Relationship between model for end-stage liver disease score and left ventricular function in patients with end-stage liver disease
2011-07-07FuRongSunYingWangBingYuanWangJingTongDaiZhangandBingChang
Fu-Rong Sun, Ying Wang, Bing-Yuan Wang, Jing Tong, Dai Zhang and Bing Chang
Shenyang, China
Relationship between model for end-stage liver disease score and left ventricular function in patients with end-stage liver disease
Fu-Rong Sun, Ying Wang, Bing-Yuan Wang, Jing Tong, Dai Zhang and Bing Chang
Shenyang, China
BACKGROUND:Decreased cardiac contractility has been observed in cirrhosis, suggesting a latent cardiomyopathy in these patients. This study was designed to evaluate left ventricular structure and function in patients with end-stage liver disease by the model for end-stage liver disease (MELD) scoring system.
METHODS:We recruited 82 patients (72 male, 10 female; mean age 50.3±8.9 years) with end-stage liver disease who underwent orthotopic liver transplantation between January 2002 and May 2008. Seventy-eight patients had cirrhosis and 4 had primary liver cancer. Patients were categorized into three groups on the basis of MELD score:≤9 (27 patients, 33%); 10-19 (40, 49%); and≥20 (15, 18%). The relationship between MELD score and cardiac structure and function was determined. Preoperative assessments of blood biochemistry, blood coagulation, serum virology, echocardiography and electrocardiography were performed.
RESULTS:MELD score was positively correlated with enlarged left atrial diameter, increased interventricular septum thickness (IVST), increased aortic flow, corrected QT interval (QTc) extension and cardiac output (P=0.033, 0.002, 0.000, 0.000 and 0.009, respectively). International normalized ratio also had a correlation with the above parameters and enlarged left ventricular end-diastolic diameter (P=0.043, 0.010, 0.000, 0.001, 0.016 and 0.008, respectively). Serum creatinine was positively correlated with IVST (r=0.257,P=0.020), but negatively correlated with early maximal ventricular filling velocity/late diastolic or atrial velocity ratio (r=-0.300,P=0.006). A difference of QTc >440 ms among the three groups was statistically significant (χ2=9.791,P=0.007).
CONCLUSIONS:Abnormalities in cardiac structure and function are common in patients with end-stage liver disease. MELD score is a practically useful approach for the assessment of cardiac function in such patients.
(Hepatobiliary Pancreat Dis Int 2011; 10: 50-54)
left ventricular dysfunction; liver cirrhosis; liver disease; model for end-stage liver disease score
Introduction
Patients with liver cirrhosis frequently display clinical features of hyperdynamic circulation and cardiovascular dysfunctions.[1-4]The commonly observed cardiovascular disorders associated with liver cirrhosis are portal hypertension and hyperdynamic circulation status. Patients with these disorders usually present with symptoms that are related to increased cardiac output (CO), increased left ventricular ejection fraction (LVEF), reduced total systemic vascular resistance, reduced mean arterial pressure, decreased blood vessel contraction, and decreased ventricular contractility. Collectively, these cardiac abnormalities are termed cirrhotic cardiomyopathy (CCM).[5,6]Although the clinical manifestations of CCM may vary among patients, four pathophysiological features have been identified as mechanisms responsible for CCM: 1) increased baseline CO; 2) attenuated systolic contraction and diastolic relaxation; 3) electrophysiological abnormalities, and 4) reduced cardiac response to direct β-adrenergic receptor stimulation.[7]If CCM is unrecognized or left untreated, or inappropriately treated, it may lead to heart failure.[5,6,8]However, only scattered clinical studies have specifically addressed the issues of CCM.
In this study, we aimed to evaluate the role of the model for end-stage liver disease (MELD) scoring system in the assessment of cardiac structure and function in patients with end-stage liver disease.
Methods
Patients
From January 2002 to May 2008, a total of 82 patients who underwent orthotopic liver transplantation were recruited to this study, and all patients provided consent. Patients with incomplete clinical data, pre-existing heart disease or respiratory disease were excluded. Of these 82 patients, 72 were male and 10 female with a mean age of 50.3±8.9 years. The distribution of etiological factors is shown in Table 1. In cases of primary liver cancer, there were three cases of hepatocellular carcinoma including one whose risk factors were attributed to HBV and HCV co-infection. Diagnosis of liver cirrhosis and hepatocellular carcinoma is based on the history, imaging characteristics and histology.
Clinical data and MELD score
Based on MELD score, patients were categorized into three groups: MELD ≤9 (27/82, 33%), MELD 10-19 (40/82, 49%), and MELD ≥20 (15/82, 18%). A full panel of blood biochemistry, including full blood count, liver function, kidney function and coagulation, was performed in all patients upon admission. MELD score was calculated using the following equation:
MELD score=3.8×LnTBIL (mg/dl)+11.2×LnINR+9.6× LnCr (mg/dl)+6.4×etiology (0 points for biliary or alcoholic causes, and 1 point for other causes), where Ln is the natural logarithm, TBIL is total bilirubin, INR is the international normalized ratio of prothrombin time, and Cr is serum creatinine. The method of MELD calculation has been previously reported.[9,10]
Measurement of cardiac dimensions and functions by echocardiography
Two-dimensional echocardiography (Philips iE33, Holland) was performed to measure cardiac dimensions and systolic and diastolic functions. The measurements were performed of left atrial diameter (LAD), interventricular septum thickness (IVST), left ventricular enddiastolic diameter (LVEDD), left ventricular posterior wall thickness (LVPWT), aortic flow (AF), LVEF, E/A ratio (E, early maximal ventricular filling velocity; A, late diastolic or atrial velocity) and stroke volume (SV).
All cardiac electrophysiological indices were measured by electrocardiography (Kenz-Cardico 1201, Japan): heart rate (HR), PR interval (PRI), QRS interval (QRSI), and corrected QT interval (QTc). CO=SV×HR.
Statistical analysis
Statistical analyses were made using SPSS version 16.0. Data were presented as mean±SD. Differences among groups were compared by analysis of variance (ANOVA), the LSD test and Chi-square test. Correlations between selected variables were assessed by Pearson's correlation coefficient. A P value of less than 0.05 was considered statistically significant.
Results
The etiological factors in each group are listed in Table 1. Differences in age among the groups were not statistically significant (ANOVA, F=2.834, P=0.065). HBV-related cirrhosis was the most common indication for liver transplantation in this series, and more than half of the subjects had a MELD score of 10-19. The mean aspartate aminotransferase (AST) concentration, alanine aminotransferase (ALT) concentration and AST/ALT ratio showed a gradual increase, but differences among the groups were not statistically significant (Table 1).
MELD score was positively correlated with LAD, IVST, AF, QTc and CO. INR also had a correlation with the above parameters and LVEDD. Cr was only associated with IVST and E/A. TBIL was associated with AF, QTc and CO (Table 2). The mean MELD score was associated with a gradual increase in the mean LAD, IVST, LVEDD, LVPWT, AF, QTc, HR and CO, and the differences between each of these parameters in different MELD groups were statistically significant except for HR (Table 3).
Thirty-two subjects had a prolonged QTc (39%, 32/82). The proportions of QTc >440 ms were 18.5%(5/27) in the MELD ≤9 group, 42.5% (17/40) in the MELD 10-19 group and 66.7% (10/15) in the MELD≥20 group. Differences among these groups werestatistically significant (χ2=9.791, P=0.007). The mean QTc increased with increasing MELD. When the etiology was taken into consideration, QTc prolongation (>440 ms) was found in 39.2% (29/74) of patients with post-viral cirrhosis, and in 50.0% (2/4) of patients with alcoholic liver cirrhosis. The proportion of E/A ≤1 in all patients was 48.8% (40/82), and the proportions in the three groups were 51.9% (14/27), 37.5% (15/40) and 73.3% (11/15) respectively. There were no statistically significant differences among the groups.
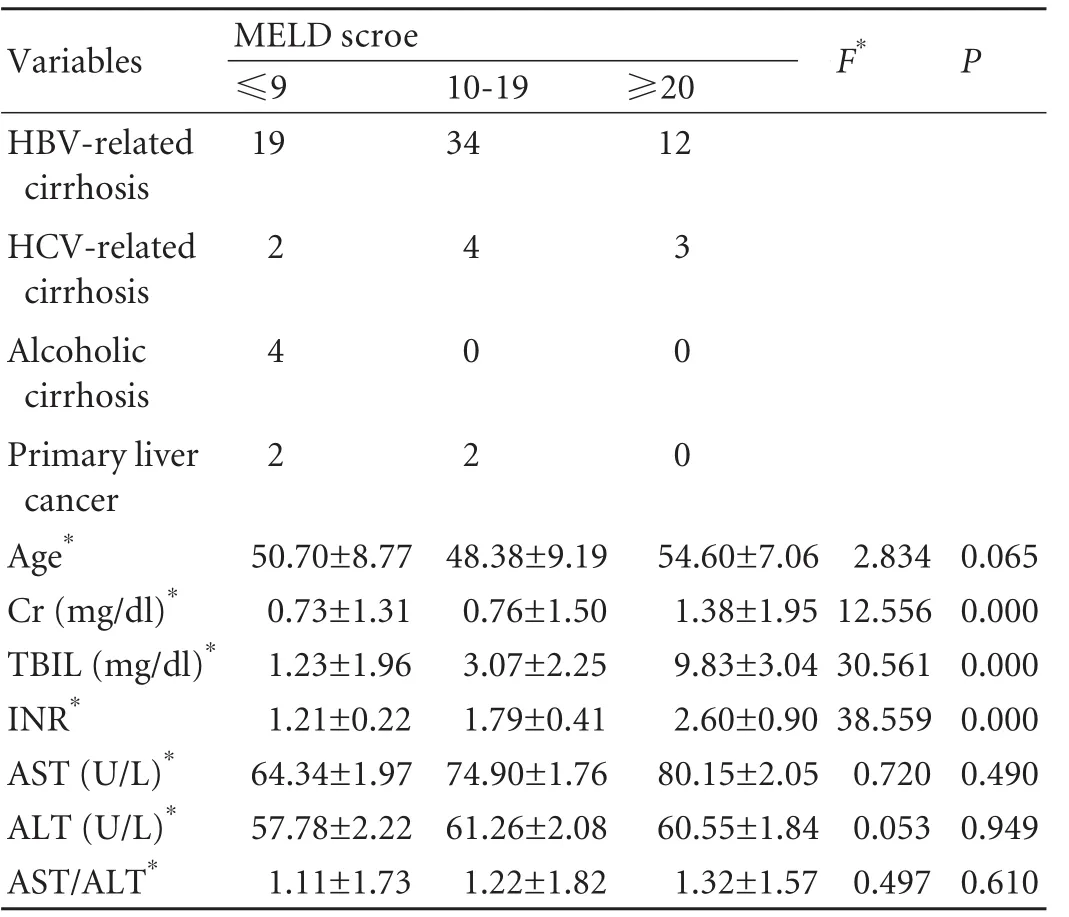
Table 1. Basic information of each group

Table 2. Relationship between MELD score and its components and left ventricular functions
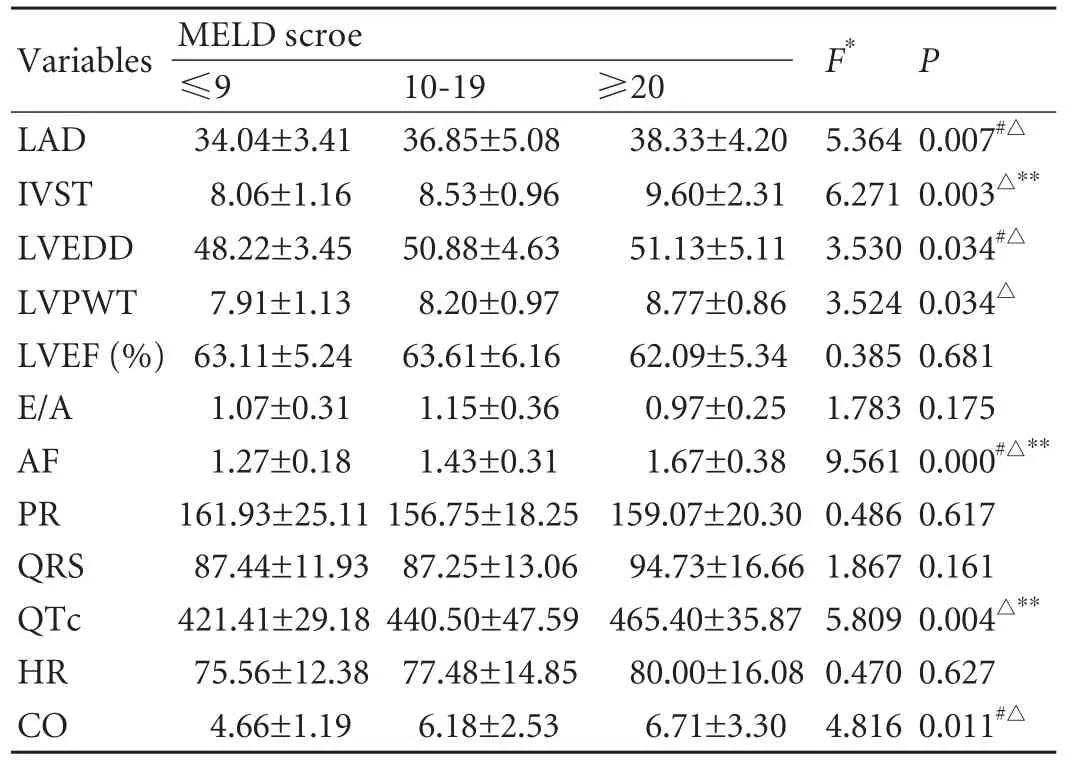
Table 3. Distribution of parameters of left ventricular structure and function
Discussion
MELD was originally used to determine the priority of organ transplant recipients.[9]It was later modified to estimate the severity of end-stage liver disease in pretransplant patients, i.e. increased MELD score was closely associated with increased mortality.[10]Recently, the MELD scoring system has been recognized as an ideal approach for evaluating end-stage liver disease.
CCM often has an insidious course in patients with cirrhosis, and no specific morphological changes or only mild ventricular enlargement may be present.[11,12]With the progression of liver disease, there may be an increased incidence of cardiac dysfunction and structural abnormality.[13]Patients may present with increased ventricular diameters, increased thickness of the left ventricular wall, and decreased E/A.[14-16]When patients with end-stage liver disease are exposed to stressful conditions such as exercise and surgery, CCM may become symptomatic.[17,18]Some abnormal changes found in the cardiovascular system of patients who undergo liver transplant may be manifestations of CCM.[17]
The histological changes of CCM include myocardial hypertrophy, intercellular edema, intracellular edema and cellular injury.[19]These changes are observed more often in the left heart than in the right heart,[13,18]and increased wall thickness of the left ventricle (as evidenced by increased IVST, LVPWT or both) was found in six cases, accounting for a small part (7.3%) of the series. This result suggests that structural changes of the heart in patients with end-stage liver disease are not significant, though they may have already presented systolic and/or diastolic dysfunction. These structural and functional changes may be due to a hyperdynamic circulation status and a decreased diastolic compliance of the left ventricle, which result in elevated LVEDD and ventricular pressure, compensatory left ventricular enlargement and increased pressure in the left atrium. If the hyperdynamic status persists for a long period, the structure and function of the right heart may change. As peripheral vascular dilation can significantly reducethe left ventricular after-load in patients with mid- and late-stage liver disease, the cardiac dysfunction may be latent or mild. Peripheral vascular dilation is regarded as a "self-treatment mechanism" of end-stage liver disease; it may thus mask some of the serious manifestations of cardiac abnormalities.[19]
In the current study, virus infection (HBV, HCV and HBV and HCV co-infection) was the most common etiology for liver cirrhosis, accounting for 90.2% (74/82) of all patients, while alcoholic cirrhosis only accounted for 4.9% (4/82) of the spectrum. Nevertheless, QTc prolongation was found in only 39% of patients with post-viral cirrhosis, but in 50% of patients with alcoholic cirrhosis. These data are partially consistent with previous reports that QTc prolongation is more common in alcoholic cirrhotics than in post-viral cirrhotics (15/18, 83.3% vs. 6/30, 20.0%).[20]Our data suggest that CCM is not rare in patients with postviral cirrhosis and cardiac dysfunctions are a common finding in end-stage liver disease regardless of the etiology. A large sample size is needed to investigate the difference of morbidity between patients with alcoholic cirrhosis and with post-viral cirrhosis.
QTc is a widely used indicator for ventricular depolarization and repolarization. A prolongation of QTc has been shown in patients with end-stage liver disease and it is considered to be a component of CCM. Prolongation and variation in QTc can affect cardiac rhythm and cause serious cardiac conditions such as ventricular fibrillation and sudden death. Liver transplantation can improve QTc in patients with QTc prolongation.[20,21]
E/A is often used to evaluate left ventricular diastolic compliance. In this study, nearly 50% of patients showed an E/A of ≤1 and the proportion exceeded 70% in the MELD ≥20 group. These figures indicate a high frequency of left ventricular diastolic dysfunction in patients with end-stage liver disease, especially in those with a high MELD score.
Ascites is one of the reported factors that may affect E/A. Valeriano et al[14]reported that the E/A ratio was decreased in patients with ascites compared to those without (0.9±0.2 vs. 1.3±0.4,P<0.05). An E/A ratio of ≤1 was proposed as a predictor of slow clearance of ascites.[22]In addition, the correlation between E/A and Cr in our study indicates a connection between left ventricular diastolic compliance and renal function. Salvetti et al[23]reported a decreased E/A in patients with chronic kidney disease such as those on hemodialysis and with chronic renal failure. Taken together, these results indicate that E/A can be affected not only by cardiac function but also by liver function and renal function.
In summary, there is progressive damage in cardiac structure and function in patients with end-stage liver disease. However, these changes may follow a silent course, hence making early diagnosis and appropriate management difficult. Full-blown CCM carries some risk of heart failure or sudden death if not appropriately treated.[18,24]Furthermore, CCM may be a mechanism involved in the pathogenesis of hepatorenal syndrome, ascites and variceal bleeding, and it can be a critical factor preventing liver transplantation.[14,16]Thus, prompt recognition and appropriate management of CCM are an integral part of the management for patients with endstage liver disease awaiting liver transplantation.
Acknowledgment
We thank Dr. Liang Qiao of the University of Sydney, Australia, for proofreading the manuscript.
Funding:This study was supported by a grant from the Science and Technology Bureau of Liaoning Province, China (2007225011-1).Ethical approval:Not needed.
Contributors:SFR and WBY proposed the study and worte the first draft. SFR, WY and ZD analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. WBY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec's cirrhosis. J Clin Invest 1953;32:1025-1033.
2 Abelmann WH, Kowalski HJ, McNeely WF. The hemodynamic response to exercise in patients with Laennec's cirrhosis. J Clin Invest 1955;34:690-695.
3 van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol 1996;24:747-752.
4 Piscaglia F, Valgimigli M, Rapezzi C, Ferlito M, Gaiani S, Siringo S, et al. Left ventricular volumes in liver cirrhosis. Dig Liver Dis 2000;32:392-397.
5 Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol 2006; 3:329-337.
6 Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology 1996;24:451-459.
7 Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol 2002;26:842-847.
8 Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart 2002;87:9-15.
9 Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts.Hepatology 2000;31:864-871.
10 Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33: 464-470.
11 Liu H, Gaskari SA, Lee SS. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol 2006;12:837-842.
12 Wong JL, Arango-Viana JC, Squires T. Heart, liver and spleen pathology in chronic alcohol and drug users. J Forensic Leg Med 2008;15:141-147.
13 Pozzi M, Redaelli E, Ratti L, Poli G, Guidi C, Milanese M, et al. Time-course of diastolic dysfunction in different stages of chronic HCV related liver diseases. Minerva Gastroenterol Dietol 2005;51:179-186.
14 Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, et al. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol 2000; 95:3200-3205.
15 De Marco M, Chinali M, Romano C, Benincasa M, D'Addeo G, D'Agostino L, et al. Increased left ventricular mass in preliver transplantation cirrhotic patients. J Cardiovasc Med (Hagerstown) 2008;9:142-146.
16 Woo JJ, Koh YY, Kim HJ, Chung JW, Chang KS, Hong SP. N-terminal pro B-type natriuretic peptide and the evaluation of cardiac dysfunction and severity of disease in cirrhotic patients. Yonsei Med J 2008;49:625-631.
17 Alexander J, Mishra P, Desai N, Ambadekar S, Gala B, Sawant P. Cirrhotic cardiomyopathy: Indian scenario. J Gastroenterol Hepatol 2007;22:395-399.
18 Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut 2001;49:268-275.
19 Milani A, Zaccaria R, Bombardieri G, Gasbarrini A, Pola P. Cirrhotic cardiomyopathy. Dig Liver Dis 2007;39:507-515.
20 Genovesi S, Prata Pizzala DM, Pozzi M, Ratti L, Milanese M, Pieruzzi F, et al. QT interval prolongation and decreased heart rate variability in cirrhotic patients: relevance of hepatic venous pressure gradient and serum calcium. Clin Sci (Lond) 2009;116:851-859.
21 Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int 2003;23:243-248.
22 Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol 2009;104:2458-2466.
23 Salvetti M, Muiesan ML, Paini A, Monteduro C, Bonzi B, Galbassini G, et al. Myocardial ultrasound tissue characterization in patients with chronic renal failure. J Am Soc Nephrol 2007;18:1953-1958.
24 Ripoll C, Catalina MV, Yotti R, Olmedilla L, Pérez-Peña J, Lo Iacono O, et al. Cardiac dysfunction during liver transplantation: incidence and preoperative predictors. Transplantation 2008;85:1766-1772.
March 29, 2010
Accepted after revision July 22, 2010
Author Affiliations: Department of Gastroenterology, First Affiliated Hospital, China Medical University, Shenyang 110001, China (Sun FR, Wang Y, Wang BY, Tong J, Zhang D and Chang B)
Bing-Yuan Wang, Professor, Department of Gastroenterology, First Affiliated Hospital, China Medical University, Shenyang 110001, China (Tel: 86-24-83282900; Fax: 86-24-83282997; Email: wangby@ medmail.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Expression oflamino acid transport system 1 and analysis of iodine-123-methyltyrosine tumor uptake in a pancreatic xenotransplantation model using fused high-resolution-micro-SPECT-MRI
- Laparoscopic liver resection for benign and malignant liver tumors
- Monday blues of deceased-donor liver transplantation
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Roles of sulfonylurea receptor 1 and multidrug resistance protein 1 in modulating insulin secretion in human insulinoma
- Atypical focal nodular hyperplasia of the liver
