Effect of the Para-substituent of the Tridentate Pyridine-based Ru(II) Complex upon the Catalytic Activity in Transfer Hydrogenation*
2011-05-15YANGGang杨刚QINGDongling秦冬玲GAOHongfei高宏飞andXUNanping徐南平
YANG Gang (杨刚), QING Dongling (秦冬玲), GAO Hongfei (高宏飞) and XU Nanping (徐南平)
Effect of the-substituent of the Tridentate Pyridine-based Ru(II) Complex upon the Catalytic Activity in Transfer Hydrogenation*
YANG Gang (杨刚)**, QING Dongling (秦冬玲), GAO Hongfei (高宏飞) and XU Nanping (徐南平)
State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
ruthenium(II) complexes, tridentate, transfer hydrogenation, catalyst
1 INTRODUCTION
Transfer hydrogenation reactions using isopropanol as the hydrogen source have been the subject of the progressing research for decades [1-3]. Considerable attentions were focused on the exploration of new chelating ligands for construction of complex catalysts. Tridentate pincer complexes with NCN and PCP ligands stabilize the catalytic metal center with unusual geometries, and subsequently were widely investigated not only in the hydrogen transfer [4] but also in reactions such as Kharasch addition [5], Heck reaction [6] and dehydrogenation[7]. It is generally thought that the metal-carbonbond in these complexes is responsible for the high thermal stability [7, 8]. Attractively, also at high temperature, the reported neutral tridentate ligands with pyridine as the skeleton [9], in which the metal-nitrogen bonds formed, were used in-cycloalkylation [10] with comparatively good catalytic stability. As an extension, the catalytic activities of pincer Pd (II) complexes withligands in Heck coupling were investigated [11].
The geometric enlargement of the complex catalyst facilitates its recovery. Therefore, the complex is preferentially tailored to anchor on the support such as a dendrimer [12]. Report [13] on dendritic catalysts concluded that the average catalytic activity usually went down, due to the geometrically crowdedness closely around the anchoring point. It might also be supposed that the dendritic substrate exerts an electronic influence on the catalysis as the functional groups near the metallic active site do. The choice of a group to link the supporting dendrimer and the complex seems important for both the chemical stability and the catalytic activity. One of our research projects on the dendritic catalysts concerned three tridentate pincer pyridine Ru(II) complexes different due to their change of a group at the para position of the pyridyl ring of the ligand. The comparative study of their catalytic activities was therefore experimentally carried out in anticipation that the results would help in making clear the electronic effects. In this paper, the syntheses of the complexes were introduced, and the effects of the substituents on the catalytic activity were discussed.
2 EXPERIMENTAL
2.1 General
All solvents were dried using common techniques and all reactions carried out under N2. RuCl2(PPh3)3was purchased from Aldrich Chemical Co. Ligand L1 [4-bromo-2,6-bis(,-diethylaminomethylene)pyridine] [11], L2 [2,6-bis(,-diethylaminomethylene)pyridine] and compound 1 (4-allyloxy-pyridyl-2,6-dicarboxylate) were prepared according to Refs. [9, 14]. Elemental analyses were performed with an Elementar Vario EL III instrument.1HNMR was recorded on Bruker AV-300 instrument and the TOF-MS was recorded on Agilent 1100 LC/MSD. The electrochemical studies were performed with Solartron SI 1287 instrument. Gas chromatography (GC) measurements for catalytic experiments were performed using a SP-6800A apparatus with PEG-20M, 30 m×0.32 mm×0.25 μm.
2.2 Preparation of (4-allyloxy-2,6-bis(N,N- diethylaminomethylene)pyridine) L3
The synthetic route for L3 is given in Fig. 1. Compound 1 (5 g, 17.9 mmol) was dissolved in pure ethanol (220 ml) at 0 °C. NaBH4(5.4 g, 143 mmol) was then added in small amount in 0.5 h. The reaction mixture was stirred for another 1 h at 0 °C and then refluxed overnight. After the solvent was removed, 100 ml of saturated NaHCO3solution were added and the mixture was subsequently refluxed for 1 h. The solvent was evaporated and the resulted crude compound 2 was used for the next reaction. Pure 2 for NMR determination was obtained by recrystallization in acetone.1H NMR (300 MHz, CDCl3):4.47 (4 H, s), 4.67 (2 H, d), 5.42 (2 H, m), 5.29 (2 H, s), 6.04 (1 H, m),6.88 (2 H, s).
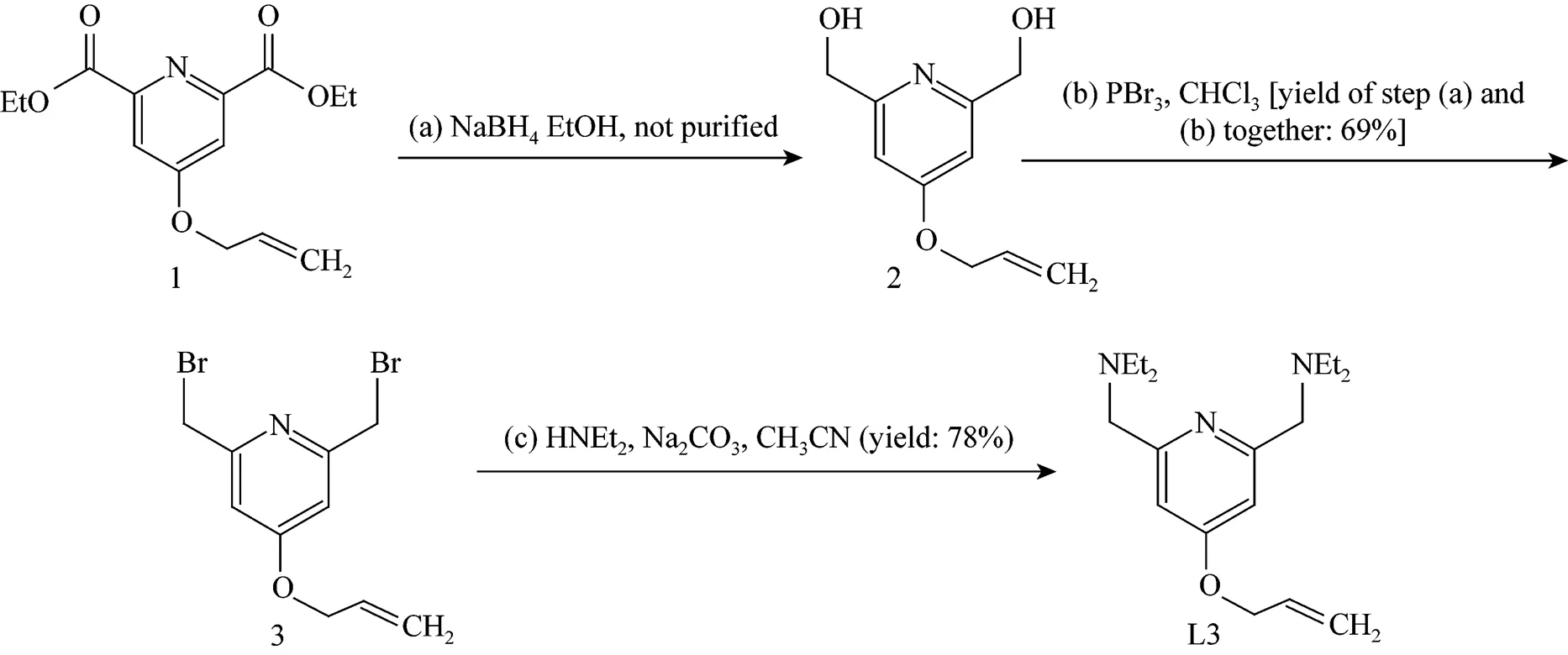
Figure 1 Synthetic route for L3
PBr3(14.6 g, 53.7 mmol) in CHCl3(70 ml) was added dropwise to the suspension of crude 2 in CHCl3(80 ml) at room temperature. The reaction mixture was refluxed overnight. Into the cooled reaction mixture was added 5% NaHCO3solution (60 ml) and stirred for another hour. The organic layer was washed with water and dried over Na2SO4. After the separation by the silica gel column chromatography with CHCl3as the eluent, compound 3 was obtained as a red oil. Yield of step a and b together: 69% (3.95 g, 12.3 mmol).1H NMR (300 MHz, CDCl3):4.47 (4 H, s), 4.62 (2 H, d), 5.42 (2 H, m), 6.04 (1 H, m), 6.90 (2 H, s).
A solution of 3 (2.89 g, 9 mmol), diethylamine (1.463 g, 20 mmol) and sodium carbonate (5.72 g, 54 mmol) in dry acetonitrile (100 ml) was stirred for 24 h at room temperature. The mixture was filtered and the solvent was removed under vacuum. The oily residue was taken up in chloroform, and the chloroform solution was washed with water and dried with Na2SO4. Evaporation under vacuum gave 2.1 g (yield: 78%) of ligand L3 as a brown oil.1H NMR (300 MHz, CDCl3):1.06 (12H, t), 2.57 (8H, q), 3.66 (4H, s), 4.59 (2H, d), 5.41 (2H, m), 6.04 (1H, m), 6.69 (2H, s).
2.3 General procedure for the synthesis of ruthenium (II) complexes
To solution of RuCl2(PPh3)3(0.60 g, 0.62 mmol) in CH2Cl2(50 ml) was added solution of 1.05 eq. of L1 in CH2Cl2(5 ml). After 30 min of stirring, the solvent was removed under vacuum. The remaining brown oil was washed with Et2O (2×10 ml), leaving complex 4 as a yellow brown solid (Fig. 2). Complexes 5 and 6 were obtained through the same approach as shown in Fig. 2.
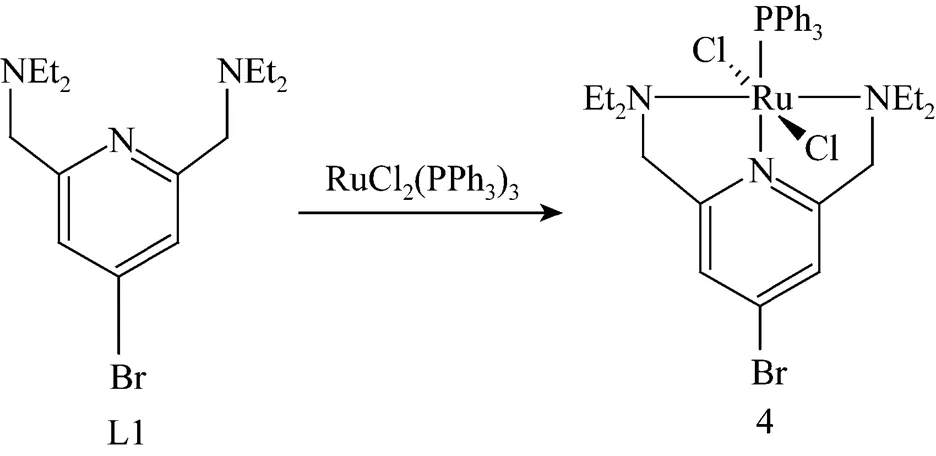
Yield: 0.44 g, 92.3%. TOF-MS:/calcd, 762.55; obsd, 761.8; Anal. calcd for C33H41BrCl2N3PRu: C 51.98, H 5.42, N 5.51; found C 51.74, H 5.48, N 5.42.
Yield: 0.385 g, 90.1%. TOF-MS:/calcd, 683.66; obsd, 683.14; Anal. calcd for C33H42Cl2N3PRu: C 57.98, H 6.19, N 6.15; found C 57.83, H 6.25, N 6.08.
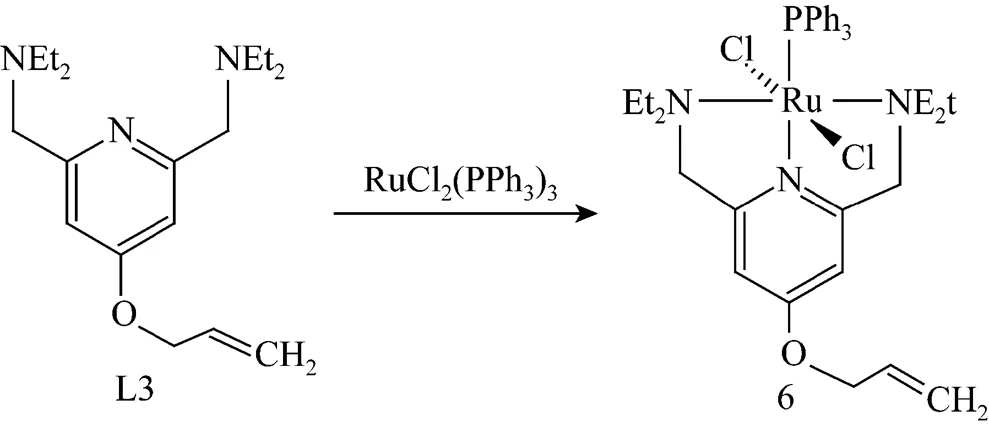
Yield: 0.422 g, 88.5%. TOF-MS:/calcd, 739.72; obsd, 738.44; Anal. calcd for C36H46Cl2N3OPRu: C 58.45, H 6.27, N 5.68; found C 58.27, H 6.38, N 5.54.
Figure 2 Synthesis of ruthenium (II) complexes (TOF-MS: time-of-flight mass spectrometry)
2.4 General procedure for transfer hydrogenation reactions
A mixture containing ketone (3.125 mmol), ruthenium complex (0.0125 mmol), and KOH (0.15 mmol) in solution in 15 ml-PrOH was heated to reflux at 82 °C for an appropriate period. The ketone conversion was equal to the product yield, and was determined by GC analysis.
3 RESULTS AND DISCUSSION
Compound 1 were reduced to compound 2 with NaBH4in anhydrous ethanol. It was possible to isolate compound 2 by crystallization in cold water, but our preliminary trial resulted in very low yields. Therefore the crude alcohol was used directly for the next step. Bromination of compound 2 with PBr3gave stable product 3 with moderate yields. In the last step the bromine was replaced using diethylamine to give L3 in dry acetonitrile. The allyloxy group was chosen herein in consideration that the group tends to donate the electron. The group is also easy to graft on the dendrimer through utilization of the double bond.
The complexes 4, 5 and 6 were explored as model catalysts in the transfer hydrogenation of ketones. As the substituents were actually limited, the ketones were chosen so that the geometric influences of the substrates were necessarily considered in evaluation of the catalytic process. The results are summarized in Table 1. In the reactions, the complexes facilitated efficiently the reduction of ketones to the corresponding alcohols with KOH as the promoter. For the three ketones, complexes 4, 5, and 6 displayed high catalytic activities. A ruthenium complex bearing pyridyl-supported pyrazolyl-imine, reported by Zhao. [15], in the transfer hydrogenation of acetophenone and cyclohexanone, the conversion rate reached 96% and 95% separately. Seen from the Table 1, the complex 6 showed higher catalytic activity. But in the transfer hydrogenation of benzophenone, the complexes reported by Zhao. [15] had higher catalytic activity 99%.
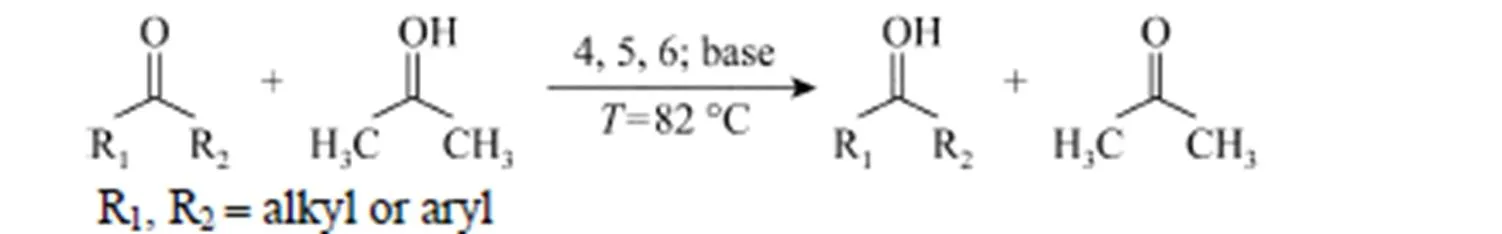
Table 1 Catalytic activity in transfer hydrogenation of ketones by ruthenium①
In general, the catalytic activity is affected by the steric and electronic effects of the nitrogen-donor ligands. The results in Table 1 suggest that the complex performance was influenced by the electronic effect of the substituent on theposition, since the steric environments of the metallic sites were the same. In complex 6, the comparatively strong electron-donating allyloxy group increased the electron density on the coordinating nitrogen atoms, and consequently on the ruthenium atom, which led to more active catalysis. In contrast, the bromine group lowered the Ru(II) activity due to its electron withdrawing property.
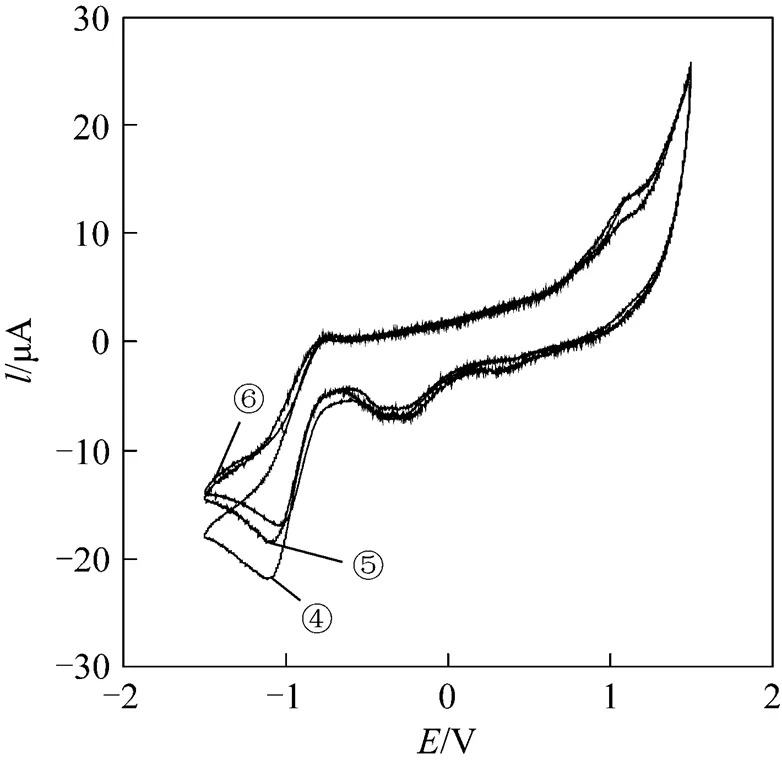
Figure 3 Cyclic voltammograms of the complexes in DMF (scan rate: 20 mV·s-1)
④ complex 4;⑤complex 5;⑥complex 6
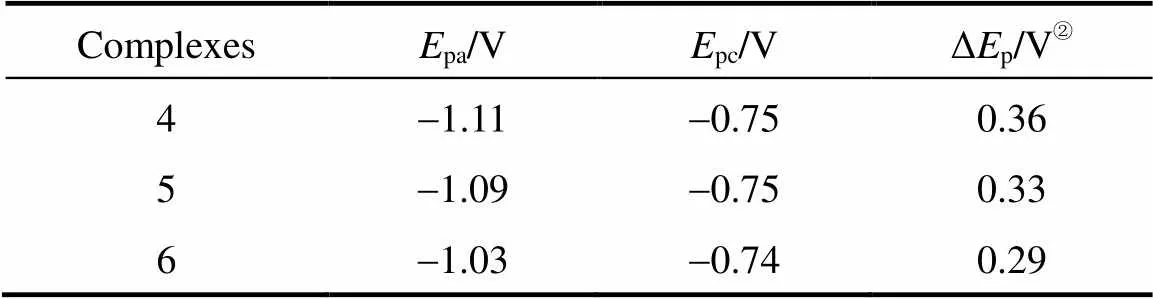
Table 2 Electrochemical parameters on the basis ofcyclic voltammograms from Fig. 3①

1 Noyori, R., Ohkuma, T., “Asymmetric catalysis by architectural and functional molecular engineering: Practical chemo- and stereoselective hydrogenation of ketones”,...., 40, 40-73 (2001).
2 Bäckval, J.E., “Transition metal hydrides as active intermediates in hydrogen transfer reactions”,.., 652, 105-111 (2002).
3 Noyori, R., Hashiguchi, S., “Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes”,..., 30, 97-102 (1997).
4 Koten, G., Dani, P., Karlen, T., Gossage, R.A., Gladiali, S., “Hydrogen-transfer catalysis with pincer-aryl ruthenium (II) complexes”,..,.., 39, 743-745 (2000).
5 van de Kuil, L.A., Grove, D.M., Gossage, R.A., Zwikker, J.W., Jenneskens, L.W., Drenth, W., van Koten, G., “Mechanistic aspects of the Kharasch addition reaction catalyzed by organonickel(II) complexes containing the monoanionic terdentate aryldiamine ligand system [C6H2(CH2NMe2)2-2,6-R-4]”,, 16, 4985-4994 (1997).
6 Chanthateyanonth, R., Alper, H., “The first synthesis of stable palladium(II) PCP-type catalysts supported on silica—Application to the Heck reaction”,...:., 201, 23-31 (2003).
7 Liu, F., Pak, E.B., Singh, B., Jensen, C.M., Goldman, A.S., “Dehydrogenation of-alkanes catalyzed by iridium “pincer” complexes: Regioselective formation of alpha-olefins”,...., 121, 4086-4087 (1999).
8 Singleton, J.T., “The uses of pincer complexes in organic synthesis”,, 59, 1837-1857 (2003).
9 Markies, B.A., Wijkens, P., Boersma, J., Kooijman, H., Veldman, N., Spek, A.L., van Koten, G., “Synthesis and structural studies of ionic monoorganopalladium(II) complexes with tridentate nitrogen-donor ligands”,, 13, 3244-3258 (1994).
10 Abbenhuis, R.A.T.M., Boersma, J., van Koten, G., “Ruthenium- complex-catalyzed-()alkylation of aromatic amines with diols. selective synthesis of-(-hydroxyalkyl)anilines of type PhNH(CH2)OH and of some bioactive arylpiperazines”,..., 63, 4282-4290 (1998).
11 Gao, H.F., Guo, X.Q., Yang, G., Xu, N.P., “Synthesis and catalytic activities of neutral palladium (II) complexes with tridentate nitrogen ligands”,,..., 27, 134-137 (2007). (in Chinese)
12 Knapen, J.W.J., van der Made, A.W., de Wilde, J.C., van Leeuwem, B.W.N.M., Wijkens, P., Grove, D.M., van koten, G., “Homogeneous catalysts based on silane dendrimers functionalized with arylnickel (II) complexes”,, 372, 659-663 (1994).
13 Kleij, A.W., Gossage, R.A., Klein Gebbink, R.J.K., Brinkmann, N., Reijerse, E.J., Kragl, U., Lutz, M., van koten, G., Spek, A.L., “A ‘dendritic effect’ in homogeneous catalysis with carbosilane-supported arylnickel (II) catalysts: Observation of active-site proximity effects in atom-transfer radical addition”,...., 122, 12112-12124 (2000).
14 Kim, I., Heui, B., Ha, C.S., Kim, J.K., Suh, H., “Preparation of silica-supported bis(imino)pyridyl iron(II) and cobalt(II) catalysts for ethylene polymerization”,, 36, 6689-6691(2003).
15 Zhao, M., Yu, Z.K., Yan, S.G., Li, Y., “Ruthenium(II) complex catalysts bearing a pyridyl-supported pyrazolyl-imine ligand for transfer hydrogenation of ketones”,, 694, 3068-3075 (2009).
16 Yi, Q.F., Zhang, J.J., Huang, W., Liu, X.P., “Electrocatalytic oxidation of cyclohexanol on a nickel oxyhydroxide modified nickel electrode in alkaline solutions”,.., 8, 1017-1022 (2007).
17 Richel, A., Demonceau, A., Noels, A.F., “Electrochemistry as a correlationtool with the catalytic activities in [RuCl2(p-cymene)(PAr3)]-catalysed Kharasch additions”,., 47, 2077-2081 (2006).
** To whom correspondence should be addressed. E-mail: yanggang@njut.edu.cn
2010-04-21,
2010-12-17.
the National Natural Science Foundation of China (20576052), the Joint Innovation Fund of Jiangsu Province (BY2009107) and the National Basic Research Program of China (2003CB615707).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effect of Boundary Layers on Polycrystalline Silicon Chemical Vapor Deposition in a Trichlorosilane and Hydrogen System*
- Experimental and CFD Study on the Role of Fluid Flow Pattern onMembrane Permeate Flux
- Separation of Eu3+ Using a Novel Dispersion Combined LiquidMembrane with P507 in Kerosene as the Carrier*
- Fabrication of SPES/Nano-TiO2 Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
- Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
- Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
