38 Grimberg A, Ferry RJ Jr, Kelly A, Koo-McCoy S, Polonsky K, Glaser B, et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes 2001;50:322-328.
September 30, 2010
Accepted after revision December 31, 2010
Author Affiliations: Department of Endocrinology (Li CJ, Zhou HL and Li WP), Department of Pathology (Li J and Yao HT), and Key Laboratory of Multi-organ Transplantation of Ministry of Public Health (Su R), First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China
Hua-Li Zhou, MD, Department of Endocrinology, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236866; Fax: 86-571-87236874; Email: lhlzhou@ hotmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Laparoscopic liver resection for benign and malignant liver tumors
- Assessment of tumor vascularization with functional computed tomography perfusion imaging in patients with cirrhotic liver disease
- Expression oflamino acid transport system 1 and analysis of iodine-123-methyltyrosine tumor uptake in a pancreatic xenotransplantation model using fused high-resolution-micro-SPECT-MRI
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Atypical focal nodular hyperplasia of the liver
- Cholangiocarcinoma: principles and current trends
