Preparation of WC@TiO2 Core-shell Nanocomposite andIts Electrocatalytic Characteristics*
2011-05-15LIGuohua李国华CHENDan陈丹YAOGuoxing姚国新SHIBinbin施斌斌andMAChunan马淳安
LI Guohua (李国华), CHEN Dan (陈丹), YAO Guoxing (姚国新), SHI Binbin (施斌斌) and MA Chun’an (马淳安)
Preparation of WC@TiO2Core-shell Nanocomposite andIts Electrocatalytic Characteristics*
LI Guohua (李国华)**, CHEN Dan (陈丹), YAO Guoxing (姚国新), SHI Binbin (施斌斌) and MA Chun’an (马淳安)
State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technology, Hangzhou 310032, China Research Center of Nano Science & Technology, School of Chemical Engineering & Materials Science, Zhejiang University of Technology, Hangzhou 310032, China
Monotungsten carbide and titania nanocomposite with core-shell (WC@TiO2) structure was prepared by a new approach of spray drying and reduction-carbonization reaction, with titania nanopowder and ammonium metatungstate as precursors, methane as carbon source, and hydrogen as reduction gas. The sample was characterized by X-ray diffraction, scanning electron microscope, high resolution transmission electron microscope and X-ray energy dispersion spectroscopy. The results show that its crystal phase is composed of brookite, tungsten and monotungsten carbide. The morphology of the sample particle is irregular sphere-like, with a diameter smaller than 100 nm. Its chemical components are titanium, tungsten, carbon and oxygen. Monotungsten carbide nanoparticles lie on the surface of titania core and form an incomplete shell around titania core in the nanocomposite. The measurement with a microelectrode system of three electrodes shows that the sample is electrocatalytic active to nitrophenol in basic solution at room temperature. Its peak potential is at-0.988 V (saturated calomel electrode (SCE)), which is more negative than the peak potential,-0.817 V (SCE), of mesoporous monotungsten carbide, and its peak current is 8.809 μA, which is higher than the peak current, 4.058 μA, of mesoporous monotungsten carbide. The hydrogen generation potential of the sample is at-1.199 V (SCE), which is more negative than that of pure nanosized monotungsten carbide at-1.100 V (SCE). These results show that the presence of titania in the sample can lower the peak potential of nitrophenol electrocatalysis and its hydrogen generation potential, and increase its peak current of nitrophenol electrocatalysis in basic solution at room temperature. This indicates a synergistic effect of titania and monotungsten carbide in electrocatalysis.
tungsten carbide, titania, nanocomposite, core-shell, electrocatalytic performance
1 Introduction
Since monotungsten carbide (WC) is found to have platinum-like behavior for the chemisorption of hydrogen and oxygen [1], it has been considered as an alternative electrocatalyst of Pt for hydrogen [2] or methanol fuel cells [3]. Although the performance of WC may be inferior in direct comparison to platinum [3], its low price and its insensitivity to catalyst poisons, such as H2S and CO [4], make it an interesting alternative to noble metal catalyst.
WC is isoelectronic to platinum [5], but it does not share the inertness of platinum. When exposed to water, the surface layer of WC undergoes continuous oxidation and dissolution. The surface oxidation of WC preferentially progresses on those sites where oxidic species already exist [6]. This leads to a simultaneous oxidation of tungsten and carbon is oxidized to CO2,.., visible as a gas formation when WC electrodes are oxidatively polarized [7]. The thickness of the tungsten oxide surface layer, formed by anodic polarization, is found to be between two and three monolayers, with the predominant species being hydrated and non-hydrated WO3[6-8]. This relatively undefined composition of the surface oxide layer may be the major reason for the varying rest potentials of monotungsten carbide electrodes, immersed in similar electrolyte solutions [6]. It also explains the irreproducible hydrogen adsorption potentials at tungsten carbide [6, 9]. WO3layers on WC are not stable in 0.2 mol·L-1H2SO4when polarized at 0.7 V (. SCE) [10, 11]. The oxides on the surface of monotungsten carbide are readily soluble in alkaline solutions as WO4. The dissolution of surface oxides will result in the degradation of electrocatalytic effect of WC.
The catalytic activity of tungsten carbide depends on many parameters. An important factor is the atomic ratio of tungsten to carbon [12] in the interstitial alloy. Other factors are particle size [13], lattice defects [13] and pseudometallic surfaces [9]. As for electrocatalysis, the most significant factor is the presence of oxygen species (tungsten oxides) at the surface of monotungsten carbide. This was testified by Böhm [14] in 1970. He demonstrated that on the surface of freshly prepared WC electrode, no appreciable hydrogen adsorption took place, only after the surface was anodized with low oxide coverage, the hydrogen adsorption could be improved.
All the above results indicate that surface oxides are very critical for the electrocatalytic activity of WC, and surface oxides of WC is not stable in electrochemical environment. How to keep the stability of surface oxide of WC in electrochemical environment is one of the key problems in an electrocatalysis.
Utilization of the synergistic effect of tungsten carbide and titania is one of the approaches to solve the stability problem, because the catalytic effect of titania is good, the electron diffusion coefficient of titania is also good, titania is stable in acidic and basic solution environment [15, 16]. The activity of the catalyst with Ti-containing support is generally higher than that of the catalyst supported on alumina, because Ti can increase the catalyst activity of the pre-hydrogenation path [17-21]. These imply that the preparation of titania and monotungsten carbide composite is interesting and valuable.
We reported tungsten carbide and titania composite (WC/TiO2), prepared with rutile as precursor and impregnation method to prepare intermediate product, and investigated the electrocatalytic characteristics of the composite for nitrobenzene with cyclic voltammetry [22]. In this study, we prepare tungsten carbide and titania nanocomposite with core-shell structure using anatase as precursor and a spray drying approach to prepare intermediate product, and investigate the electrocatalytic characteristics of the nanocomposite for nitrophenol by cyclic voltammetry. The electrocatalytic synergistic effect of titania and monotungsten carbide is examined.
2 Experimental
2.1 Sample preparation
An appropriate amount of ammonium metatungstate was dissolved into deionized water to form aqueous solution with a mass percentage of 5%. The solution was stirred by ultrasonic wave for 15 min. Anatase nanoparticle, prepared according to reference [23], was added into the solution at a molar ratio of W to Ti 1︰1 with stirring. The turbid liquid was stirred by ultrasonic wave for 15 min, and was guided into a spray desiccator while stirred with magnetic stirrer at room temperature. During the drying process in the spray desiccator, the flow rate of hot air at the entrance was 30 m3·h-1and that of the liquid was 5 ml·min-1, the temperature of drying air at the entrance was 438 K and that of the mixture of gas and powder at the exit was 378 K. In this way, a precursor was prepared.
Five grams of the precursor was put into a quartz boat, which was sent into a tubal resistance furnace. After pure nitrogen gas passed through the furnace at a flow rate of 50 ml·min-1for 30 min, a mixture gas at a ratio of CH4to H24︰1 passed through the furnace for 15 min, and the precursor was reduced and carbonized at 900°C for 3 h under the atmosphere of the mixture gas in the furnace. During this process, the exhaust fume was disposed by passing through 2.0 mol·L-1NaOH solution. After the furnace cooled down to room temperature under the protection of pure nitrogen gas, the quartz boat was drawn out from the furnace to get the final product.
2.2 Sample characterization

Figure 1 XRD patterns of the sample
B—brookite; M—monotungsten carbide; T—tungsten

Figure 2 SEM image of the sample
2.3 Electrocatlytic characteristics
The electrocatalytic characteristic of the sample was examined with an EG&G M273A potentiostat/ galvanostat by a microelectrode at room temperature (298 K), which was stated in detail in reference [24]. A saturated calomel electrode (SCE) was used as the reference electrode and a platinum sheet (1.0 cm2) was used as the counter electrode. Sodium hydroxide (A.R.) and nitrophenol (A.R.) were distilled with deionized water, which was obtained from a Millipore-Milli-Q system before use.
3 Results and Discussion
3.1 XRD
Figure 1 shows XRD patterns of the sample. Ten diffraction peaks, with double diffraction angles (2) around 31.5°, 35.7°, 40.3°, 48.4°, 58.4°, 64.1°, 73.3°, 75.5°, 77.2°and 84.1°, are obvious, and one diffraction peak is weak, with 2value around 25.4°. The weak diffraction peak is assigned to brookite (JCPDS: 29-1360). Diffraction peaks with 2values around 31.5°, 35.7°, 48.4°, 64.1°, 75.5°, 77.2°and 84.1°are assigned to monotungsten carbide (JCDPS: 25-1047). Diffraction peaks with 2values around 40.3°, 58.4°and 73.3°are assigned to tungsten (JCPDS: 04-0806). Thus the sample is composed of monotungsten carbide, tungsten and brookite.
3.2 SEM
Figure 2 displays SEM image of the sample. The particles are non-spherical. The diameters of most particles are smaller than 100 nm. Some particles are congregate. The white dots are those particles whose conductivity is not good enough during scanning electron microscope characterization.
3.3 HRTEM
Figure 3 shows HRTEM images of the sample. The shape and size are consistent with SEM result. Most particles of the sample display dark crust. In order to see the crust structure more clearly, one selected area, as indicated in Fig. 3 (a), is enlarged and shown in Fig. 3 (b). The crust is composed of irregular crystallites, some of which intercross each other, as shown in the middle part of Fig. 3 (b). The enlarged middle part of Fig. 3 (b) is shown in Fig. 3 (c). Two directions of lattice stripe indicate intercrossing of two particles. The distances between the two lattice stripes are 0.285 nm and 0.252 nm, which are close to the distances of the crystal plane (001) (0.28400 nm) and (100) (0.25180 nm) of monotungsten carbide (JCPDS: 25-1047), respectively. The differences of the distances between the values of JCPDS card and the estimated values of the sample may result from the error of measurement. These results indicate that the crystallite that constructs the dark crust in Fig. 3 (a) can be assigned to monotungsten carbide. Since XRD results show that the sample is composed of monotungsten carbide and titania, the core of the particle should be titania. Fig. 3 (d) shows that titania and monotungsten carbide nanocomposite is of a core-shell structure, with a titania core and monotungsten carbide shell, though the shell is incomplete.

Figure 3 TEM and HRTEM images of the sample
Figure 4 EDS result of the sample
3.4 EDS
Figure 4 shows EDS patterns of the sample, indicating the peaks of tungsten, titanium, oxygen and carbon elements. Based on this result, the atomic and mass percentages of the above elements are estimated and shown in Table 1. The atomic percentage of W is 27.89%, and that of C is 26.87%. This indicates that the ratio of W to C is larger than 1 and some metal tungsten atoms may exist in the sample. This conclusion is consistent with XRD results. The difference between W atomic percentage and C atomic percentage is only 1.02%. This can explain why the diffraction peaks of self-existent tungsten metal phase can be seen in the XRD patterns, but it is not easy to find the lattice structure of metal tungsten lattice structure in HRTEM images.

Table 1 Estimated atomic and mass percentages of thesample based on EDS analysis
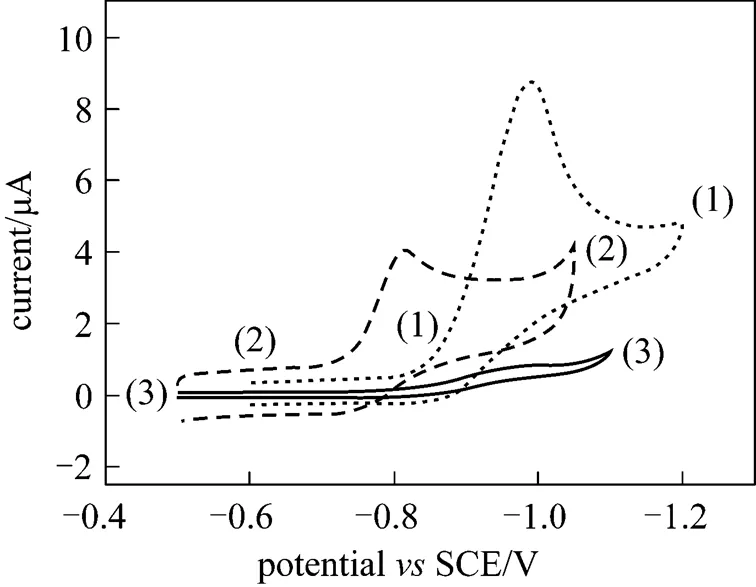
Figure 5 Cyclic voltammogram of the microelectrode of different samples in 0.5 mol·L-1sodium hydroxide +0.01 mol·L-1nitrophenol solutions (scan rate: 0.05 V·s-1)
(1)—WC@TiO2nanocomposite; (2)—hollow mesoporous WC; (3)—WC (200 nm)
3.5 Electrocatalytic performance
Figure 5 displays the electrocatalytic activities of WC, hollow mesoporous WC and the sample to nitrophenolin basic solution at room temperature. Nanosized monotungsten carbide (200 nm) is not electrocatalytically active to nitrophenol, without any apparent peak current from-0.5 V to-1.0 V. Its hydrogen generation potential is at-1.1 V (SCE), with a weak peak current of 1.205 μA. Hollow mesoporous WC, prepared according to reference [24], is electrocatalytically active to nitrophenol, with a peak current of 4.058 μA and a peak potential at-0.817 V (SCE). Its hydrogen generation potential is at-1.049 V (SCE) with a current of 4.173 μA. Titania and monotungsten carbide core-shell nanocomposite is electrocatalytically active to nitrophenol, with a peak current of 8.809 μA and a peak potential of-0.988 V (SCE). Its hydrogen generation potential is at-1.19 V (SCE) with a current of 4.884 μA. These results show that the presence of titania in the sample can lower the peak potential of nitrophenol electrocatalysis and its hydrogen generation potential, and increase its peak current of nitrophenol electrocatalysis in basic solution at room temperature. This indicates a synergistic effect of titania and monotungsten carbide in electrocatalysis.

Table 2 The values of peak currents and potentials of different samples for nitrophenol
The comparison of electrocatalytic activity of titania and monotungsten carbide core-shell nanocomposite with that of hollow mesoporous monotungsten carbide in Table 2 show that the peak potential and the hydrogen generation potential of the core-shell nanocomposite are more negative than those of hollow mesoporous WC. The peak current and the hydrogen generation current of the core-shell nanocomposite are higher than those of hollow mesoporous WC. These results show that the core-shell nanocomposite has higher overpotential and wider electrochemical window than hollow mesoporous WC in basic environment. Higher overpotential corresponds to more stable electrode at the same potential under redox in electrochemical environment. Wider electrochemical window means that wider potential range of the electrode can be used in electrochemical environment. Thus monotungsten carbide and titania core-shell nanocomposite will be found more applications in electrocatalytic area.
4 Conclusions
Based on the above results and discussion, the following conclusions can be drawn.
(1) Titania and monotungsten carbide core-shell nanocomposite, with an irregular sphere-like morphology, can be prepared by a new approach of spray drying and reduction-carbonization reaction approach.
(2) The crystal phase of titania and monotungsten carbide core-shell nanocomposite is composed of brookite, tungsten and monotungsten carbide. The core is composed of titania, and the shell is composed of monotungsten carbide mainly.
(3) Titania and monotungsten carbide core-shell nanocomposite is electrocatalytically active to nitrophenol, with a peak current of 8.809 μA and a peak potential of-0.988 V (SCE), a hydrogen generation potential of-1.19 V (SCE) and a hydrogen generation current of 4.884 μA.
(4) Titania and monotungsten carbide nanocomposite has higher overpotential and wider electrochemical window than hollow mesoporous WC in basic environment.
(5) An electrocatalytic synergistic effect exists between titania and monotungsten carbide of core-shell nanocomposite.
1 Böhm, H., “New non-noble metal anode catalysts for acid fuel cells”,, 227, 484-485(1970).
2 Böhm, H., Pohl, F.A., “The electrocatalytic property of tungsten carbide”,.., 41, 46-50 (1968).
3 Baresel, D., Gellert, W., Heidemeyer, J., Scharner, P., “Tungsten carbide as anode material for fuel cells”,...., 10, 194-195 (1971).
4 Palanker, V.S., Gajyev, R.A., Sokolsky, D.V., “On adsorption and electro-oxidation of some compounds on tungsten carbide; their effect on hydrogen electro-oxidation”,., 22, 133-136 (1977).
5 Levy, R. B., Boudar, M. T., “Platinum-like behavior of tungsten carbide in surface catalysis”,, 181, 547-548 (1973).
6 Bozzini, B., De Gaudenzi, G.P., Fanigliulo, M.A.C., “Electrochemical oxidation of WC in acidic sulphate solution”,., 46, 453-469 (2004).
7 Fleischmann, R., Bohm, H., “Water oxidation on versatile tungsten carbide materials”,., 22, 1123-1128 (1977).
8 Vidick, B., Lemaitre, J., Delmon, B., “Control of the catalytic activity of tungsten carbides (II) Physicochemical characterizations of tungsten carbides”,.., 99, 428-438 (1986).
9 Johnson, J.W., Wu, C.L., “The surface property and catalytic activity of tungsten carbide”,..., 109, 531-533 (1971).
10 Weigert, E.C., Stottlemyer, A.L., Zellner, M.B., Jingguang, G.C., “Tungsten monocarbide as potential replacement of platinum for methanol electrooxidation”,..., 111, 14617-14620 (2007).
11 Patil, P.R., Pawar, S.H., Patil, P.S., “The electrochromic properties of tungsten oxide thin films deposited by solution thermolysis”,, 136–137, 505-511 (2000).
12 Ross Jr.,P.N., Macdonald, J., Stonehart, P., “Surface composition of catalytically active tungsten carbide (WC)”,, 63, 450-455 (1975).
13 Armstrong, R.D., “The crystal structure and catalytic activity of tungsten carbide”,..., 118, 568-571 (1971).
14 Böhm, H., “Adsorption and anodic oxidation of water on tungsten carbide”,., 15, 1273-1280 (1970).
15 Diamant, Y., Chappel, S., Chen, S.G., Melamed, O., Zaban, A., “Core-shell nanoporous electrode for dye sensitized solar cells: The effect of shell characteristics on the electronic properties of the electrode”,, 248, 1271-1276 (2004).
16 Kambe, S., Nakade, S., Wada, Y., Kitamura, T., Yanagida, S., “Effects of crystal structure, size, shape and surface structural differences on photo-induced electron transport in TiO2mesoporous electrodes”,,12, 723-728 (2002).
17 Pophal, C., Kameda, F., Hoshino, K., Yoshinaka, S., Segawa, K., “Hydrodesulfurization of dibenzothiophene derivatives over TiO2-Al2O3supported sulfided molybdenum catalyst”,.,39, 21-32 (1997).
18 Wei, Z.B., Yan, W., Zhang, H., Ren, T., Xin, Q., Li, Z., “Hydrodesulfurization activity of NiMo/TiO2-Al2O3catalysts”,..:., 167, 39-48 (1998).
19 Segawa, K., Takahashi, K., Satoh, S., “Development of new catalysts for deep hydrodesulfurization of gas oil”,., 63, 123-131 (2000).
20 Grzechowiak, R.J., Wereszczako-Zielinska, I., Rinkowski, J., Ziolek, M., “Hydrodesulphurisation catalysts supported on alumina-titania”,..:., 250, 95-103 (2003).
21 Lecrenay, E., Sakanishi, K., Nagamatsu, T., Mochida, I., Suzuka, T., “Hydrodesulfurization activity of CoMo and NiMo supported on Al2O3-TiO2for some model compounds and gas oils”,..:., 18, 325-330 (1998).
22 Wang, X.J., Ma, C.A., Li, G.H., Zheng, Y.F., “Preparation and electrocatalytic properties of WC/TiO2nanocomposite”,, 67, 367-371 (2009). (in Chinese)
23 Li, G.H., Wang, D.W., Xu, Z.D., “The Experimental study about the control of TiO2nanopowder crystal phase prepared by the hydrolysis-sediment approach”,, 17, 422-427 (2002). (in Chinese)
24 Li, G.H., Ma, C.A., Zheng, Y.F., Zhang, W.M., “Preparation and electrocatalytic activity of hollow global tungsten carbide with mesoporosity”,, 85, 234-239 (2005).
** To whom correspondence should be addressed. E-mail: nanozjut@zjut.edu.cn
2010-06-29,
2010-11-06.
the National Natural Science Foundation of China (20476097), the Zhejiang Natural Science Foundation (Y4080209,Y406094), the Science Plan of Zhejiang Province (2007F70039) and the Scientific Starting Fund of Zhejiang University of Technology.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-, at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
