利用给体-π-受体分子模板在Au(111)电极表面构筑富勒烯带状结构
2010-12-12严会娟温国永张德清万立骏
严会娟 王 栋 温国永 张德清 万立骏
(中国科学院化学研究所,分子纳米结构与纳米技术院重点实验室,北京分子科学国家实验室,北京 100190)
The unique physical and chemical properties of fullerenes endow them with a great deal of application potential in many fields[1-3].In particular,due to their strong π-electron accepting ability,fullerenes and their derivatives have been extensively used in solar cell devices involving electron transfer processes between fullerenes and organic electron donor molecules,such as poly(3-hexylthiophene)[2]and porphyrin[4-6].“Bottom-up”strategy,as a promising supplement or even replacement for traditional“top-down”techniques,has been adopted to build these molecular devices[4-9].One representative method of“bottom-up”strategy is to fabricate the composite structure via supramolecular self-assembly of functional molecules on various electrode surfaces[10].The controllable assembly of fullerenes and functional molecules on electrode surfaces is important for the fabrication of molecular devices with improved electronic properties.
High resolution scanning tunneling microscopy(STM)has been proved to be a powerful tool to study the two dimensional self-assembly structures of functional molecules on various surfaces[11-13].The composite assembly structures of fullerene with porphyrin or phthalocyanine through π-π or donor-acceptor interaction on electrode surfaces have been studied extensively by STM in different environments[14-17].For example,Itaya and coworkers[14-15]reported a series of supramolecular assembly arrays of C60molecules on metaloctaetylporphyrin(MOEP)-modified Au(111)surfacepreparedthroughsuccessiveimmersioninto benzene solution containing MOEP and C60molecules.In situ electrochemical STM(ECSTM)study in HClO4solution indicated that the underlying organic molecule modified gold electrode surface could guide the assembly process of the C60molecular adlayerandcontroltheorientationofthefullerenederivatives[10,15]. The self-assembly of ordered hybrid bilayers of C60and bis(3-cyanophenyl)-substituted monomer or triply fused diporphyrins was fabricated on Ag(111)and Ag(100)surface by the“bottomup”method in an ultrahigh vacuum(UHV)environment by Bonifazi et al.[16].The obtained high resolution STM images demonstrated the C60guests organized into long chains and/or two-dimensional arrays.The precise location of C60molecules on a zinc porphyrin derivative array was determined to be on top of the 3-cyanophenyl substituent.Bipyridine compounds carrying multiple fullerene units(Py2F3)were found to form bright spots on the periphery of dendritic molecules composed of multiple zinc porphyrin units(DP12),which was visualized by STM under UHV conditions[17].Furthermore,supramolecular assembly network structures with proper cavities fabricated by a variety of different molecules[18-22]were used to selectively recognize fullerenes as guest molecules.
Since Aviram and Ratner provided the theoretical basis of donor-bridge-acceptor as the organic counterpart of p-n junction in 1974[23],organic donor-bridge-acceptor molecules have attracted great attention in building molecular electronic devices[24-26]. Z-β-(5-hexadecyloxy-1,3,3-trimethyl-2-indolium)-α-cyano-4-styryl dicyanomethanide(C16H33O-I3CNQ)is a typical donor-π bridgeacceptor molecule synthesized on the basis of tetracyanoquinodimethane(TCNQ),a typical electron acceptor molecule,and indolium,a typical donor molecule.The chemical structure of C16H33O-I3CNQ is illustrated in Scheme 1.The molecule includes mainly two sections:a planar,conjugated donor-π-acceptor moiety and a long alkyl chain.Organic field electronic transistor (OFET)devices based on C16H33O-I3CNQ showed high performance with p-type semiconductor characteristics[25-26].Studying the arrangement of C16H33O-I3CNQ on electrode surface will benefit the understanding of the structure-property relationship of this type of materials.Meanwhile,to the best of our knowledge,few studies have been reported on the assembly of donorπ-acceptor molecules on electrode surface.
In the present work,the two-component self-assembly structures of C60and C16H33O-I3CNQ on Au(111)electrode were fabricated and investigated by electrochemical STM.The structure details of C16H33O-I3CNQ adlayer and the composite adlayer were revealed by high resolution STM images.The intermolecular interaction was discussed to explain the observed results.
1 Experimental
C16H33O-I3CNQ molecules were synthesized and characterized according to previous reference[26].The solution containing the C16H33O-I3CNQ molecules(ca 1 mmol·L-1)was prepared with ethanol(Acros Organics,USA).10 μmol·L-1C60solution was prepared with toluene(Acros Organics,USA).
TheECSTMapparatus was a Nanoscope E microscopy(Veeco Co.,USA).The STM observation was performed in HClO4solution under potential control.The HClO4electrolyte solutions were prepared by diluting ultrapure HClO4(60%,Kanto Chemical Co.,Japan)with Milli-Q water.The potentials were positioned at the double layer region.A platinum wire and a reversible hydrogen electrode(RHE)were used as the counter elecctrode and the reference electrode,respectively.All potentials are reported with respect to the RHE.The tunneling tips were prepared by electrochemically etching a tungsten wire(0.25 mm in diameter)in 0.6 mol·L-1KOH.An AC voltage of 12-15 V was applied until the etching process stopped.Then the W tips were coated with a clear nail polish to minimize the faradic current.The STM images shown here were obtained in the con-stant-current mode to evaluate corrugation heights of the adsorbed molecules.

Scheme 1 Chemical structures of C16H33O-I3CNQ and C60
The(111)facets on an Au singe-crystal bead,prepared by melting an Au wire(0.8 mm,99.999%),were used for STM experiments.The molecular adlayers were prepared by immersing the single-crystal Au bead into the solution containing sample molecules for 10-30 s.After the formation of molecular adlayers,the Au bead was rinsed with Milli-Q water to remove the remnant molecules and then mounted into STM electrochemical cell.The composite structures of C16H33O-I3CNQ and C60were prepared by immersing the C16H33O-I3CNQ modified Au(111) surface in C60solution for 10-20 s.
2 Results and discussion
2.1 Adlayer of C16H33O-I3CNQ
Fig.1(a)shows a typical large scale STM image of C16H33OI3CNQmoleculeonAu(111)electrodesurfacewithascaleof100 nm×100 nm.It can be seen that C16H33O-I3CNQ molecules selforganize into lamellar-like structures with alternately arranged bright and shaded stripes.The bright stripes should be attributed to the donor-π-acceptor moieties because the high electron density of large conjugated planar structures is well-known to give high contrast in STM[27-28].Naturally,the long alkyl chains should contribute to the shade space in the nanostructures of C16H33OI3CNQ.Although previous results demonstrated the〈110〉direction of the Au(111)substrate was the preferable orientation for long alkyl chains[29-30],the alkyl chains of C16H33O-I3CNQ orientate randomly relative to the Au(111)substrate because the stripes in the 2D nanostructure are not arranged orderly.Furthermore, the whole 2D nanostructure of C16H33O-I3CNQ is short-range ordered,which may be related to the strong molecule-substrate interaction[31].
More detailed information about the arrangement of C16H33OI3CNQ on Au(111)was disclosed in the high resolution STM image(Fig.1(b)).The bright stripes in Fig.2(a)split into ordered bright spots as two closely packed long bars,which are outlined by two black frames in Fig.2(b).The separation between neighboring long bars along molecular rows is measured to be(0.82± 0.03)nm.Each long bar is measured with a length of(1.60±0.03) nm,which matches well with the length of donor-π-acceptor moieties of C16H33O-I3CNQ.Therefore the two long bars should be attributed to two donor-π-acceptor moieties arranged in a head-to-head configuration.According to previous results[32-33], there exists strong electrostatic repulsion if two cyanic groups arrange head-to-head because of their strong electron-withdrawing ability.However,partial electron transfer between the TCNQ moieties and indole moieties via their π bridge should lower the electron-withdrawing ability of cyanic groups and thus weaken the repulsion between them.
The alkyl chains in the shaded space in Fig.2(a)appear as interdigitated lines with a separation of(0.48±0.03)nm,which is in agreement with previous results[29-30].The length of the long alkyl lines was measured with(2.12±0.03)nm.The size is in agreement with the length of hexadecyloxy chain.There is an angle β of 160°±2°between hexadecyloxy chains and donor-π-acceptor moieties.The angle between hexadecyloxy chains and molecular rows is 100°±2°.

Fig.1 (a)Large scale STM image of C16H33O-I3CNQ nanostructure on Au(111)electrode surface,(b)high resolution STM image of C16H33O-I3CNQ nanostructure,(c)proposal model for the C16H33O-I3CNQ nanostructureE=470 mV,Ebias=120 mV,Itip=1 nA
According to the obtained STM data,a unit cell can be ob-tained and superimposed in Fig.1(b)with a=(0.82±0.03)nm,b= (6.21±0.03)nm,and α=(100±2)°.Nevertheless,since the whole adlayer of C16H33O-I3CNQ is short-range ordered,the unit cell parameters are only applicable for the ordered area.Based on the chemical structure of C16H33O-I3CNQ and STM data,a structural model obtained with HyperChem software was proposed in Fig.1(c).The C16H33O-I3CNQ molecules in this model are arranged with the head-to-head orientation.This model agrees well with the STM image.
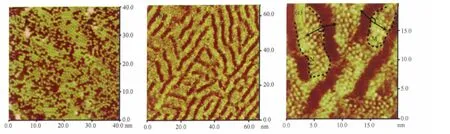
Fig.2 (a)STM image of C60nanostructure on Au(111)surface,E=507 mV,Ebias=100 mV,Itip=1 nA;(b)large scale STM image of C60 and C16H33O-I3CNQ composite structure on Au(111)surface,66.5 nm×66.5 nm,E=505 mV,Ebias=107 mV,Itip=1 nA;(c)high resolution STM image of C60and C16H33O-I3CNQ composite structure,E=505 mV,Ebias=107 mV,Itip=1 nA,19.7 nm×19.7 nm
2.2 Composite structure of C60and C16H33O-I3CNQ
Pure C60molecules adsorb randomly on Au(111)surface and form disordered structure,as illustrated in the STM image of Fig.2(a).The adlayer structure of C60is the same as the previous results[34].
Different from the stripe-like nanostructure of pure C16H33OI3CNQ molecules and random structure of C60molecules,the composite adlayer of C60and C16H33O-I3CNQ shows an interesting pattern on Au(111)substrate.As demonstrated in the large scale STM image of Fig.2(b),a series of bright spots appear on the bright stripes formed by donor-π-acceptor moietie of C16H33OI3CNQ in the 2D composite nanostructure.However,no change can be observed from the shaded space formed by alkyl chains.
From the high resolution STM image in Fig.2(c),it can be seen that the shaded space is still occupied by interdigitated alkyl chains and no C60molecule is adsorbed on the alkyl chains.The bright stripes composed of donor-π-acceptor moieties,however, are covered by bright and round spots with a diameter of(0.70± 0.03)nm.The size is in good agreement with the diameter of C60.So each bright spot can be ascribed to one C60molecule. That is to say,induced by the template of C16H33O-I3CNQ,C60molecules only deposit along the bright stripes of donor-πacceptor moieties and self-organize into band-like structures with a width of 2-5 nm.The width of C60band structures is varied consistently with that of donor-π-acceptor stripes.There are 3-6 C60molecules with a separation of(0.8±0.1)nm along the transverse orientation of C60arrays.Although the space for the assembly of C60molecules is limited by the bright stripes of donorπ-acceptor moieties,C60molecules are arranged into some shortranged ordered domains in their band-like assembly,as circled by the doted curves in Fig.2(c),marked by M and N,respectively.In domain M and N,there is an angle δ of 160°±2°between the long alkyl chains and the orientation of C60molecular rows indicated by black arrows in Fig.2(c).This angle is the same as the angle(β)between alkyl chains and donor-π-acceptor moieties in the adlayer of C16H33O-I3CNQ itself.Hence,it can be inferred that the orientation of C60molecular rows in the band arrays is affected by the arrangement of donor-π-acceptor moieties.The donor-acceptor interaction or π-π stacking interaction between C60and donor-π-acceptor moieties of C16H33O-I3CNQ may contribute to the formation of C60band assembly.This is also the reason why no C60adsorption occurs on the alkyl chains.The detailed information about the interaction between C60and C16H33OI3CNQ is under investigation.
3 Conclusions
Wehave investigated the 2D nanostructures of C16H33O-I3CNQ and C60molecules on Au(111)electrode surface by ECSTM.Pure C16H33O-I3CNQ molecules form stripe-like nanostructures.The donor-π-acceptor moieties are arranged in a head-to-head orientation.C60band structures are successfully fabricated by using C16H33O-I3CNQ adlayer as template.There are 3-6 C60molecules along the transverse orientation of every C60band structure.The orientation of C60molecular rows in the band structures is affected by the arrangement of donor-π-acceptor moieties.π-π stacking interactions and charge transfer between C60molecules and C16H33O-I3CNQ are thought to be associated with the formation of C60band-like assembly.This result provides a novel approach to control the nanostructure of fullerene,which would benefit the related organic molecular devices fabrication in the future.
1 Allemand,P.M.;Khemani,K.C.;Koch,A.;Wudl,F.;Holczer,K.; Donovan,S.;Grüner,G.;Thompson,J.D.Science,1991,253:301
2 Kim,Y.;Cook,S.;Tuladhar,S.M.;Choulis,S.A.;Nelson,J.; Durrant,J.R.;Bradley,D.D.C.;Giles,M.;Mcculloch,I.;Ha,C. S.;Ree,M.Nature Mater.,2006,5:197
3 Liu,H.B.;Li,Y.L.;Jiang,L.;Luo,H.Y.;Xiao,S.Q.;Fang,H.J.; Li,H.M.;Zhu,D.B.;Yu,D.P.;Xu,J.;Xiang,B.J.Am.Chem. Soc.,2002,124:13370
4 Imahori,H.;Fukuzmi,S.Adv.Funct.Mater.,2004,14:525
5 Hasobe,T.;Imahori,H.;Kamat,P.V.;Ahn,T.K.;Kim,S.K.; Kim,D.;Fujimoto,A.;Hirakawa,T.;Fukuzumi,S.J.Am.Chem. Soc.,2005,127:1216
6 Boyd,P.D.W.;Reed.C.A.Acc.Chem.Res.,2005,38:235
7 Lu,W.;Lieber,C.M.Nature Mater.,2007,6:841
8 Li,G.;Shrotriya,V.;Huang,J.S.;Yao,Y.;Moriarty,T.;Emery, K.;Yang,Y.Nature Mater.,2005,4:864
9 Schiek,M.;Balzer,F.;Al-Shamery,K.;Brewer,J.R.;Lützen,A.; Rubahn,H.G.Small,2008,4:176
10 Yoshimoto,S.;Honda,Y.;Ito,O.;Itaya,K.J.Am.Chem.Soc., 2008,130:1085
11 Jiang,P.;Ma,X.C.;Ning,Y.X.;Song,C.L.;Chen,X.;Jia,J.F.; Xue,Q.K.J.Am.Chem.Soc.,2008,130:7790
12 Shao,X.;Luo,X.C.;Hu,X.Q.;Wu,K.J.Phys.Chem.B,2006, 110:15393
13 (a)Wan,L.J.Acc.Chem.Res.,2006,39:334 (b)Li,S.S.;Northrop,B.;Yuan,Q.H.;Wan,L.J.;Stang,P.J.Acc. Chem.Res.,2009,42:249
14 (a)Yoshimoto,S.;Tsutsumi,E.;Honda,Y.;Ito,O.;Itaya,K. Chem.Lett.,2004,33:914 (b)Yoshimoto,S.;Sugawara,S.;Itaya,K.Electrochemistry,2006, 74:175
15 (a)Yoshimoto,S.;Tsutsumi,E.;Honda,Y.;Murata,Y.;Murata, M.;Komatsu,K.;Ito,O.;Itaya,K.Angew.Chem.Int.Edit.,2004, 43:3044 (b)Yoshimoto,S.;Saito,A.;Tsutsumi,E.;D′Souza,F.;Ito,O.; Itaya,K.Langmuir,2004,20:11046
16 Bonifazi,D.;Kiebele,A.;Stöhr,M.;Cheng,F.Y.;Jung,T.; Diederich,F.;Spillmann,H.Adv.Funct.Mater.,2007,17:1051
17 Li,W.S.;Kim,K.S.;Jiang,D.L.;Tanaka,H.;Kawai,T.;Kwon,J. H.;Kim,D.;Aida,T.J.Am.Chem.Soc.,2006,128:10527
18 Pan,G.B.;Cheng,X.H.;Höger,S.;Freyland,W.J.Am.Chem. Soc.,2006,128:4218
19 Piot,L.;Silly,F.;Tortech,L.;Nicolas,Y.;Blanchard,P.;Roncali J.;Fichou,D.J.Am.Chem.Soc.,2009,131:12864
20 MacLeod,J.M.;Ivasenko,O.;Fu,C.;Taerum,T.;Rosei,F.; Perepichka,D.F.J.Am.Chem.Soc.,2009,131:16844
21 Theobald,J.A.;Oxtoby,N.S.;Phillips,M.A.;Champness,N.R.; Beton,P.H.Nature,2003,424:1029
22 Xu,B.;Tao,C.G.;Williams,E.D.;Reutt-Robey,J.E.J.Am. Chem.Soc.,2006,128:8493
23 Aviram,A.;Ratner,M.Chem.Phys.Lett.,1974,29:277
24 Ashwell,G.J.;Tyrrell,W.D.;Whittam,A.J.J.Am.Chem.Soc., 2004,126:7102
25 Metzger,R.M.;Chen,B.;Höpfner,U.;Lakshmikantham,M.V.; Vuillaume,D.;Kawai,T.;Wu,X.;Tachibana,H.;Hughes,T.V.; Sakurai,H.;Baldwin,J.W.;Hosch,C.;Cava,M.P.;Brehmer,L.; Ashwell,G.J.J.Am.Chem.Soc.,1997,119:10455
26 Wen,G.Y.;Wang,Y.;Song,Y.B.;Lu,Z.L.;Zhang,D.Q.;Liu, Y.Q.;Zhu,D.B.Chem.Phy.Lett.,2006,431:370
27 Fisher,J.;Blöchl,P.E.Phys.Rev.Lett.,1993,70:3263
28 Katsonis,N.;Marchenko,A.;Fichou,D.J.Am.Chem.Soc.,2003, 125:13682
29 Uosaki,K.;Yamada,R.J.Am.Chem.Soc.,1999,121:4090
30 Marchenko,A.;Lukyanets,S.;Cousty,J.Phys.Rev.B,2002,65: 45414
31 Wang,L.;Yan,H.J.;Wan,L.J.J.Nanosci.Nanotechnol.,2007, 7:3111
32 Yan,H.J.;Li,S.S.;Yan,C.J.;Chen,Q.;Wan,L.J.Sci.China Ser. B-Chem.,2009,52:559
33 Kamna,M.M.;Graham,T.M.;Love,J.C.;Weiss,P.S.Surf.Sci., 1998,419:12
34 Su,G.J.;Gan,L.H.,Yang,Z.Y.;Pan,G.B.;Wan,L.J.;Wang.C. R.J.Phys.Chem.B,2006,110:5559
