Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
2011-05-15ZHANGQiaoling张巧玲LIUYouzhi刘有智LIGuangming李光明andLIJunping李军平
ZHANG Qiaoling (张巧玲), LIU Youzhi (刘有智), LI Guangming (李光明) and LI Junping (李军平)
Preparation of-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
ZHANG Qiaoling (张巧玲), LIU Youzhi (刘有智)**, LI Guangming (李光明) and LI Junping (李军平)
Research Center of Shanxi Province for High Gravity Engineering and Technology, College of Chemical Engineering and Environment, North University of China, Taiyuan 030051, China
A new type of reactor, featured with impinging stream-rotating packed bed (IS-RPB) and coil pipes, was designed and used to prepare-hydroxybenzaldehyde (PHB) by hydrolysis from diazonium salts. The influence of operating parameters, such as reaction temperature, reaction time and high gravity factor, on the yield of PHB was investigated. Compared with the traditional kettle-type reactor, the yield of PHB with the new reactor is increased significantly and the reaction time is much shorter. Under the optimum conditions, the yield of PHB is increased from 51% to 84.1%. The reactor offers an opportunity for replacing the traditional batch mode operation with a continuous process.
impinging stream-rotating packed bed, coil pipe, diazonium salt,-hydroxybenzaldehyde
1 INTRODUCTION
-Hydroxybenzaldehyde (PHB) is an important intermediate for producing fine chemicals and is extensively used in perfume, medicine, agrochemical and cosmetic industries. One of the important synthesis methods for PHB is diazotization and hydrolysis of-amino benzaldehyde, which has advantages of easy separation of reaction system and high purity product. In most cases the yield from the reaction is discouragingly low due to the coupling of phenol with un-decomposed diazonium. When PHB is prepared by hydrolysis of diazonium, the main and side reactions are competitive series reaction. The mechanism is as follows:
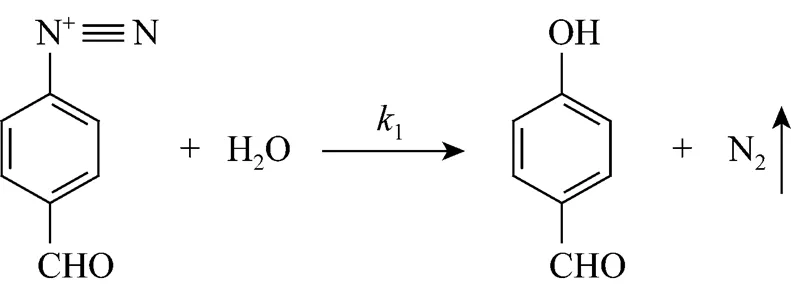

The activation energy of hydrolysis reaction (1) is 95-139 kJ·mol-1and that of coupling reaction (2) is 59-72 kJ·mol-1, that is, high temperature and rapid heating are favorable for the hydrolysis reaction.
Traditionally, commercial hydrolysis processes are carried out in a kettle-type reactor in batch mode, in which diazonium salts solution is added dropwise to the mixture (hydrolysate). As the reaction proceeds, the product reacts with diazonium salts easily, forming more by-products. Several problems frequently encountered are long operation time, large amount of by-product, and low yield of about 50%-60% [1]. To improve the yield of phenol (PHB), many measures have been proposed. An improved method is to remove phenol immediately by steam distillation after its formation to suppress the side reactions [2, 3], so that the yield of phenol is increased considerably. The process with the steam distillation is suitable for liquid phenol slightly soluble or insoluble in water, such as 2,4-dimethylphenol and guaiacol. PHB is a white crystal and easily dissolves in hot water, so the steam distillation can not be adopted. New types of reactors are needed to improve the yield of PHB. Because the hydrolysis is a consecutive complex reaction system, the reactor should have following features: shorter heating time and good micromixing to dilute the product from the reaction zone.
As an intensification device, rotating packed bed (RPB) has many advantages such as highly efficient mass and heat transfer, small size, easy operation and good flexibility [4]. Since the invention of RPB by Ramshaw [5], it has been widely applied to processes such as absorption [6-8], distillation [9, 10], extraction [11], desulfurization of flue gas [12], synthesis of nanoparticles [13-16], preparation of polymers [17], and organic synthesis [18, 19]. Recently, our group reports a new equipment, impinging stream-rotating packed bed (IS-RPB), which exhibits excellent micro- mixing efficiency and greatly improve the mass and heat transfer performance [4, 20]. In this work, a new IS-RPB reactor with coil pipes is designed and used to synthesize PHB by hydrolysis of diazonium salts.
2 EXPERIMENTAL
2.1 Preparation of diazonium salt solution
2.2 Kettle-type reactor and experimental procedure
Figure 1 shows a kettle-type reactor, which consists of a 2000 ml four-necked round-bottom flask, a stirrer, a water bath with a digital temperature controller, a thermometer, a condenser, and a funnel.

Figure 1 Schematic diagram of kettle-type reactor
The four-necked flask equipped with a reflux condenser, an overhead stirrer and a thermometer was charged with 400 ml of water and 180 ml of sulfuric acid (purity of 98%). Then, the mixture (hydrolysate) was heated to the prescribed temperature (65-95°C), 800 ml of aqueous solution of diazonium salts was added dropwise into the hydrolysate under stirring, and the hydrolysis of diazonium salts took place. After the reaction, the mixture was put into a filter (equipped withactivated carbon) to remove the black, oily-byproduct (floating over the aqueous phase). Thereafter, the solution (filtrate) containing crude product was cooled to room temperature, and the crude product of PHB was precipitated from the supersaturated solution. PHB was separated from the solution by a Buchner funnel, washed with cold distilled water for several times until the washing water became neutral, and then dried in vacuum at 60°C until the mass was constant. The purity was analyzed by high performance liquid chromatogram (HPLC) and measured concentrations were used for calculating the yield, which is defined as

whereis the mass of PAB transformed to PHB (g) and0is the mass of PAB added initially.
2.3 IS-RPB reactor and experimental procedure
Figure 2 shows the apparatus of IS-RPB, the parameters of which are given in Table 1. Fig. 3 shows the flow chart for the new reactor system for hydrolysis reaction, consisting of two pumps, an IS-RPB, a coil pipe, a filter and a condenser.

Figure 2 Structure diagram of IS-RPB
Figure 3 Flow chart of the reactor system

Table 1 Parameters of IS-RPB used in this study
The hydrolysate in tank 1 was pumped to the preheatera rotor flowmeter and preheated to 85-90°C, and then went to the nozzle of liquid distributor. At the same time, the cold diazonium salts solution in tank 2 was pumped to another nozzle of the liquid distributora rotor flowmeter. The two liquid streams flowed as jets from nozzles in the opposite direction, then collided and dispersed into the packing quickly, and the mixture solution was heated to 65-95°C by heat air of 80-100°C. In order to achieve optimal collision, the volumetric flow rates of the two streams were equal within the range of 10-30 L·h-1. From the IS-RPB chamber, the liquid was introduced into the coil pipe with length of 30 m and inner diameter of 21 mm (temperature of the mixture in coil pipe being maintained with heat-conducting oil). In preliminary tests, the length of the coil was varied between 3 amd 30 m. It was found that 30 m can assure the completion of hydrolysis and high selectivity of PHB, so the coil pipe of this length was used for all subsequent experiments. The reaction mixture in the coil pipe was nearly in plug-flow and the back-mixing was negligible. After the reaction, the treatment procedure of the resulting mixture was similar to that described in Section 2.2.
2.4 Analysis of product
HPLC was used for determining the purity of PHB. A Diamonsil C18 (220 cm×4.6 mm) was used as the analytic column, methanol/water (50/50, by volume) as the mobile phase, UV detection, wavelength at 220 nm, 1.0 ml·min-1as the flow rate, and 10 μl as the injection volume. The accuracy of the data collected was within ±1%.
3 RESULTS AND DISCUSSION
3.1 Effect of temperature on the yield of PHB
The hydrolysis reaction was carried out in the kettle-type reactor by adding diazonium salts solution dropwise in 60 min, at the stirrer speed of 800 r·min-1. The reaction temperature was controlled by the water bath. For the new reactor system, the rotational speed of IS-RPB was 1000 r·min-1and the volumetric flow rates of the two streams were 20 L·h-1. The reaction temperature was controlled by the heating air, and it was measured by thermocouple at the RPB outlet.
The effect of temperature on the yield of PHB was studied in the two reactors separately, and the results are shown in Fig. 4. The yield with the IS-RPB reactor is higher than that with the kettle-type reactor at the same temperature, and the increase is more remarkable at higher temperature.

Figure 4Effect of temperature on the yield of PHB in the two reactors
(dropping time of 60 min, stirrer speed of 800 r·min-1,of 37, residence time of 160 s)
■ kettle-type reactor;● IS-RPB reactor
Since the activation energy of coupling reaction (2) is lower than that of hydrolysis reaction (1), higher temperature is favorable for hydrolysis reaction [21]. For the kettle-type reactor, hydrolysate in the flask is heated to the prescribed temperature, and then the cool aqueous solution of diazonium salts is added dropwise into the hydrolysate, so that the temperature in the reaction system can be maintained the best for hydrolysis. However, the addition of cool solution of diazonium salts to hydrolysate drop by drop results in many small droplets of diazonium salts, so that the product (PHB) on the droplet surface reacts with the un-decomposed diazonium inside the droplet and forms more by-products. Because of the macro-mixing and longer reaction time in the kettle-type reactor, the diazo-coupling reaction may increase, resulting in a very low yield of PHB in the kettle-type reactor generally.
For the IS-RPB reactor, two liquid streams flow in the opposite direction and collision with quick mixing, then enter the high speed rotating packing, and are broken into droplets, strips and films with large interfacial area, so that the micro-mixing is greatly improved. The micro-mixing efficiency of IS-RPB is 5-10 times greater than that of kettle-type reactor [4]. With the excellent micro-mixing of diazonium salts and hydrolysate in the IS-RPB, the raw material will not accumulate locally as in the kettle- type reactor. In addition, the mixture can be further heated to the hydrolysis temperature in very short time by heat air through direct contact heat transfer of gas/liquid, so that the reaction system is always kept at higher temperature that is favorable for hydrolysis. Moreover, a coil pipe is used, in which the reaction mixture is nearly in plug flow, the backmixing is negligible and the coupling reaction is reduced.
As shown in Fig. 4, when the temperature is above 85°C, the yield decreases slightly as temperature increases, probably due to that the water evaporation increases with temperature, resulting in lower water concentration, which is unfavorable for hydrolysis reaction.
3.2 Effect of reaction time on the yield of PHB
3.2.1
Figure 5 shows the influence of time of adding diazonium salt solution on the yield of PHB. The dropping starts when all cool diazonium salts solution is added into the hydrolysate at 85°C in the flask. When addition is done in a short time, the temperature of the system decreases rapidly to below 85°C which is necessary for hydrolysis, thus more by-product will be produced in this period. Moreover, the dispersion of feed material is poor, and the product PHB formed at the drop surface will react rapidly with un-decomposed diazonium to produce more by-products. When the dropping time is longer,.., the diazonium salt solution is added more slowly, the effects of above two unfavorable factors are suppressed, and the yield of PHB becomes higher. When the dropping time is more than 60 min, the yield of PHB decreases slightly.
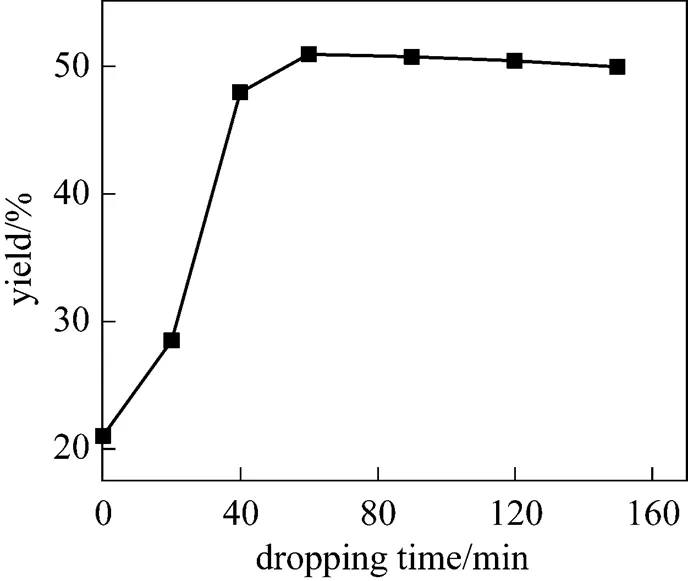
Figure 5 Effect of dropping time on the yield in kettle-type reactor
(at temperature of 85°C, stirrer speed of 800 r·min-1)
3.2.2
The residence time, which is defined as the sum of from exiting the nozzles to exiting the coil pipe, was controlled by adjusting the volumetric flow rate of the two streams. Fig. 6 illustrates the effect of residence time on the yield. The yield of PHB first increases rapidly and then decreases slightly with the increase of residence time. At 160 s, the yield is 83.4%, which is greatly higher than that with traditional kettle-type reactor. High velocity is favorable for micro-mixing efficiency of the two liquid streams and hydrolysis reaction. In addition, since the main and side reactions are in series, longer residence time leads to lower concentration of diazonium and lower reaction rate of main reaction.
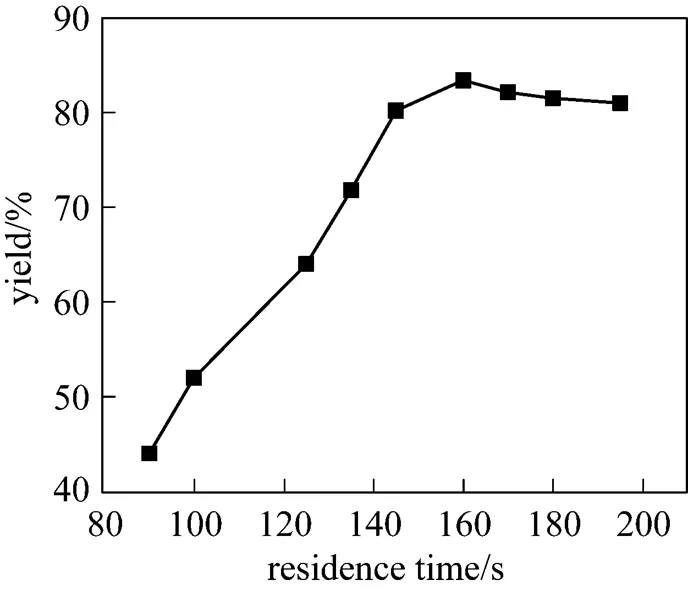
Figure 6 Effect of residence time on the yield in IS-RPB reactor
(at temperature of 85°C,of 37)
3.3 Effect of high gravity factor β on the yield of PHB
As a dimensionless factor, the high gravity factor () is defined as the ratio of the high gravitational (centrifugal) acceleration in the IS-RPB to the gravitational acceleration:


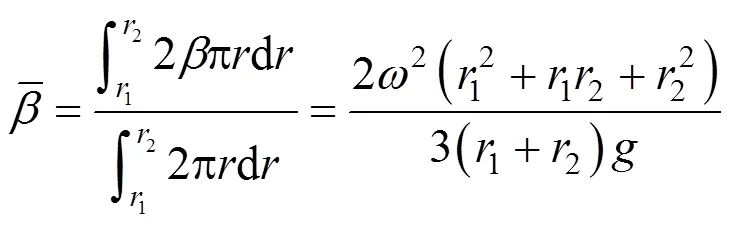
The influence ofon the yield of PHB is shown in Fig. 7. The yield is first improved greatly with. Whenis 37.4, the yield is the optimum (84%). With the further increase of, the yield changes a little only. The reason may be as follows. First, greater relative velocity among liquid elements (.. droplets, strips and films) and packing are obtained by increasing the rotational speed, so that the impingement is vigorous and coalescence-dispersion of liquid elements is improved, resulting in good mixing. Secondly, the mixture of the two streams is directly heated by heat air. The higher gravity factor leads to higher heat transfer rate between gas and liquid phases and less time required for increasing the temperature of the mixed liquid.
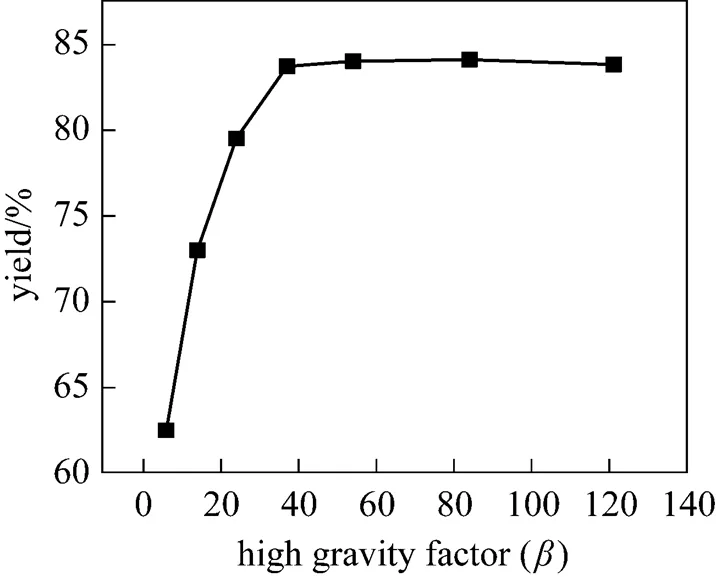
Figure 7 Effect of high gravity factor on the yield in IS-RPB reactor
(at temperature of 85°C, residence time of 160 s)
4 CONCLUSIONS
A new type of reactor, comprising IS-RPB and a coil pipe, exhibits better performance for the synthesis of PHB than the traditional kettle-type reactor due to its good heat and mass transfer performance. The IS-RPB can provide an ideal mirco-mixing environment to make diazonium salt solution dispersed into hydrolysate quickly and uniformly, overcoming the poor dispersing effect in the kettle-type reactor. Meanwhile, the quick heating process ensures that the temperature may maintain at higher operating temperature for hydrolysis reaction to reduce side reaction. The coil pipe is nearly a plug flow reactor to reduce the back-mixing and improve the yield of product. Under the optimum conditions, the yield of PHB is increased from 51% to 84.1%. Such a new reactor offers an opportunity for continuous operation for product quality rather than batch mode.
1 Luo, D.X., The Synthetic Basis of Chemical Reagents and Fine Chemicals, Higher Education Press, Beijing, 213 (1991). (in Chinese)
2 Lambooy, P. “An improved method for the hydrolysis of diazonium salts”,...., 72 (11), 5327-5328 (1950).
3 Hao, Y.X., Chen, S.S., “Study on the preparation technique of-cresol from-toluidine”,.., 14 (1), 64-66 (2002). (in Chinese)
4 Liu, Y.Z., Chemical Engineering Process and Technology in High Gravity, National Defense Industry Press, Beijing, 47-70 (2009). (in Chinese)
5 Ramshaw, C., “Higee distillation—An example of process intensification”,.., 389, 13-14 (1983).
6 Pao, D., Prahlada, K.M., “Studies on a high-gravity gas-liquid contactor”,...., 29 (5), 917-920 (1990).
7 Reddy, K.J., Gupta, A., Rao, D.P., “Process intensification in a HIGEE with split packing”,...., 45, 4270-4277 (2006).
8 Lin, C.C., Chen, B.C., Chen, Y.S., Hsu, S.K., “Feasibility of a cross-flow rotating packed bed in removing carbon dioxide from gaseous streams”,..., 62, 507-512 (2008).
9 Li, X.P., Liu, Y.Z., Li, Z.Q., Wang, X.L., “Continuous distillation experiment with rotating packed bed”,...., 16 (4), 656-662 (2008).
10 Li, X.P., Liu, Y.Z., “Characteristics of fin baffle packing used in rotating packed bed”,...., 18 (1), 55-60 (2008).
11 Liu, Y.Z., Qi, G.S., Yang, L.R., “Study on the mass transfer characteristics in impinging stream rotating packed bed extractor”,...., 22 (10), 1108-1111 (2003). (in Chinese)
12 Zhang, Y.H., Liu, L.S., Liu, Y.Z., “Experimental study on flue gas dedusting by high gravity rotary bed”,...., 21, 42-43 (2003). (in Chinese)
13 Li, Y., Liu, Y.Z., “Synthesis and catalytic activity of copper(II) resorcylic acid nanoparticles”,...., 23 (2), 217-220 (2007).
14 Chen, J.F., Zhou, M.Y., Shao, L., Wang, Y.Y., Yun, J., Chew, N.Y.K., Chan, H.K., “Feasibility of preparing nanodrugs by high-gravity reactive precipitation”,..., 269 (1), 267-274 (2004).
15 Chen, J.F., Wang, Y.H., Guo, F., Wang, X.M., “Synthesis of nanoparticles with new technology: High-gravity reactive precipitation”,...., 39, 948-954 (2003).
16 Li, Y., Guo, Y., Liu, Y.Z., “Synthesis of high purity TiO2nanoparticles from Ti(SO4)2in presence of EDTA as complexing agent”,, 3, 240-242 (2005).
17 Zhang, L., Gao, H., Zhou, H.K., “Preparation of butyl rubber by new high-gravity technology”,..... (), 59 (11), 260-263 (2008). (in Chinese)
18 Zhang, D., Zhang, P.Y., Zou, H.K., Chu, G.W., Wu, W., Zhu, Z.W., Shao, L., Chen, J.F., “Application of HIGEE process intensification technology in synthesis of petroleum sulfonate surfactant”,.., 49, 508-513 (2010).
19 Ding, J.S., Chen, J.F., Hua, W.Q., Chu, G.W., Yao, Y., Zou, H.K., Luo, P.C., Zhang, P.Y., Yu, T.J., Xu, L.M., Zhang, G., Ma, D.Q., “A method of preparing isocyanate”, Chin. Pat., 200710098040.4 (2007).
20 Jiao, W.Z., Liu, Y.Z., Qi, G.S., “A new impinging stream-rotating packed bed reactor for improvement of micromixing iodide and iodate”,..., 157, 168-173 (2010).
21 Chen, R.Y., Organic Synthesis Process Optimization, Chemical Industry Press, Beijing, 182-195 (2006).
** To whom correspondence should be addressed. E-mail: qlzhang_65@sohu.com
2010-05-19,
2010-11-07.
the Science and Technology Key Projects of Shanxi Province (20090321113).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-, at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
- Alkylation of p-Cresol with tert-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
